Diagnostic Accuracy of Vitreous Cytology in Patients with Vitreoretinal Lymphoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion and Exclusion Criteria
2.2. Diagnostic Procedures and Statistical Methods
3. Results
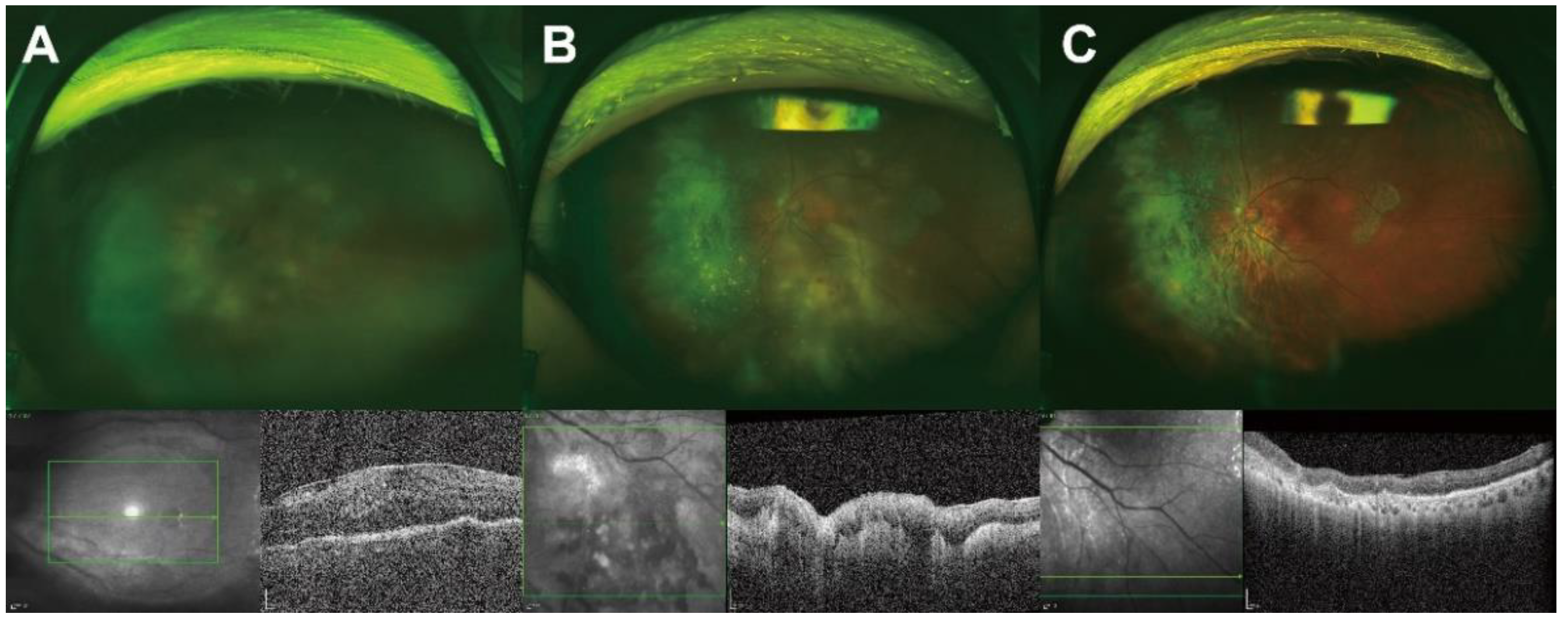
3.1. Ocular Findings at Diagnosis
3.2. Origin and Involvement Patterns of Vitreoretinal Lymphomas
3.3. Comparing the Diagnostic Values of Tests
3.4. Effect of Steroid Pretreatment on Diagnostic Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, L.J.; Gu, C.L.; Zhang, P. Intraocular lymphoma. Int. J. Ophthalmol. 2017, 10, 1301–1307. [Google Scholar] [PubMed]
- Reichstein, D. Primary vitreoretinal lymphoma: An update on pathogenesis, diagnosis and treatment. Curr. Opin. Ophthalmol. 2016, 27, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Coupland, S.E.; Damato, B. Understanding intraocular lymphomas. Clin. Exp. Ophthalmol. 2008, 36, 564–578. [Google Scholar] [CrossRef]
- Levasseur, S.D.; Wittenberg, L.A.; White, V.A. Vitreoretinal lymphoma: A 20-year review of incidence, clinical and cytologic features, treatment, and outcomes. JAMA Ophthalmol. 2013, 131, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Whitcup, S.M.; de Smet, M.D.; Rubin, B.I.; Palestine, A.G.; Martin, D.F.; Burnier, M., Jr.; Chan, C.C.; Nussenblatt, R.B. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology 1993, 100, 1399–1406. [Google Scholar] [CrossRef]
- Peterson, K.; Gordon, K.B.; Heinemann, M.H.; DeAngelis, L.M. The clinical spectrum of ocular lymphoma. Cancer 1993, 72, 843–849. [Google Scholar] [CrossRef]
- Coupland, S.E.; Bechrakis, N.E.; Anastassiou, G.; Foerster, A.M.; Heiligenhaus, A.; Pleyer, U.; Hummel, M.; Stein, H. Evaluation of vitrectomy specimens and chorioretinal biopsies in the diagnosis of primary intraocular lymphoma in patients with masquerade syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 860–870. [Google Scholar] [CrossRef]
- Steffen, J.; Coupland, S.E.; Smith, J.R. Primary vitreoretinal lymphoma in hiv infection. Ocul. Immunol. Inflamm. 2020, 3, 621–627. [Google Scholar] [CrossRef]
- Woei, A.J.F.J.; Kersting, S.; Bollemeijer, J.G. Primary intraocular lymphoma in a patient with systemic lupus erythematosus. Ann. Hematol. 2012, 91, 1821–1822. [Google Scholar] [CrossRef][Green Version]
- Laver, N.M.V. Ocular cytology: Diagnostic features and ongoing practices. Cancer Cytopathol. 2020, 129, 419–431. [Google Scholar] [CrossRef]
