Clinical Application of Gait Retraining in the Injured Runner
Abstract
:1. Background
2. Methods
3. Biomechanical Risk Factors for Running-Related Injury (RRI)
4. Gait Retraining Overview
5. Interventions Characterizing Gait Retraining Variables
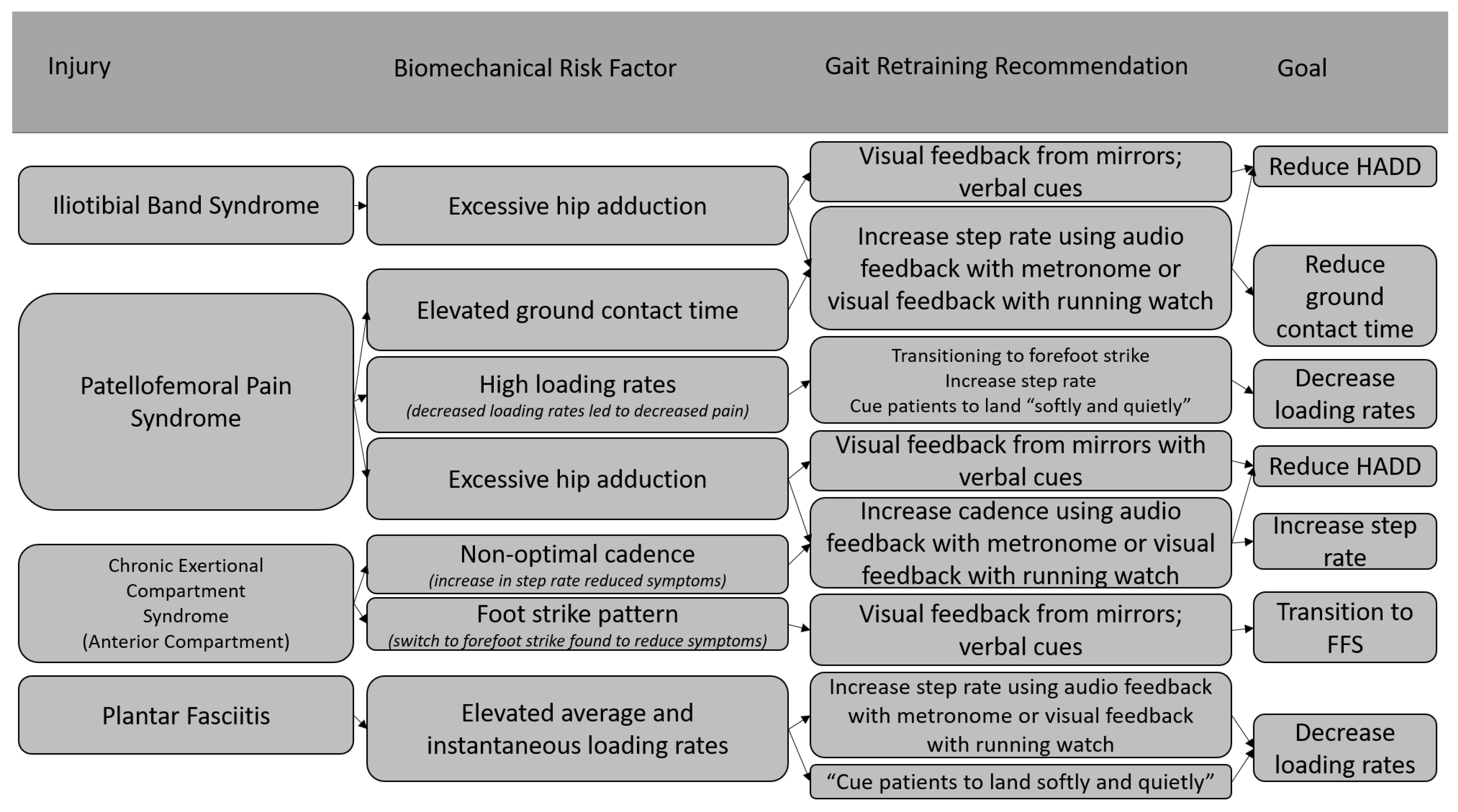
6. Clinical Application of Gait Retraining
7. Limitations of Current Gait Retraining Strategies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.C.; Pate, R.R.; Lavie, C.J.; Sui, X.; Church, T.S.; Blair, S.N. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 2014, 64, 472–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, F.; Campbell, J.; Williamson, C.; Richards, J.; Kelly, P. A scoping review of the relationship between running and mental health. Int. J. Environ. Res. Public Health 2020, 17, 8059. [Google Scholar] [CrossRef] [PubMed]
- Lun, V.; Meeuwisse, W.H.; Stergiou, P.; Stefanyshyn, D. Relation between running injury and static lower limb alignment in recreational runners. Br. J. Sports Med. 2004, 38, 576–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelsey, J.L.; Bachrach, L.K.; Procter-Gray, E.; Nieves, J.; Greendale, G.A.; Sowers, M.; Brown, B.W.; Matheson, K.A.; Crawford, S.L.; Cobb, K.L. Risk factors for stress fracture among young female cross-country runners. Med. Sci. Sports Exerc. 2007, 39, 1457–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauh, M.J.; Margherita, A.J.; Rice, S.G.; Koepsell, T.D.; Rivara, F.P. High School Cross Country Running Injuries: A Longitudinal Study. J. Clin. Sport Med. 2000, 10, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M.D.; Caldwell, W.; Schlueter, E.L.; Walters, C.; Hassen, A. Hip abductor strength and lower extremity running related injury in distance runners: A systematic review. J. Sci. Med. Sport 2017, 20, 349–355. [Google Scholar] [CrossRef]
- Willy, R.W.; Davis, I.S. The effect of a hip-strengthening program on mechanics during running and during a single-leg squat. J. Orthop. Sports Phys. Ther. 2011, 41, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Noehren, B.; Scholz, J.; Davis, I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br. J. Sports Med. 2011, 45, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Willy, R.W.; Scholz, J.P.; Davis, I.S. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin. Biomech. 2012, 27, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.-L.; Dilauro, A.; Weeks, C.; Odell, C.; Kincaid, H.; VanDine, B.; Wu, W.F. Short-term effects of a trunk modification program on patellofemoral joint stress in asymptomatic runners. Phys. Ther. Sport 2020, 44, 107–113. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Nakagawa, T.H.; Lessi, G.C.; Luz, B.C.; Matsuo, H.T.; Nakashima, G.Y.; Maciel, C.D.; Serrão, F.V. Effects of three gait retraining techniques in runners with patellofemoral pain. Phys. Ther. Sport 2019, 36, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Crowell, H.P.; Davis, I.S. Gait retraining to reduce lower extremity loading in runners. Clin. Biomech. 2011, 26, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clansey, A.C.; Hanlon, M.; Wallace, E.S.; Nevill, A.; Lake, M.J. Influence of Tibial shock feedback training on impact loading and running economy. Med. Sci. Sports Exerc. 2014, 46, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Bowser, B.J.; Fellin, R.; Milner, C.E.; Pohl, M.B.; Davis, I.S. Reducing impact loading in runners: A one-year follow-up. Med. Sci. Sports Exerc. 