1H-NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Ethical Aspects
2.3. Psychiatric Evaluation
2.4. Sample Preparation and Data Analysis
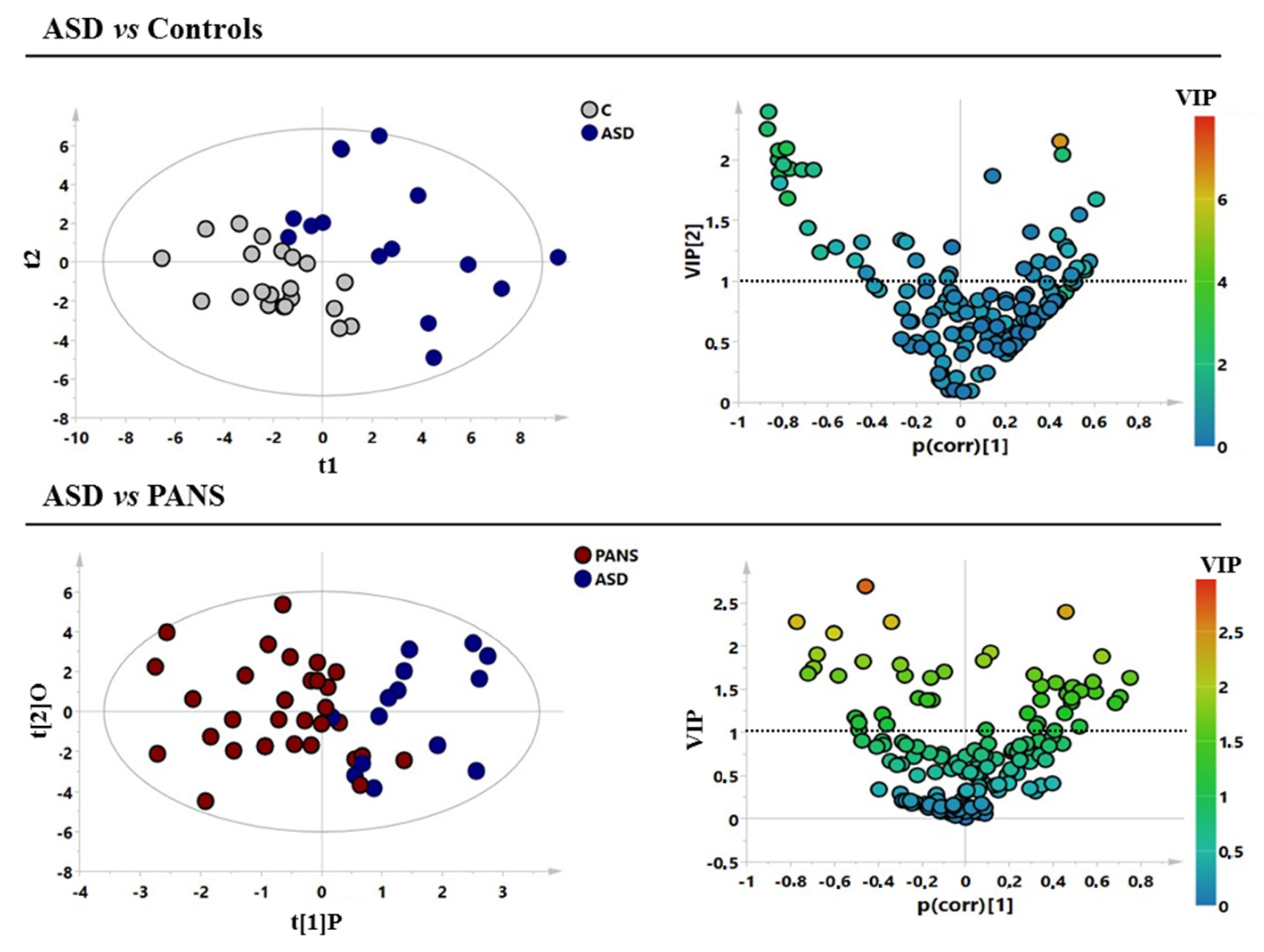
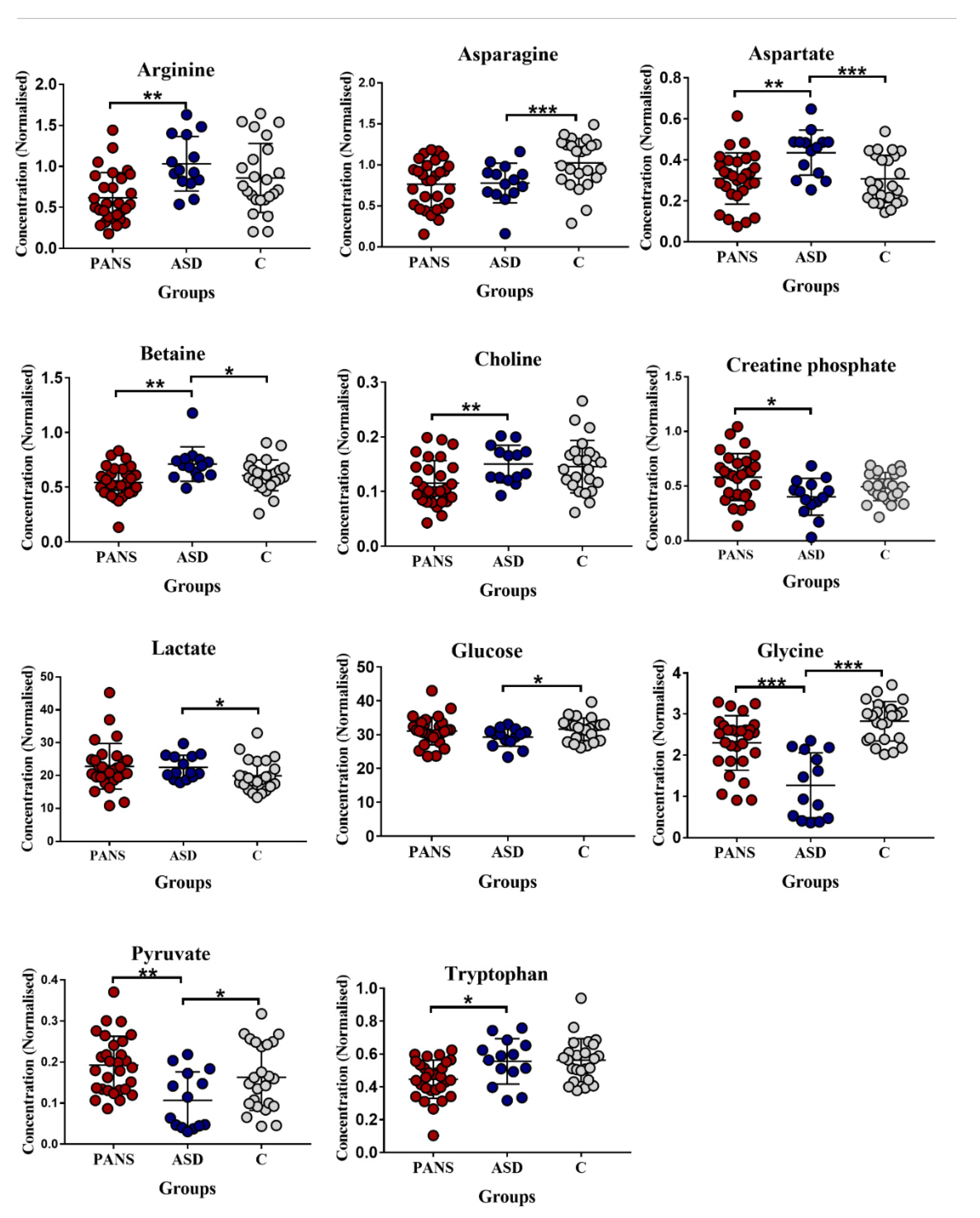
3. Results
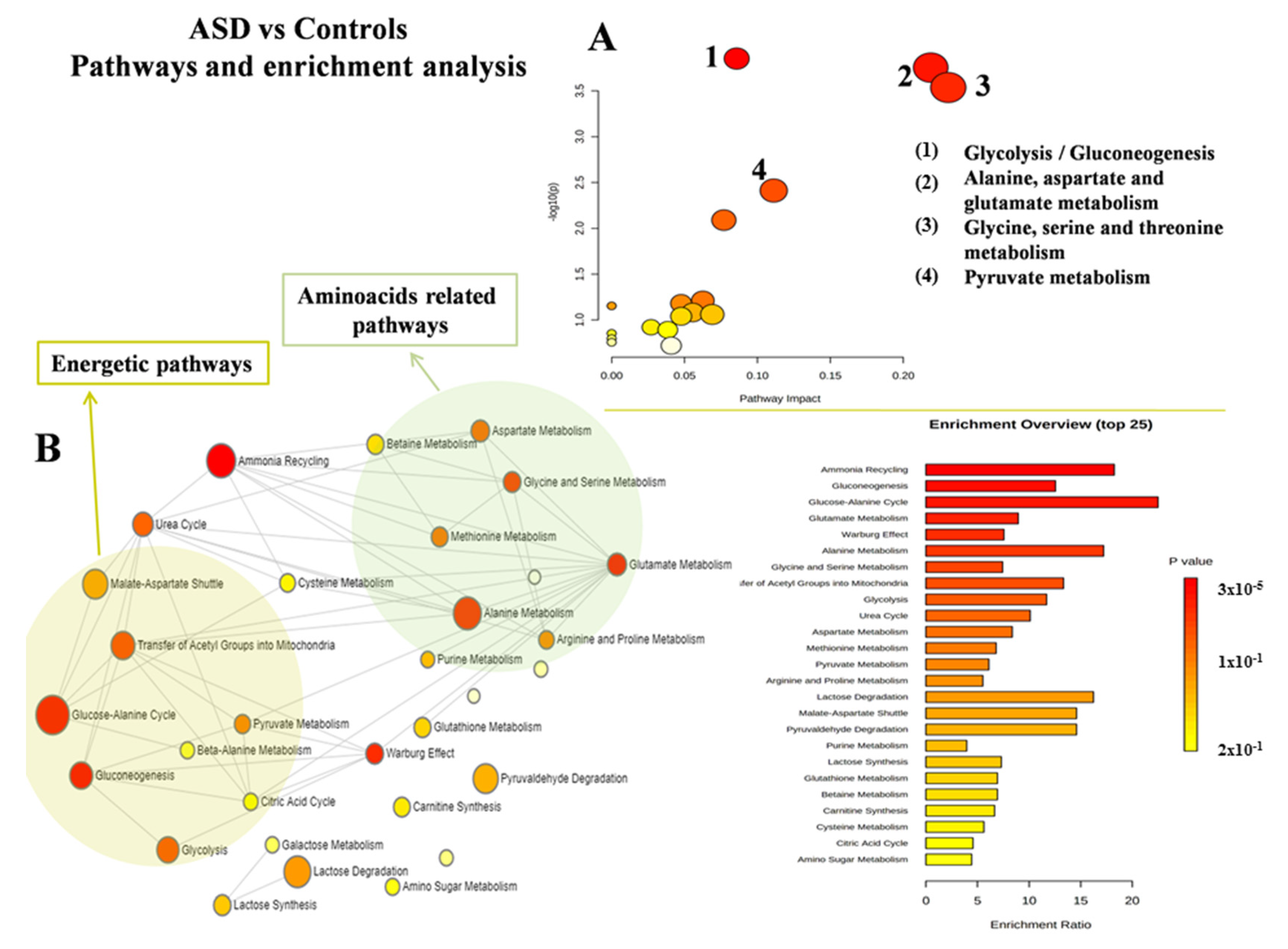
4. Discussion
4.1. Decrease in Glycine (Gly) and Asparagine Concentrations Is a Shared Biomarker of ASD and PANS
4.2. Impaired Glucose Metabolism Appears to Be the Key Feature of ASD
4.3. Decreased Tryptophan Concentration Is a Relevant Feature of PANS, but Not of ASD
5. Conclusions and Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, K.; Frankovich, J.; Cooperstock, M.; Cunningham, M.W.; Latimer, M.E.; Murphy, T.K.; Pasternack, M.; Thienemann, M.; Williams, K.; Walter, J. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): Recommendations from the 2013 PANS Consensus Conference. J. Child Adolesc. Psychopharmacol. 2015, 25, 3–13. [Google Scholar] [CrossRef]
- Swedo, S.E.; Leckman, J.F.; Rose, N.R. From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Ther. 2012, 2, 113. [Google Scholar] [CrossRef]
- Hoekstra, P.J.; Manson, W.L.; Steenhuis, M.-P.; Kallenberg, C.G.M.; Minderaa, R.B. Association of Common Cold with Exacerbations in Pediatric but not Adult Patients with Tic Disorder: AProspective Longitudinal Study. J. Child Adolesc. Psychopharmacol. 2005, 15, 285–292. [Google Scholar] [CrossRef]
- Müller, N.; Riedel, M.; Blendinger, C.; Oberle, K.; Jacobs, E.; Abele-Horn, M. Mycoplasma pneumoniae infection and Tourette’s syndrome. Psychiatry Res. 2004, 129, 119–125. [Google Scholar] [CrossRef]
- Spinello, C.; Laviola, G.; Macrì, S. Pediatric autoimmune disorders associated with streptococcal infections and Tourette’s syndrome in preclinical studies. Front. Neurosci. 2016, 10, 310. [Google Scholar] [CrossRef]
- Swedo, S.E.; Seidlitz, J.; Kovacevic, M.; Latimer, M.E.; Hommer, R.; Lougee, L.; Grant, P. Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections in research and community settings. J. Child Adolesc. Psychopharmacol. 2015, 25, 26–30. [Google Scholar] [CrossRef]
- Zheng, J.; Frankovich, J.; McKenna, E.S.; Rowe, N.C.; MacEachern, S.J.; Ng, N.N.; Tam, L.T.; Moon, P.K.; Gao, J.; Thienemann, M. Association of pediatric acute-onset neuropsychiatric syndrome with microstructural differences in brain regions detected via diffusion-weighted magnetic resonance imaging. JAMA Netw. Open 2020, 3, e204063. [Google Scholar] [CrossRef]
- Frankovich, J.; Thienemann, M.; Pearlstein, J.; Crable, A.; Brown, K.; Chang, K. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: Presenting characteristics of the first 47 consecutive patients. J. Child Adolesc. Psychopharmacol. 2015, 25, 38–47. [Google Scholar] [CrossRef]