- Kimura, K.; Usui, Y.; Goto, H. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn. J. Ophthalmol. 2012, 56, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Char, D.H.; Ljung, B.M.; Miller, T.; Phillips, T. Primary intraocular lymphoma (ocular reticulum cell sarcoma) diagnosis and management. Ophthalmology 1988, 95, 625–630. [Google Scholar] [CrossRef]
- Pochat-Cotilloux, C.; Bienvenu, J.; Nguyen, A.M.; Ohanessian, R.; Ghesquières, H.; Sève, P.; Garnier, L.; Kodjikian, L. Use of a threshold of interleukin-10 and il-10/il-6 ratio in ocular samples for the screening of vitreoretinal lymphoma. Retin. 2018, 38, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, B.; Meijers, R.W.J.; Rijntjes, J.; van der Klift, M.Y.; Möbs, M.; Steinhilber, J.; Reigl, T.; van den Brand, M.; Kotrová, M.; Ritter, J.M.; et al. Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: A technical feasibility study by euroclonality-ngs. Leukemia 2019, 33, 2227–2240. [Google Scholar] [CrossRef]
- Weber, A.N.R.; Cardona Gloria, Y.; Çınar, Ö.; Reinhardt, H.C.; Pezzutto, A.; Wolz, O.O. Oncogenic myd88 mutations in lymphoma: Novel insights and therapeutic possibilities. Cancer Immunol. Immunother. 2018, 67, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Pulido, J.S.; Johnston, P.B.; Nowakowski, G.S.; Castellino, A.; Raja, H. The diagnosis and treatment of primary vitreoretinal lymphoma: A review. Int. J. Retin. Vitr. 2018, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, D.; Mahajan, S.; Chee, S.P.; Sobolewska, B.; Agrawal, R.; Bülow, T.; Gupta, V.; Jones, N.P.; Accorinti, M.; Agarwal, M.; et al. Consensus recommendations for the diagnosis of vitreoretinal lymphoma. Ocul. Immunol. Inflamm. 2021, 29, 507–520. [Google Scholar] [CrossRef]
- Tanaka, R.; Kaburaki, T.; Taoka, K.; Karakawa, A.; Tsuji, H.; Nishikawa, M.; Yatomi, Y.; Shinozaki-Ushiku, A.; Ushiku, T.; Araki, F. More accurate diagnosis of vitreoretinal lymphoma using a combination of diagnostic test results: A prospective observational study. Ocul. Immunol. Inflamm. 2021, 1–7. [Google Scholar] [CrossRef]
- Sugita, S.; Takase, H.; Sugamoto, Y.; Arai, A.; Miura, O.; Mochizuki, M. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn. J. Ophthalmol. 2009, 53, 209–214. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.W.; Kim, H.; Lee, C.S.; Kim, M.; Lee, S.C. Differential diagnosis for vitreoretinal lymphoma with vitreoretinal findings, immunoglobulin clonality tests, and interleukin levels. Retin. 2019, 39, 1165–1176. [Google Scholar] [CrossRef]