2018, 50, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Ching, E.; An, W.W.-K.; Au, I.P.H.; Zhang, J.H.; Chan, Z.Y.; Shum, G.; Cheung, R.T. Impact Loading during Distracted Running before and after Auditory Gait Retraining. Int. J. Sports Med. 2018, 39, 1075–1080. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chan, Z.Y.S.; Au, I.P.H.; An, W.W.; Cheung, R.T.H. Can runners maintain a newly learned gait pattern outside a laboratory environment following gait retraining? Gait Posture 2019, 69, 8–12. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chan, Z.Y.-S.; Au, I.P.-H.; An, W.W.; Shull, P.B.; Cheung, R.T.-H. Transfer Learning Effects of Biofeedback Running Retraining in Untrained Conditions. Med. Sci. Sports Exerc. 2019, 51, 1904–1908. [Google Scholar] [CrossRef]
- Sheerin, K.R.; Reid, D.; Taylor, D.; Besier, T.F. The effectiveness of real-time haptic feedback gait retraining for reducing resultant tibial acceleration with runners. Phys. Ther. Sport 2020, 43, 173–180. [Google Scholar] [CrossRef]
- Esculier, J.-F.; Bouyer, L.J.; Dubois, B.; Fremont, P.; Moore, L.; McFadyen, B.; Roy, J.-S. Is combining gait retraining or an exercise programme with education better than education alone in treating runners with patellofemoral pain? A randomised clinical trial. Br. J. Sports Med. 2018, 52, 659–666. [Google Scholar] [CrossRef]
- Willy, R.W.; Buchenic, L.; Rogacki, K.; Ackerman, J.; Schmidt, A.; Willson, J.D. In-field gait retraining and mobile monitoring to address running biomechanics associated with tibial stress fracture. Scand. J. Med. Sci. Sports 2016, 26, 197–205. [Google Scholar] [CrossRef]
- Baumgartner, J.; Gusmer, R.; Hollman, J.; Finnoff, J.T. Increased stride-rate in runners following an independent retraining program: A randomized controlled trial. Scand. J. Med. Sci. Sports 2019, 29, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Kliethermes, S.A.; Stiffler-Joachim, M.R.; Wille, C.M.; Sanfilippo, J.L.; Zavala, P.; Heiderscheit, B.C. Lower step rate is associated with a higher risk of bone stress injury: A prospective study of collegiate cross country runners. Br. J. Sports Med. 2021, 55, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Doyle, E.; Doyle, T.L.A.; Bonacci, J.; Fuller, J.T. The Effectiveness of Gait Retraining on Running Kinematics, Kinetics, Performance, Pain, and Injury in Distance Runners: A Systematic Review with Meta-analysis. J. Orthop. Sports Phys. Ther. 2022, 52, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Gardner, E.R.; McNeal, D.R.; Barto, P.S.; Nicholson, D.E. Standing balance training: Effect on balance and locomotion in hemiparetic adults. Arch. Phys. Med. Rehabil. 1989, 70, 755–762. [Google Scholar]
- Vannatta, C.N.; Heinert, B.L.; Kernozek, T.W. Biomechanical risk factors for running-related injury differ by sample population: A systematic review and meta-analysis. Clin. Biomech. 2020, 75, 104991. [Google Scholar] [CrossRef]
- Ceyssens, L.; Vanelderen, R.; Barton, C.; Malliaras, P.; Dingenen, B. Biomechanical Risk Factors Associated with Running-Related Injuries: A Systematic Review. Sports Med. 2019, 49, 1095–1115. [Google Scholar] [CrossRef] [Green Version]