- Gagliano, A.; Galati, C.; Ingrassia, M.; Ciuffo, M.; Alquino, M.A.; Tanca, M.G.; Carucci, S.; Zuddas, A.; Grossi, E. Pediatric acute-onset neuropsychiatric syndrome: A data mining approach to a very specific constellation of clinical variables. J. Child Adolesc. Psychopharmacol. 2020, 30, 495–511. [Google Scholar] [CrossRef]
- Gładysz, D.; Krzywdzińska, A.; Hozyasz, K.K. Immune abnormalities in autism spectrum disorder—could they hold promise for causative treatment? Mol. Neurobiol. 2018, 55, 6387–6435. [Google Scholar] [CrossRef]
- Benedetto, L.; Cucinotta, F.; Maggio, R.; Germanò, E.; De Raco, R.; Alquino, A.; Impallomeni, C.; Siracusano, R.; Vetri, L.; Roccella, M. One-year follow-up diagnostic stability of autism spectrum disorder diagnosis in a clinical sample of children and toddlers. Brain Sci. 2021, 11, 37. [Google Scholar] [CrossRef]
- Trifiletti, R.; Lachman, H.M.; Manusama, O.; Zheng, D.; Spalice, A.; Chiurazzi, P.; Schornagel, A.; Serban, A.M.; van Wijck, R.; Cunningham, J.L. Identification of ultra-rare genetic variants in pediatric acute onset neuropsychiatric syndrome (PANS) by exome and whole genome sequencing. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K. Meta-analysis of SHANK mutations in autism spectrum disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef]
- Smolinska, A.; Blanchet, L.; Buydens, L.M.C.; Wijmenga, S.S. NMR and pattern recognition methods in metabolomics: From data acquisition to biomarker discovery: A review. Anal. Chim. Acta 2012, 750, 82–97. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Murgia, F.; Muroni, A.; Puligheddu, M.; Polizzi, L.; Barberini, L.; Orofino, G.; Solla, P.; Poddighe, S.; Del Carratore, F.; Griffin, J.L. Metabolomics as a tool for the characterization of drug-resistant epilepsy. Front. Neurol. 2017, 8, 459. [Google Scholar] [CrossRef]
- Murgia, F.; Lorefice, L.; Poddighe, S.; Fenu, G.; Secci, M.A.; Marrosu, M.G.; Cocco, E.; Atzori, L. Multi-platform characterization of cerebrospinal fluid and serum metabolome of patients affected by relapsing–Remitting and primary progressive multiple sclerosis. J. Clin. Med. 2020, 9, 863. [Google Scholar] [CrossRef]
- Murgia, F.; Gagliano, A.; Tanca, M.G.; Or-Geva, N.; Hendren, A.; Carucci, S.; Pintor, M.; Cera, F.; Cossu, F.; Sotgiu, S. Metabolomic characterization of pediatric acute-onset neuropsychiatric syndrome (PANS). Front. Neurosci. 2021, 15, 597. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Fanos, V. Metabolomics of autism spectrum disorders: Early insights regarding mammalian-microbial cometabolites. Expert Rev. Mol. Diagn. 2016, 16, 869–881. [Google Scholar] [CrossRef]