- Frenkel, S.; Pe’er, J.; Kaufman, R.; Maly, B.; Habot-Wilner, Z. The importance of cytokines analysis in the diagnosis of vitreoretinal lymphoma. Acta Ophthalmol. 2020, 98, e668–e673. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Arai, A.; Iwasaki, Y.; Imai, A.; Nagao, T.; Kawagishi, M.; Ishida, T.; Mochizuki, M. Challenges in the diagnosis and management of vitreoretinal lymphoma—Clinical and basic approaches. Prog. Retin. Eye Res. 2022, 90, 101053. [Google Scholar] [CrossRef] [PubMed]
- Baehring, J.M.; Androudi, S.; Longtine, J.J.; Betensky, R.A.; Sklar, J.; Foster, C.S.; Hochberg, F.H. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer 2005, 104, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Agarwal, A.; Lee, C.S.; Chhablani, J.; Gupta, V.; Khatri, M.; Nirmal, J.; Pavesio, C.; Agrawal, R. Management of noninfectious posterior uveitis with intravitreal drug therapy. Clin. Ophthalmol. 2016, 10, 1983–2020. [Google Scholar] [CrossRef] [PubMed]
- Touhami, S.; Audo, I.; Terrada, C.; Gaudric, A.; LeHoang, P.; Touitou, V.; Bodaghi, B. Neoplasia and intraocular inflammation: From masquerade syndromes to immunotherapy-induced uveitis. Prog. Retin. Eye Res. 2019, 72, 100761. [Google Scholar] [CrossRef]
| Count/Mean ± Standard Deviation | |
|---|---|
| Patients (n) | 38 |
| Laterality (right/left) | 17/21 |
| Age (years) | 62.5 ± 11.9 |
| Sex (male/female) | 14/24 |
| Comorbidities | |
| Hypertension | 10 (35.7%) |
| Diabetes mellitus | 8 (21.1%) |
| Mean follow-up period (months) | 36.3 ± 33.7 |
| Treatment patterns for VRL | 38 (100.0%) |
| IVit MTX (%, count) | 34 (89.5%, 14.7 ± 6.9) |
| None | 4 (10.5%) |
| Treatment patterns for CNS lymphoma | 22 (57.9%) |
| IVit. MTX + Systemic CTx | 20 (90.9%) |
| IVit. MTX + Systemic CTx + Brain/Eye RTx | 9 (40.9%) |
| Death during follow-up | 11 (28.9%) |
| Count/Mean ± Standard Deviation | |
|---|---|
| LogMAR BCVA (Snellen equivalent) | 0.8 ± 0.9 (0.2 ± 0.1) |
| IOP (mmHg) | 13.4 ± 3.9 |
| Anterior segment findings | |
| Keratic precipitates | 2 (5.3%) |
| Corneal edema | 1 (2.6%) |
| Cells/SUN grading | 17 (44.7%)/1.4 ± 1.3 |
| Posterior segment findings | |
| Vitreous cells or haziness | 37 (97.4%) |
| SubRPE infiltration | 25 (65.8%) |
| Retinal hemorrhage | 8 (21.1%) |
| Count/Mean ± Standard Deviation | |
|---|---|