- Willwacher, S.; Kurz, M.; Robbin, J.; Thelen, M.; Hamill, J.; Kelly, L.; Mai, P. Running-Related Biomechanical Risk Factors for Overuse Injuries in Distance Runners: A Systematic Review Considering Injury Specificity and the Potentials for Future Research. Sports Med. 2022, 52, 1863–1877. [Google Scholar] [CrossRef]
- Duffey, M.J.; Martin, D.F.; Cannon, D.W.; Craven, T.; Messier, S.P. Etiologic factors associated with anterior knee pain in distance runners. Med. Sci. Sports Exerc. 2000, 32, 1825–1832. [Google Scholar] [CrossRef]
- Messier, S.P.; Davis, S.E.; Curl, W.W.; Lowery, R.B.; Pack, R.J. Etiological factors associated with patellofemoral pain in runners. Med. Sci. Sports Exerc. 1991, 23, 1008–1015. [Google Scholar] [CrossRef]
- Becker, J.; Nakajima, M.; Wu, W.F.W. Factors Contributing to Medial Tibial Stress Syndrome in Runners: A Prospective Study. Med. Sci. Sports Exerc. 2018, 50, 2092–2100. [Google Scholar] [CrossRef]
- Becker, J.; James, S.; Wayner, R.; Osternig, L.; Chou, L. Biomechanical Factors Associated With Achilles Tendinopathy and Medial Tibial Stress Syndrome in Runners. Am. J. Sports Med. 2017, 45, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Bramah, C.; Preece, S.J.; Gill, N.; Herrington, L. Is There a Pathological Gait Associated with Common Soft Tissue Running Injuries? Am. J. Sports Med. 2018, 46, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Tenforde, A.S.; Outerleys, J.; Reilly, J.; Davis, I.S. Impact-Related Ground Reaction Forces Are More Strongly Associated With Some Running Injuries Than Others. Am. J. Sports Med. 2020, 48, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.; João, S.M.A.; Dinato, R.C.; Tessutti, V.D.; Sacco, I.C.N. Dynamic patterns of forces and loading rate in runners with unilateral plantar fasciitis: A cross-sectional study. PLoS ONE 2015, 10, e0136971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, M.B.; Hamill, J.; Davis, I.S. Biomechanical and Anatomic Factors Associated with a History of Plantar Fasciitis in Female Runners. Clin. J. Sport Med. 2009, 19, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Heiderscheit, B.C.; Chumanov, E.S.; Michalski, M.P.; Wille, C.M.; Ryan, M.B. Effects of step rate manipulation on joint mechanics during running. Med. Sci. Sports Exerc. 2011, 43, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.S.; Tenforde, A.S.; Neal, B.S.; Roper, J.L.; Willy, R.W. Gait Retraining as an Intervention for Patellofemoral Pain. Curr. Rev. Musculoskelet. Med. 2020, 13, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Napier, C.; Cochrane, C.K.; Taunton, J.E.; Hunt, M.A. Gait modifications to change lower extremity gait biomechanics in runners: A systematic review. Br. J. Sports Med. 2015, 49, 1382–1388. [Google Scholar] [CrossRef]
- Cheung, R.T.H.; An, W.W.; Au, I.P.H.; Zhang, J.H.; Chan, Z.Y.S.; MacPhail, A.J. Control of impact loading during distracted running before and after gait retraining in runners. J. Sports Sci. 2018, 36, 1497–1501. [Google Scholar] [CrossRef]
- Neto, W.C.d.; Lopes, A.D.; Ribeiro, A.P. Gait Retraining with Visual Biofeedback Reduces Rearfoot Pressure and Foot Pronation in Recreational Runners. J. Sport Rehabil. 2022, 31, 165–173. [Google Scholar] [CrossRef]