- Likhitweerawong, N.; Thonusin, C.; Boonchooduang, N.; Louthrenoo, O.; Nookaew, I.; Chattipakorn, N.; Chattipakorn, S.C. Profiles of urine and blood metabolomics in autism spectrum disorders. Metab. Brain Dis. 2021, 36, 1641–1671. [Google Scholar] [CrossRef]
- Piras, C.; Pintus, R.; Pruna, D.; Dessì, A.; Atzori, L.; Fanos, V. Pediatric acute-onset neuropsychiatric syndrome and mycoplasma pneumoniae infection: A case report analysis with a metabolomics approach. Curr. Pediatric Rev. 2020, 16, 183–193. [Google Scholar]
- Sotgiu, S.; Manca, S.; Gagliano, A.; Minutolo, A.; Melis, M.C.; Pisuttu, G.; Scoppola, C.; Bolognesi, E.; Clerici, M.; Guerini, F.R. Immune regulation of neurodevelopment at the mother–foetus interface: The case of autism. Clin. Transl. Immunol. 2020, 9, e1211. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- Ma, Y.; Zhou, H.; Li, C.; Zou, X.; Luo, X.; Wu, L.; Li, T.; Chen, X.; Mao, M.; Huang, Y. Differential metabolites in Chinese autistic children: A multi-center study based on urinary 1H-NMR metabolomics analysis. Front. Psychiatry 2021, 12, 624767. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qian-Qian, L.; Cong, Y.; Xiao-Bing, Z.; Hong-Zhu, D. Reduction of essential amino acid levels and sex-specific alterations in serum amino acid concentration profiles in children with autism spectrum disorder. Psychiatry Res. 2021, 297, 113675. [Google Scholar] [CrossRef]
- Guerrant, R.L.; Oriá, R.B.; Moore, S.R.; Oriá, M.O.B.; Lima, A.A.M. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr. Rev. 2008, 66, 487–505. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Quagliariello, A.; Del Chierico, F.; Russo, A.; Reddel, S.; Conte, G.; Lopetuso, L.R.; Ianiro, G.; Dallapiccola, B.; Cardona, F.; Gasbarrini, A. Gut microbiota profiling and gut–brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front. Microbiol. 2018, 9, 675. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, M.; Xie, D.; Wu, X.; Hong, C.; Fu, J.; Fan, L.; Wang, S.; Han, S. Glycinergic Signaling in Macrophages and Its Application in Macrophage-Associated Diseases. Front. Immunol. 2021, 12, 762564. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, Z.; Ke, X.; Yao, P.; Chen, Y.; Lin, L.; Lu, J. Urinary metabonomic profiling discriminates between children with autism and their healthy siblings. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e926634. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Desbarats, L.; Aïdoud, N.; Emond, P.; Blasco, H.; Filipiak, I.; Sarda, P.; Bonnet-Brilhault, F.; Mavel, S.; Andres, C.R. Combined 1 H-NMR and 1 H–13 C HSQC-NMR to improve urinary screening in autism spectrum disorders. Analyst 2014, 139, 3460–3468. [Google Scholar] [CrossRef] [PubMed]