| Primary origin of the lymphomas | |
| Eye | 28 (73.7%) |
| CNS involvement during follow-up | 12 (42.9%) |
| MRI at routine checkup | 9 (75.0%) |
| Neurological symptoms | 2 (16.7%) |
| Visual-field defects | 1 (8.3%) |
| Brain | 10 (26.3%) |
| Count/Mean ± Standard Deviation | |
|---|---|
| Vitreous cytology | 38 (100.0%) |
| Unsatisfactory specimen | 11 (28.9%) |
| Satisfactory specimen | 27 (71.1%) |
| Positive | 12 (44.4%) |
| Negative | 15 (55.6%) |
| Interleukin analysis | 28 (73.7%) |
| IL-10/IL-6 ratio > 1 | 23 (82.1%) |
| IL-10 > 50 pg/mL | 25 (89.3%) |
| IGH/IGK gene clonality assay | 30 (78.9%) |
| IGH-positive | 18 (60.0%) |
| IGK-positive | 19 (63.3%) |
| IGH- or IGK-positive | 25 (83.3%) |
| Steroid Pretreatment (n = 12) | No Steroid Pretreatment (n = 26) | p-Value | |
|---|---|---|---|
| Number/Mean ± Standard Deviation | Number/Mean ± Standard Deviation | ||
| Vitreous cytology | 12 (100.0%) | 26 (100.0%) | |
| Unsatisfactory specimen | 6 (50.0%) | 5 (19.2%) | 0.068 * |
| Satisfactory specimen | 6 (50.0%) | 21 (80.8%) | |
| Positive | 0 (0.0%) | 12 (57.1%) | 0.020 * |
| Negative | 6 (100.0%) | 9 (42.9%) | |
| Interleukin analysis | 9 (75.0%) | 19 (73.1%) | |
| IL-10/IL-6 ratio > 1 | 8 (88.9%) | 15 (78.9%) | 0.999 † |
| IL-10 > 50 pg/mL | 9 (100.0%) | 16 (84.2%) | 0.530 † |
| IGH/IGK gene clonality assay | 11 (91.7%) | 19 (73.1%) | |
| IGH-positive | 8 (72.7%) | 10 (52.6%) | 0.442 † |
| IGK-positive | 7 (63.6%) | 12 (63.2%) | 0.999 † |
| IGH- or IGK-positive | 9 (81.8%) | 16 (84.2%) | 0.999 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, J.; Nahm, J.-H.; Kim, M. Diagnostic Accuracy of Vitreous Cytology in Patients with Vitreoretinal Lymphoma. J. Clin. Med. 2022, 11, 6450. https://doi.org/10.3390/jcm11216450
Lee D, Lee J, Nahm J-H, Kim M. Diagnostic Accuracy of Vitreous Cytology in Patients with Vitreoretinal Lymphoma. Journal of Clinical Medicine. 2022; 11(21):6450. https://doi.org/10.3390/jcm11216450
Chicago/Turabian StyleLee, Donghyun, Junwon Lee, Ji-Hae Nahm, and Min Kim. 2022. "Diagnostic Accuracy of Vitreous Cytology in Patients with Vitreoretinal Lymphoma" Journal of Clinical Medicine 11, no. 21: 6450. https://doi.org/10.3390/jcm11216450
APA StyleLee, D., Lee, J., Nahm, J.-H., & Kim, M. (2022). Diagnostic Accuracy of Vitreous Cytology in Patients with Vitreoretinal Lymphoma. Journal of Clinical Medicine, 11(21), 6450. https://doi.org/10.3390/jcm11216450