- Cheung, R.T.H.; Davis, I.S. Landing pattern modification to improve patellofemoral pain in runners: A case series. J. Orthop. Sports Phys. Ther. 2011, 41, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.L.; Harding, E.M.; Doerfler, D.; Dexter, J.G.; Kravitz, L.; Dufek, J.S.; Mermier, C.M. The effects of gait retraining in runners with patellofemoral pain: A randomized trial. Clin. Biomech. 2016, 35, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chan, Z.Y.S.; Zhang, J.H.; Ferber, R.; Shum, G.; Cheung, R.T.H. The effects of midfoot strike gait retraining on impact loading and joint stiffness. Phys. Ther. Sport 2020, 42, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Luo, Z.; Wang, X.; Ye, D.; Fu, W. Alterations in running biomechanics after 12 week gait retraining with minimalist shoes. Int. J. Environ. Res. Public Health 2020, 17, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, P.P.K.; Chan, Z.Y.S.; Au, I.P.H.; Lam, B.M.F.; Lam, W.K.; Cheung, R.T.H. Biomechanical effects following footstrike pattern modification using wearable sensors. J. Sci. Med. Sport 2021, 24, 30–35. [Google Scholar] [CrossRef]
- Helmhout, P.H.; Diebal, A.R.; van der Kaaden, L.; Harts, C.C.; Beutler, A.; Zimmermann, W.O. The effectiveness of a 6-week intervention program aimed at modifying running style in patients with chronic exertional compartment syndrome: Results from a series of case studies. Orthop. J. Sports Med. 2015, 3, 2325967115575691. [Google Scholar] [CrossRef] [Green Version]
- Futrell, E.E.; Gross, K.D.; Reisman, D.; Mullineaux, D.R.; Davis, I.S. Transition to forefoot strike reduces load rates more effectively than altered cadence. J. Sport Health Sci. 2020, 9, 248–257. [Google Scholar] [CrossRef]
- Miller, E.M.; Crowell, M.S.; Morris, J.B.; Mason, J.S.; Zifchock, R.; Goss, D.L. Gait Retraining Improves Running Impact Loading and Function in Previously Injured U.S. Military Cadets: A Pilot Study. Mil. Med. 2021, 186, E1077–E1087. [Google Scholar] [CrossRef]
- Bonacci, J.; Hall, M.; Saunders, N.; Vicenzino, B. Gait retraining versus foot orthoses for patellofemoral pain: A pilot randomised clinical trial. J. Sci. Med. Sport 2018, 21, 457–461. [Google Scholar] [CrossRef]
- Molina-Molina, A.; Latorre-Román, P.Á.; Mercado-Palomino, E.; Delgado-García, G.; Richards, J.; Soto-Hermoso, V.M. The effect of two retraining programs, barefoot running vs increasing cadence, on kinematic parameters: A randomized controlled trial. Scand. J. Med. Sci. Sports 2022, 32, 533–542. [Google Scholar] [CrossRef]
- Bittencourt, N.F.N.; Meeuwisse, W.H.; Mendonça, L.D.; Nettel-Aguirre, A.; Ocarino, J.M.; Fonseca, S.T. Complex systems approach for sports injuries: Moving from risk factor identification to injury pattern recognition-Narrative review and new concept. Br. J. Sports Med. 2016, 50, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Willy, R.W.; Hoglund, L.T.; Barton, C.J.; Bolgla, L.A.; Scalzitti, D.A.; Logerstedt, D.S.; Lynch, A.D.; Snyder-Mackler, L.; McDonough, C.M. Patellofemoral pain clinical practice guidelines linked to the international classification of functioning, disability and health from the academy of orthopaedic physical therapy of the American physical therapy association. J. Orthop. Sports Phys. Ther. 2019, 49, CPG1–CPG95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramah, C.; Preece, S.J.; Gill, N.; Herrington, L. A 10% Increase in Step Rate Improves Running Kinematics and Clinical Outcomes in Runners With Patellofemoral Pain at 4 Weeks and 3 Months. Am. J. Sports Med. 2019, 47, 3406–3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrener, A.; Tamai, R.; Lieberman, D.E. The effect of trunk flexion angle on lower limb mechanics during running. Hum. Mov. Sci. 2021, 78, 102817. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, P.; Wang, R.; Wang, D.; Liu, J.; Zhou, H. Effects of Foot Strike Techniques on Running Biomechanics: A Systematic Review and Meta-analysis. Sports Health 2021, 13, 71–77. [Google Scholar] [CrossRef]