- Comhair, J.; Devoght, J.; Morelli, G.; Harvey, R.J.; Briz, V.; Borrie, S.C.; Bagni, C.; Rigo, J.-M.; Schiffmann, S.N.; Gall, D. Alpha2-containing glycine receptors promote neonatal spontaneous activity of striatal medium spiny neurons and support maturation of glutamatergic inputs. Front. Mol. Neurosci. 2018, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ho, T.N.T.; Harvey, R.J.; Lynch, J.W.; Keramidas, A. Structure-function analysis of the GlyR α2 subunit autism mutation p. R323L reveals a gain-of-function. Front. Mol. Neurosci. 2017, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Pilorge, M.; Fassier, C.; Le Corronc, H.; Potey, A.; Bai, J.; De Gois, S.; Delaby, E.; Assouline, B.; Guinchat, V.; Devillard, F. Genetic and functional analyses demonstrate a role for abnormal glycinergic signaling in autism. Mol. Psychiatry 2016, 21, 936–945. [Google Scholar] [CrossRef]
- Humer, E.; Probst, T.; Pieh, C. Metabolomics in psychiatric disorders: What we learn from animal models. Metabolites 2020, 10, 72. [Google Scholar] [CrossRef]
- Erjavec, G.N.; Konjevod, M.; Perkovic, M.N.; Strac, D.S.; Tudor, L.; Barbas, C.; Grune, T.; Zarkovic, N.; Pivac, N. Short overview on metabolomic approach and redox changes in psychiatric disorders. Redox Biol. 2018, 14, 178–186. [Google Scholar] [CrossRef]
- Cai, H.-L.; Li, H.-D.; Yan, X.-Z.; Sun, B.; Zhang, Q.; Yan, M.; Zhang, W.-Y.; Jiang, P.; Zhu, R.-H.; Liu, Y.-P. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J. Proteome Res. 2012, 11, 4338–4350. [Google Scholar] [CrossRef]
- De Bartolomeis, A.; Manchia, M.; Marmo, F.; Vellucci, L.; Iasevoli, F.; Barone, A. Glycine signaling in the framework of dopamine-glutamate interaction and postsynaptic density. Implications for treatment-resistant schizophrenia. Front. Psychiatry 2020, 11, 369. [Google Scholar] [CrossRef]
- Zheng, P.; Gao, H.C.; Li, Q.; Shao, W.H.; Zhang, M.L.; Cheng, K.; Yang, D.Y.; Fan, S.H.; Chen, L.; Fang, L. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J. Proteome Res. 2012, 11, 1741–1748. [Google Scholar] [CrossRef]
- Zhang, Y.; Filiou, M.D.; Reckow, S.; Gormanns, P.; Maccarrone, G.; Kessler, M.S.; Frank, E.; Hambsch, B.; Holsboer, F.; Landgraf, R. Proteomic and metabolomic profiling of a trait anxiety mouse model implicate affected pathways. Mol. Cell. Proteom. 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, A.S.; Gerasch, S.; García-García, I.; Lampe, L.; Pampel, A.; Anwander, A.; Near, J.; Möller, H.E.; Müller-Vahl, K. Pathological glutamatergic neurotransmission in Gilles de la Tourette syndrome. Brain 2017, 140, 218–234. [Google Scholar] [CrossRef] [PubMed]
- McCracken, L.M.; Lowes, D.C.; Salling, M.C.; Carreau-Vollmer, C.; Odean, N.N.; Blednov, Y.A.; Betz, H.; Harris, R.A.; Harrison, N.L. Glycine receptor α3 and α2 subunits mediate tonic and exogenous agonist-induced currents in forebrain. Proc. Natl. Acad. Sci. USA 2017, 114, E7179–E7186. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.W.; Zhang, Y.; Talwar, S.; Estrada-Mondragon, A. Glycine receptor drug discovery. Adv. Pharmacol. 2017, 79, 225–253. [Google Scholar]
