Sustainability of C-Reactive Protein Apheresis in Acute Myocardial Infarction—Results from a Supplementary Data Analysis of the Exploratory C-Reactive Protein in Acute Myocardial Infarction-1 Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Study Approval
3. Results
3.1. Comparison of CMR1 and CMR2
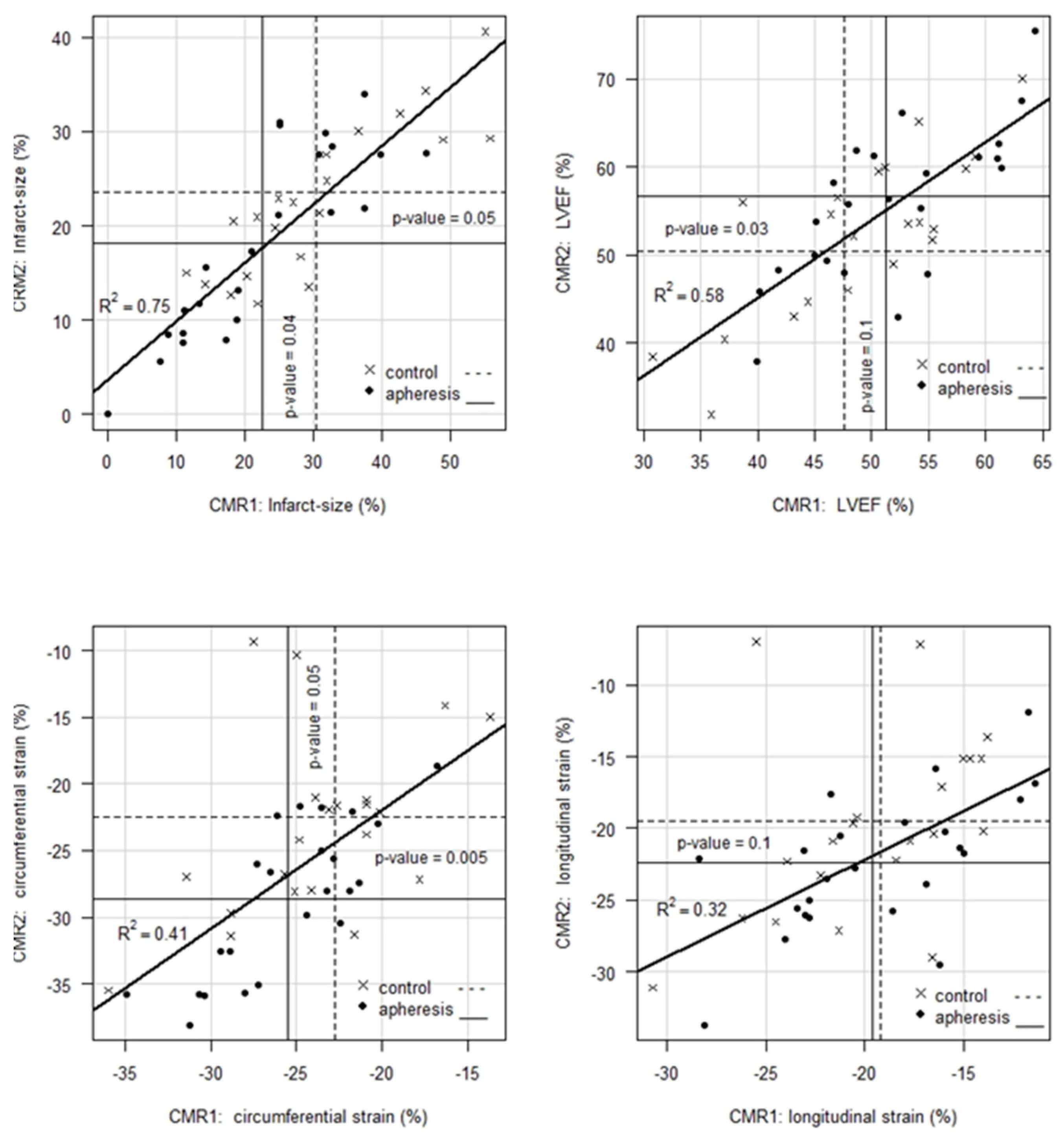
- For IS, CMR1 reveals an ~8% absolute or 26% relative improvement in patients with CRP apheresis compared to controls. Whereas CMR2 shows an improvement of IS in both, control and apheresis group (23.52% vs. 18.10%), the gap between both groups remains significant with a p-value = 0.05 in favor of the patients in the CRP apheresis group.
- For LVEF, CMR1 shows, on average, a 3.7% absolute or 7.8% relative better value in patients with CRP apheresis compared to controls. This initial trend, however, is not statistically significant (p = 0.1). Notably, CMR2 shows a 6.4% absolute or 13% relative improvement of LVEF in favor of patients in the CRP apheresis group. This improvement is statistically significant with a p-value = 0.03. Thus, CRP apheresis has a significant long-term effect on LVEF in patients with a CRPgrad > 0.6 mg/L/h.