- Teng, H.L.; Powers, C.M. Hip-extensor strength, trunk posture, and use of the knee-extensor muscles during running. J. Athl. Train. 2016, 51, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Chan, Z.Y.; Zhang, J.H.; Au, I.P.; An, W.W.; Shum, G.L.; Ng, G.Y.; Cheung, R.T.H. Gait Retraining for the Reduction of Injury Occurrence in Novice Distance Runners: 1-Year Follow-up of a Randomized Controlled Trial. Am. J. Sports Med. 2018, 46, 388–395. [Google Scholar] [CrossRef]
- Daoud, A.I.; Geissler, G.J.; Wang, F.; Saretsky, J.; Daoud, Y.A.; Lieberman, D.E. Foot strike and injury rates in endurance runners: A retrospective study. Med. Sci. Sports Exerc. 2012, 44, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.M.; Bonanno, D.R.; Hart, H.F.; Barton, C.J. What are the Benefits and Risks Associated with Changing Foot Strike Pattern During Running? A Systematic Review and Meta-analysis of Injury, Running Economy, and Biomechanics. Sports Med. 2020, 50, 885–917. [Google Scholar] [CrossRef]
- Hoenig, T.; Rolvien, T.; Hollander, K. Footstrike patterns in runners: Concepts, classifications, techniques, and implications for running-related injuries. Dtsch. Z. Sportmed. 2020, 71, 55–61. [Google Scholar] [CrossRef]
- Pohl, M.B.; Mullineaux, D.R.; Milner, C.E.; Hamill, J.; Davis, I.S. Biomechanical predictors of retrospective tibial stress fractures in runners. J. Biomech. 2008, 41, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.E.; Hamill, J.; Davis, I.S. Distinct hip and rearfoot kinematics in female runners with a history of tibial stress fracture. J. Orthop. Sports Phys. Ther. 2010, 40, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ferber, R.; Noehren, B.; Hamill, J.; Davis, I. Competitive female runners with a history of iliotibial band syndrome demonstrate atypical hip and knee kinematics. J. Orthop. Sports Phys. Ther. 2010, 40, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noehren, B.; Davis, I.; Hamill, J. ASB Clinical Biomechanics Award Winner 2006. Prospective study of the biomechanical factors associated with iliotibial band syndrome. Clin. Biomech. 2007, 22, 951–956. [Google Scholar] [CrossRef]
- Noehren, B.; Pohl, M.B.; Sanchez, Z.; Cunningham, T.; Lattermann, C. Proximal and distal kinematics in female runners with patellofemoral pain. Clin. Biomech. 2012, 27, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Neal, B.S.; Barton, C.J.; Birn-jeffery, A.; Morrissey, D. Increased hip adduction during running is associated with patellofemoral pain and differs between males and females: A case-control study. J. Biomech. 2019, 91, 133–139. [Google Scholar] [CrossRef]
- Noehren, B.; Hamill, J.; Davis, I. Prospective Evidence for a Hip Etiology in Patellofemoral Pain. Med. Sci. Sports Exerc. 2013, 45, 1120–1124. [Google Scholar] [CrossRef]
| Variable Name | Variable Definition |
|---|---|
| Vertical Impact Peak (VIP) | The local maximum found between initial foot strike and the maximum ground reaction force [12] |