- Belforte, J.E.; Zsiros, V.; Sklar, E.R.; Jiang, Z.; Yu, G.; Li, Y.; Quinlan, E.M.; Nakazawa, K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci. 2010, 13, 76–83. [Google Scholar] [CrossRef]
- Gagliano, A.; Puligheddu, M.; Ronzano, N.; Congiu, P.; Tanca, M.G.; Cursio, I.; Carucci, S.; Sotgiu, S.; Grossi, E.; Zuddas, A. Artificial neural networks analysis of polysomnographic and clinical features in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS): From sleep alteration to “Brain Fog”. Nat. Sci. Sleep 2021, 13, 1209. [Google Scholar] [CrossRef]
- Meng, D.; Yang, Q.; Wang, H.; Melick, C.H.; Navlani, R.; Frank, A.R.; Jewell, J.L. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 2020, 295, 2890–2899. [Google Scholar] [CrossRef]
- Soriano-Correa, C.; Barrientos-Salcedo, C.; Campos-Fernández, L.; Alvarado-Salazar, A.; Esquivel, R.O. Importance of asparagine on the conformational stability and chemical reactivity of selected anti-inflammatory peptides. Chem. Phys. 2015, 457, 180–187. [Google Scholar] [CrossRef]
- Naushad, S.M.; Jain, J.M.N.; Prasad, C.K.; Naik, U.; Akella, R.R.D. Autistic children exhibit distinct plasma amino acid profile. Natl. Libr. Medsine 2013, 50, 474–478. [Google Scholar]
- Saleem, T.H.; Shehata, G.A.; Toghan, R.; Sakhr, H.M.; Bakri, A.H.; Desoky, T.; Hamdan, F.R.A.; Mohamed, N.F.; Hassan, M.H. Assessments of amino acids, ammonia and oxidative stress among cohort of Egyptian autistic children: Correlations with electroencephalogram and disease severity. Neuropsychiatr. Dis. Treat. 2020, 16, 11. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Mitelman, S.A.; Bralet, M.-C.; Mehmet Haznedar, M.; Hollander, E.; Shihabuddin, L.; Hazlett, E.A.; Buchsbaum, M.S. Positron emission tomography assessment of cerebral glucose metabolic rates in autism spectrum disorder and schizophrenia. Brain Imaging Behav. 2018, 12, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Guerrera, S.; Ravà, L.; Ciofi degli Atti, M.; Di Vara, S.; Valeri, G.; Vicari, S. Cross-sectional investigation of insulin resistance in youths with autism spectrum disorder. Any role for reduced brain glucose metabolism? Transl. Psychiatry 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.W.; Taudorf, S. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Parisi, L.; Salerno, M.; Maltese, A.; Tripi, G.; Romano, P.; Di Folco, A.; Di Filippo, T.; Messina, G.; Roccella, M. Emotional intelligence and obstructive sleep apnea syndrome in children: Preliminary case-control study. Acta Med. Mediterr. 2017, 33, 485–489. [Google Scholar]
- Essa, M.M.; Subash, S.; Braidy, N.; Al-Adawi, S.; Lim, C.K.; Manivasagam, T.; Guillemin, G.J. Role of NAD+, oxidative stress, and tryptophan metabolism in autism spectrum disorders. Int. J. Tryptophan Res. 2013, 6, IJTR-S11355. [Google Scholar] [CrossRef]
- Glinton, K.E.; Elsea, S.H. Untargeted metabolomics for autism spectrum disorders: Current status and future directions. Front. Psychiatry 2019, 10, 647. [Google Scholar] [CrossRef]
- Gál, E.M.; Sherman, A.D. l-Kynurenine Its synthesis and possible regulatory function in brain. Neurochem. Res. 1980, 5, 223–239. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Wang, M.; Gao, J.; Sun, C.; Wang, J.; Xia, W.; Wu, S.; Sumner, S.J.; Zhang, F. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. 2016, 41, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Möller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Role of the kynurenine metabolism pathway in inflammation-induced depression: Preclinical approaches. Inflamm.-Assoc. Depress. Evid. Mech. Implic. 2016, 31, 117–138. [Google Scholar]
- Fishman, M.A.; Perelson, A.S. Th1/Th2 differentiation and cross-regulation. Bull. Math. Biol. 1999, 61, 403–436. [Google Scholar] [CrossRef]
- Vécsei, L.; Beal, M.F. Comparative behavioral and pharmacological studies with centrally administered kynurenine and kynurenic acid in rats. Eur. J. Pharmacol. 1991, 196, 239–246. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Parrott, K. 3-Monooxygenase: An Influential Mediator of Neuropathology. Front. Psychiatry 2015, 6, 116. [Google Scholar] [CrossRef]
- Santoro, J.D.; Frankovich, J.; Bhargava, S. Continued presence of period limb movements during REM sleep in patients with chronic static pediatric acute-onset neuropsychiatric syndrome (PANS). J. Clin. Sleep Med. 2018, 14, 1187–1192. [Google Scholar] [CrossRef]
- Lord, C.; Charman, T.; Havdahl, A.; Carbone, P.; Anagnostou, E.; Boyd, B.; Carr, T.; De Vries, P.J.; Dissanayake, C.; Divan, G. The Lancet Commission on the future of care and clinical research in autism. Lancet 2022, 399, 271–334. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Seminar Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Lin, Y.; Yerukala Sathipati, S.; Ho, S.Y. Predicting the Risk Genes of Autism Spectrum Disorders. Front. Genet. 2021, 12, 665469. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chen, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Neurological Diseases With Autism Spectrum Disorder: Role of ASD Risk Genes. Front. Neurosci. 2019, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Huguet, G.; Benabou, M.; Bourgeron, T. The genetics of autism spectrum disorders. A Time Metab. Horm. 2016, 101–129. [Google Scholar]