| Vertical Average Loading Rate (VALR) | Slope of the ground reaction force curve from 20% to 80% of the vertical impact peak, measured in body weights per second (BW/s) [12] |
| Vertical Instantaneous Loading Rate (VILR) | Maximum slope of the ground reaction force curve from 20% to 80% of the vertical impact peak, measured in BW/s [12] |
| Braking Impulse | A measure of the total force applied in the posterior direction during stance phase. Area under the anteroposterior ground reaction force curve from initial contact until midstance [36] |
| Peak Tibial Acceleration | Maximum tibial acceleration at time of initial contact (also known as “impacts”) [12] |
| Adjusted Variable | Feedback | Subjects | Retraining Design | Outcomes | |
|---|---|---|---|---|---|
| Noehren et al., 2011 [8] | HADD | Visual display and verbal cues | 10 female runners with PFPS and high HADD | Faded, 8 sessions over 2 weeks | 86% reduction in pain with 11-point increase in LEFI. Significant reduction in HADD and contralateral pelvic drop. All changes persisted at 1-month follow-up |
| Willy et al., 2012 [9] | HADD | Visual feedback from mirror and verbal cues | 10 female runners with PFPS | Faded, 8 sessions over 2 weeks | Reduced HADD, thigh adduction and contralateral pelvic drop. All changes persisted at the 1- and 3-month follow-ups, although HADD increased from post-trial to 1- and 3-month follow-ups |
| Esculier et al., 2018 [19] | Step rate | Not clear | 69 runners with PFPS | Not faded, 5 sessions over 8 weeks | No difference in KOS-ADLS scores between runners who received both education and gait retraining compared to runners who only received education on load management |
| Willy et al., 2016 [20] | Step rate | Visual feedback from Garmin Forerunner | 30 healthy runners with high loading rates | Faded, 8 runs, no feedback on 4th, 6th or 8th run | Significant increase in step rate, significant reduction in VALR, VILR, HADD and knee eccentric work |
| Baumgartner et al., 2019 [21] | Step rate | Visual feedback from watch | 38 healthy runners, step rate <170 | Not faded | Significant increase in step rate from 79.9 +/− 4.8 to 86.8 +/− 5.7 strides per leg per minute |
| Crowell and Davis 2011 [12] | Tibial acceleration | Visual feedback | 10 healthy RFS runners with high tibial acceleration | Faded, 8 sessions over 2 weeks | Significant reductions in tibial acceleration, VALR, VILR that persisted at 1-month follow-up |
| Clansey et al., 2014 [13] | Tibial acceleration | Visual feedback | 22 healthy RFS male runners with high tibial accelerations | Not faded, 6 sessions over 3 weeks | Significant reductions in tibial acceleration, VALR, VILR at post-trial. Only tibial acceleration remained significant at the 1-month follow-up |
| Bowser et al., 2018 [14] | Tibial acceleration | Visual feedback | 19 healthy RFS runners with high tibial acceleration | Faded, 8 sessions over 2 weeks | Significant reductions in tibial acceleration, VIP, VALR, VILR, at follow-up timepoints of 1, 6, and 12 months |
| Cheung et al., 2018 [39] | Tibial acceleration | Visual feedback | 16 healthy runners with high tibial accelerations | Faded, 8 sessions over 2 weeks | In the post-trial participants were distracted but still had significant reduction in VALR, VILR and tibial acceleration compared to pre-trial |