- Courchesne, E.; Gazestani, V.H.; Lewis, N.E. Prenatal origins of ASD: The when, what, and how of ASD development. Trends Neurosci. 2020, 43, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pramparo, T.; Gazestani, V.H.; Lombardo, M.V.; Pierce, K.; Lewis, N.E. The ASD Living Biology: From cell proliferation to clinical phenotype. Mol. Psychiatry 2019, 24, 88–107. [Google Scholar] [CrossRef]
- Jansen, A.G.; Jansen, P.R.; Savage, J.E.; Kraft, J.; Skarabis, N.; Polderman, T.J.C.; Dieleman, G.C. The predictive capacity of psychiatric and psychological polygenic risk scores for distinguishing cases in a child and adolescent psychiatric sample from controls. J. Child Psychol. Psychiatry 2021, 62, 1079–1089. [Google Scholar] [CrossRef]
- Larsen, E.; Menashe, I.; Ziats, M.N.; Pereanu, W.; Packer, A.; Banerjee-Basu, S. A systematic variant annotation approach for ranking genes associated with autism spectrum disorders. Mol. Autism 2016, 7, 44. [Google Scholar] [CrossRef]
- O’Connell, K.S.; McGregor, N.W.; Lochner, C.; Emsley, R.; Warnich, L. The genetic architecture of schizophrenia, bipolar disorder, obsessive-compulsive disorder and autism spectrum disorder. Mol. Cell. Neurosci. 2018, 88, 300–307. [Google Scholar] [CrossRef]
- Riddle, M.A.; Greenhill, L.L. The Research Units on Pediatric Psychopharmacology Anxiety Study Group: The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 1061–1069. [Google Scholar]
- Scahill, L.; Riddle, M.A.; McSwiggin-Hardin, M.; Ort, S.I.; King, R.A.; Goodman, W.K.; Cicchetti, D.; Leckman, J.F. Children’s Yale-Brown obsessive compulsive scale: Reliability and validity. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 844–852. [Google Scholar] [CrossRef]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.; Gould, M.S.; Brasic, J.; Ambrosini, P.; Fisher, P.; Bird, H.; Aluwahlia, S. A children’s global assessment scale (CGAS). Arch. Gen. Psychiatry 1983, 40, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Jr, A.L.; Maia, D.P.; Cardoso, F. UFMG Sydenham’s chorea rating scale (USCRS): Reliability and consistency. Mov. Disord. Off. J. Mov. Disord. Soc. 2005, 20, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV); The Psychological Corporation: San Antonio, TX, USA, 2003; Volume 3. [Google Scholar]

| Classes | n | Female/Male | Age | ||
|---|---|---|---|---|---|
| Mean Value | SD | Range | |||
| PANS | 34 | 10/24 | 9.1 | 2.90 | 5–16 |
| ASD | 15 | 0/15 | 9 | 4.28 | 3–17 |
| Controls | 25 | 9/16 | 12 | 2.17 | 8–17 |
| Models | |||||
|---|---|---|---|---|---|
| Models | R2X | R2Y | Q2 | p-Value | Permutation Test: Intercept R2\Q2 |
| ASD vs. Controls | 0.49 | 0.61 | 0.49 | 0.0001 | 0.29/−0.22 |
| ASD vs. PANS | 0.41 | 0.51 | 0.25 | 0.02 | 0.32/−0.18 |