| Ching et al., 2018 [15] | Tibial acceleration | Audio feedback | 16 healthy runners with high tibial acceleration | Faded, 8 sessions over 2 weeks | In the post-trial participants were distracted but still had significant reduction in VALR, VILR and tibial acceleration compared to pre-trial. Additional feedback did not change loading rates in runners that had already undergone gait retraining |
| Zhang et al., 2019 [17] | Tibial acceleration | Visual feedback | 13 healthy runners with high tibial acceleration | Faded, 8 sessions over 2 weeks | 37.3% reduction in peak tibial acceleration, runners maintained lower tibial accelerations at +/− 10% of their self-selected pace |
| Zhang et al., 2019 [16] | Tibial acceleration | Visual feedback | 12 healthy runners with high tibial acceleration | Faded, 8 sessions over 2 weeks | Runners were able to maintain lower tibial accelerations during overground running and treadmill slope running, but not overground slope running |
| Sheerin et al., 2020 [18] | Tibial acceleration | Haptic feedback through watch | 18 healthy runners with high tibial acceleration | Faded, 8 sessions over 2 weeks | 41% reduction in average tibial acceleration on a treadmill. 17% reduction in tibial acceleration during overground running |
| da Silva Neto et al., 2022 [40] | Vertical ground reaction force | Visual feedback | 24 healthy RFS runners | Not faded, 8 sessions over 2 weeks | Reduced maximum force in the midfoot and medial rearfoot. Showed gait retraining can be performed overground rather than with a treadmill |
| Cheung and Davis 2011 [41] | Forefoot strike pattern | Audio feedback from buzzer in shoe | 3 female runners with PFPS | Faded, 8 sessions over 2 weeks | All 3 participants had decreased VALR and VILR by 10.9–35.1%. Pain scores were improved by 10.4–19.5 points |
| Roper et al., 2016 [42] | Forefoot strike pattern | Visual feedback from mirror and verbal cues | 16 RFS runners with running-related knee pain | Faded, 8 sessions over 2 weeks | Significant reduction in pain from 5.3 to 1.0 at post-trial and 1-month follow-up |
| Chan et al., 2020 [43] | Midfoot strike pattern | Visual display of footstrike pattern | 20 healthy RFS male runners | Faded, 8 sessions over 2 weeks | Only 40% of participants successfully transitioned to midfoot strike pattern, those who did displayed no difference in vertical loading rate |
| Yang et al., 2020 [44] | Forefoot strike pattern | Audio feedback from mobile app | 17 healthy RFS runners | Not faded | Significantly lower loading rates, significantly higher ankle joint moment from pre- to post-study. Significantly lower loading rates in participants who underwent gait retraining and switched to minimalist shoes compared to those who just switched to minimalist shoes |
| Chan et al., 2021 [45] | Forefoot strike pattern | Audio feedback | 16 healthy runners | Faded, 8 sessions over 2 weeks | 75% of participants switched to non rearfoot striking over level ground, 94% over uphill running and 88% over downhill running |
| Teng et al., 2020 [10] | Trunk lean | Visual display of trunk lean | 12 healthy RFS runners | Faded, 5 sessions over 8 weeks | Significant reduction in PFJ stress, knee extensor moment, peak ankle plantar flexor moment, significant increase in peak hip extensor moment |