| Serum Samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | ASD | p-Value | p-Value Corrected | ROC-CURVE | ||||
| AUC | Std. Error | CI | p-Value | |||||
| ASD vs. Controls | Asparagine | − | 0.006 | 0.06 | 0.77 | 0.07 | 0.6–0.9 | 0.007 |
| Aspartate | + | 0.0008 | 0.1 | 0.82 | 0.07 | 0.7–0.9 | 0.001 | |
| Betaine | + | 0.02 | 0.06 | 0.71 | 0.08 | 0.5–0.9 | 0.02 | |
| Glucose | − | 0.03 | 0.01 | 0.66 | 0.08 | 0.5–0.8 | 0.09 | |
| Glycine | − | <0.0001 | 0.1 | 0.96 | 0.02 | 0.9–1 | <0.0001 | |
| Lactate | + | 0.02 | 0.03 | 0.72 | 0.08 | 0.5–0.8 | 0.02 | |
| Pyruvate | − | 0.04 | 0.1 | 0.70 | 0.08 | 0.5–0.9 | 0.04 | |
| ASD vs. PANS | Arginine | + | 0.0003 | 0.004 | 0.82 | 0.06 | 0.7–0.94 | 0.0005 |
| Aspartate | + | 0.002 | 0.02 | 0.82 | 0.06 | 0.7–0.95 | 0.001 | |
| Betaine | + | 0.001 | 0.005 | 0.71 | 0.08 | 0.54–0.88 | 0.01 | |
| Choline | + | 0.006 | 0.01 | 0.76 | 0.07 | 0.61–0.90 | 0.007 | |
| Creatine Phosphate | − | 0.01 | 0.74 | 0.07 | 0.59–0.88 | 0.01 | ||
| Glycine | − | <0.0001 | 0.005 | 0.85 | 0.05 | 0.74–0.96 | 0.0001 | |
| Pyruvate | − | 0.002 | 0.70 | 0.08 | 0.52–0.87 | 0.04 | ||
| Tryptophan | + | 0.01 | 0.02 | 0.73 | 0.08 | 0.56–0.9 | 0.021 | |
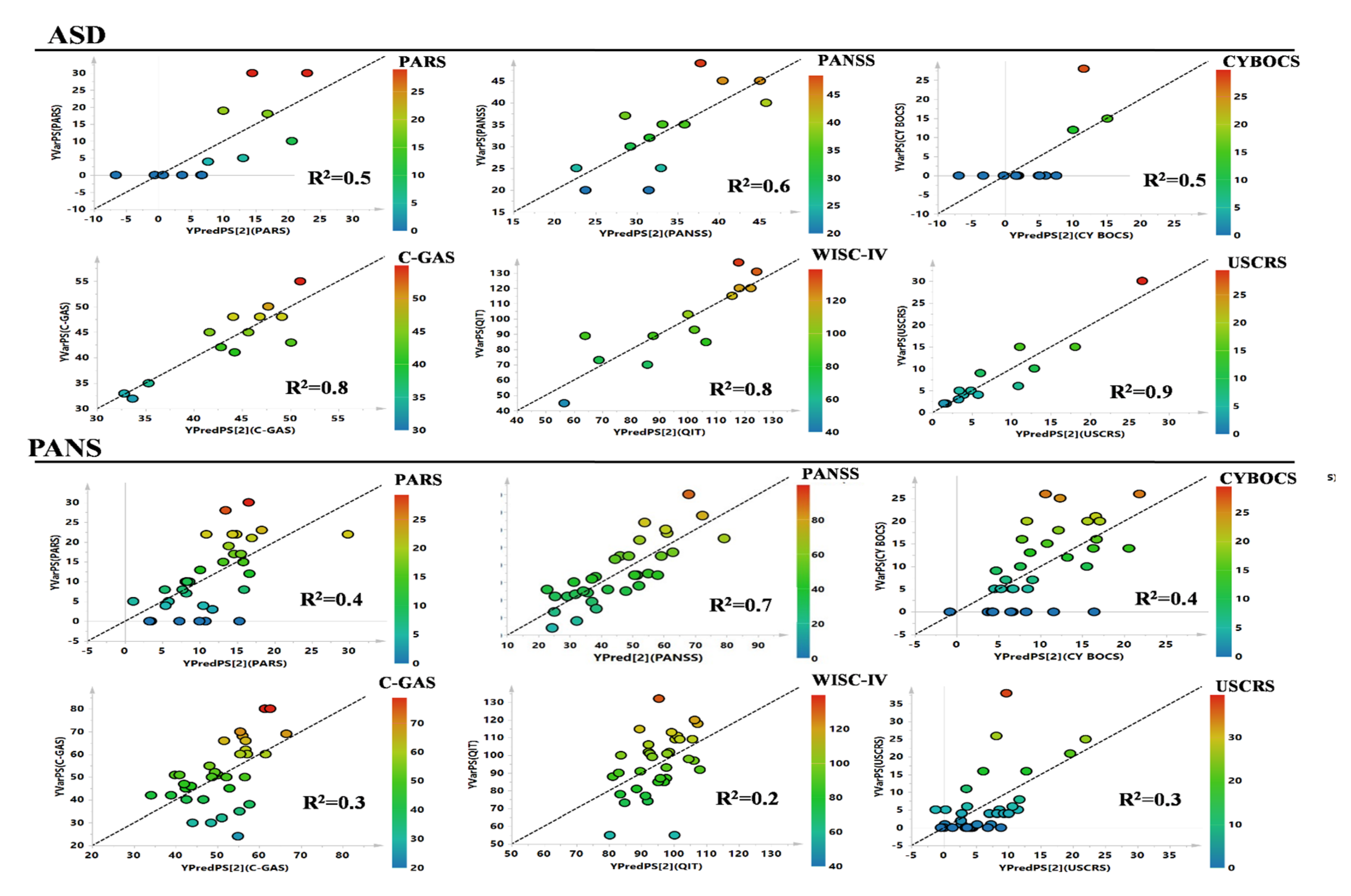
| Class | Clinical Parameters | ||||||
|---|---|---|---|---|---|---|---|
| PARS R2 | PANSS R2 | CYBOCS R2 | YGTSS R2 | C-GAS R2 | TIQ R2 | USCRS R2 | |
| PANS | 0.4 | 0.7 | 0.4 | 0.2 | 0.3 | 0.2 | 0.3 |
| ASD | 0.5 | 0.6 | 0.5 | - | 0.8 | 0.8 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliano, A.; Murgia, F.; Capodiferro, A.M.; Tanca, M.G.; Hendren, A.; Falqui, S.G.; Aresti, M.; Comini, M.; Carucci, S.; Cocco, E.; et al. 1H-NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome. J. Clin. Med. 2022, 11, 6493. https://doi.org/10.3390/jcm11216493
Gagliano A, Murgia F, Capodiferro AM, Tanca MG, Hendren A, Falqui SG, Aresti M, Comini M, Carucci S, Cocco E, et al. 1H-NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome. Journal of Clinical Medicine. 2022; 11(21):6493. https://doi.org/10.3390/jcm11216493
Chicago/Turabian StyleGagliano, Antonella, Federica Murgia, Agata Maria Capodiferro, Marcello Giuseppe Tanca, Aran Hendren, Stella Giulia Falqui, Michela Aresti, Martina Comini, Sara Carucci, Eleonora Cocco, and et al. 2022. "1H-NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome" Journal of Clinical Medicine 11, no. 21: 6493. https://doi.org/10.3390/jcm11216493
APA StyleGagliano, A., Murgia, F., Capodiferro, A. M., Tanca, M. G., Hendren, A., Falqui, S. G., Aresti, M., Comini, M., Carucci, S., Cocco, E., Lorefice, L., Roccella, M., Vetri, L., Sotgiu, S., Zuddas, A., & Atzori, L. (2022). 1H-NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome. Journal of Clinical Medicine, 11(21), 6493. https://doi.org/10.3390/jcm11216493