| Helmhout et al., 2015 [46] | Forefoot strike pattern and step rate | Education and audio feedback from verbal cues | 19 military members with chronic extertional compartment syndrome for at least 2 months | Not faded | Significant increase in running distance, significant increase in SANE and LLOS, significant decrease in PSC |
| Futrell et al., 2020 [47] | Forefoot strike pattern and step rate | Audio feedback from metronome for step rate group, audio feedback for footstrike pattern group | 39 healthy RFS runners without a history of bone stress injuries and with step rates below 170 | Faded, 8 sessions over 2 weeks | 41% reduction in VALR in the footstrike pattern group compared to 14% reduction in VALR in the step rate group at 1-week post-trial. Changes were maintained at 6 months post-trial |
| Miller et al., 2021 [48] | Forefoot strike pattern and step rate | Audio feedback from metronome and verbal cues | 9 injured military service members | Not faded | Significant reduction in VALR, increase in step rate, significant improvement in patient SANE scores. All participants remained injury free at 6-month follow-up |
| Bonacci et al., 2018 [49] | Footwear and step rate | Audio feedback from metronome | 14 RFS runners with PFPS | Faded, 10 sessions over 6 weeks | All subjects in gait retraining had reduction in pain and improvement in function. Significantly lower anterior knee pain compared to orthotics group |
| Molina-Molina et al., 2022 [50] | Footwear and step rate | Audio feedback from a metronome for step rate group, removal of shoes for barefoot group | 70 healthy runners | Not faded, 30 sessions over 3 weeks | Significant decrease in rearfoot strike angle in barefoot group and step rate group. Significant increase in step rate at comfortable speed for step rate group. At a high speed, step rate increased for the barefoot group and decreased for the step rate group. |
| dos Santos et al., 2019 [11] | Forefoot strike pattern, step rate and forward trunk lean | Audio feedback from clinician for footstrike and forward trunk lean groups, audio feedback from metronome for step rate group | 18 runners with PFPS | Faded, 8 sessions over 2 weeks | All 3 groups had decreased pain, increased functionality and decreased LEFS scores from pre- to post-trial. All changes were maintained at a 6-month follow-up. AKPS scores decreased from pre-trial to post-trial in the footstrike and trunk lean groups and between pre-trial and 6-month follow-up in all groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudette, L.W.; Bradach, M.M.; de Souza Junior, J.R.; Heiderscheit, B.; Johnson, C.D.; Posilkin, J.; Rauh, M.J.; Sara, L.K.; Wasserman, L.; Hollander, K.; et al. Clinical Application of Gait Retraining in the Injured Runner. J. Clin. Med. 2022, 11, 6497. https://doi.org/10.3390/jcm11216497
Gaudette LW, Bradach MM, de Souza Junior JR, Heiderscheit B, Johnson CD, Posilkin J, Rauh MJ, Sara LK, Wasserman L, Hollander K, et al. Clinical Application of Gait Retraining in the Injured Runner. Journal of Clinical Medicine. 2022; 11(21):6497. https://doi.org/10.3390/jcm11216497
Chicago/Turabian StyleGaudette, Logan W., Molly M. Bradach, José Roberto de Souza Junior, Bryan Heiderscheit, Caleb D. Johnson, Joshua Posilkin, Mitchell J. Rauh, Lauren K. Sara, Lindsay Wasserman, Karsten Hollander, and et al. 2022. "Clinical Application of Gait Retraining in the Injured Runner" Journal of Clinical Medicine 11, no. 21: 6497. https://doi.org/10.3390/jcm11216497