- For CS, CRP apheresis shows a significant effect of 2.8 units absolute or 12% relative in CMR1 already. In CMR2, this advantage increases to 6.1 units absolute or 27% relative (p-value = 0.005). Hence, CRP apheresis has a significant long-term effect on CS in patients with a CRPgrad > 0.6 mg/L/h.
- For LS, there is no significant difference between the two groups in neither CMR1 nor CMR2. However, this parameter shows a significant improvement by 2.8 units in the CRP apheresis group in the period between CMR1 and CMR2, while control patients do not show a corresponding improvement.
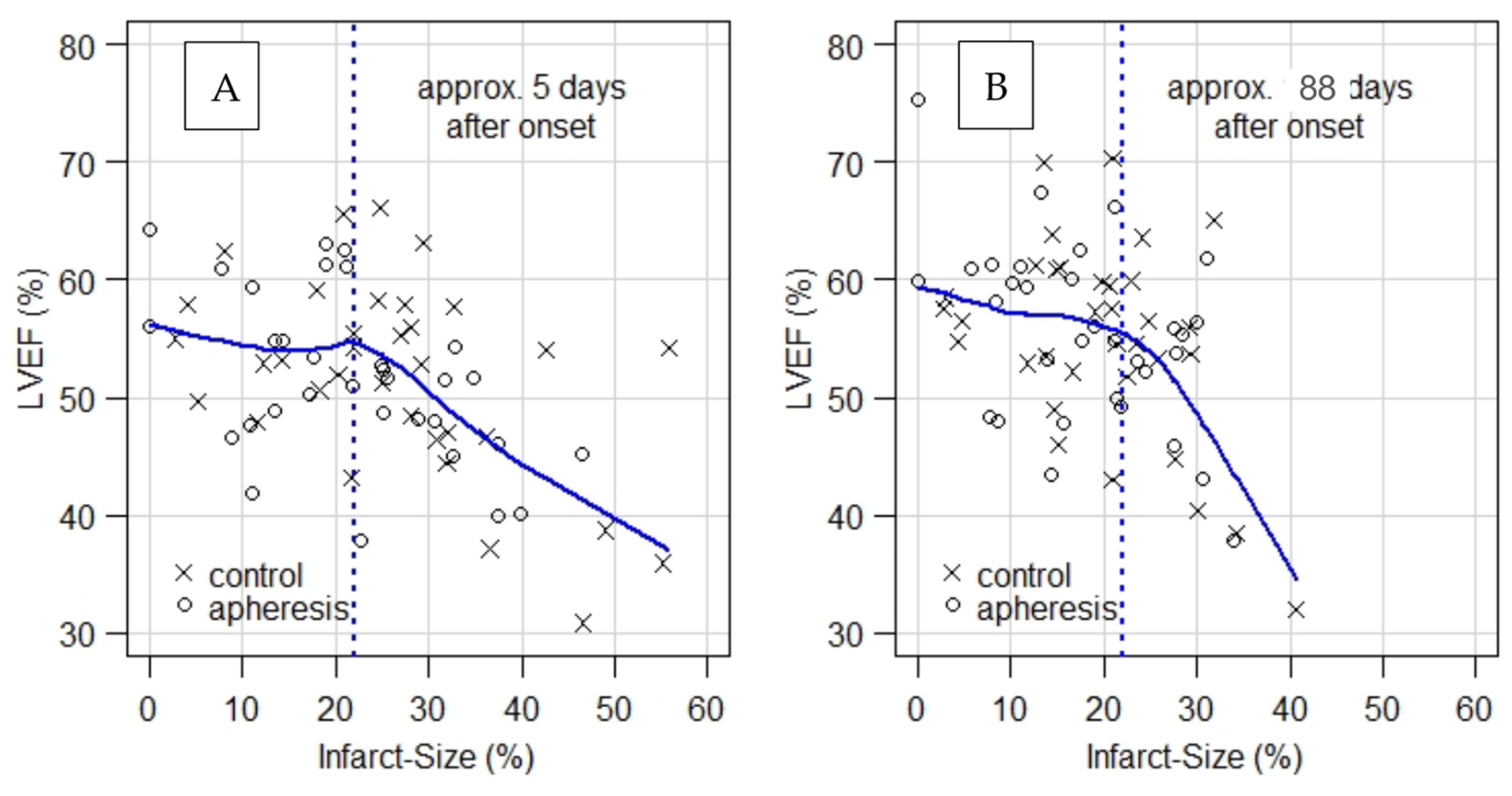
- When LVEF is plotted as a function of infarct size (Figure 1), plots for CMR1 and CMR2 show a kink at infarct size ≈ 22%. That is, LVEF remains unchanged on average at the level of LVEF ≈ 55% up to about 22% of infarct size and decreases significantly beyond that point.
- As an aside, it should be noted that the IS of apheresis patients with a mean of 22.51% in CMR1 almost reach the kink in the curve regarding LVEF (Figure 1A) as a function of IS in CMR1 already, while controls with a mean of 30.49% are still well within the range of strongly decreasing LVEF. For CMR2, it should be noted that the mean of IS in apheresis patients is clearly left of the above-mentioned kink (Figure 1B), while control patients with a mean of 23.52% still remain in the range of decreasing LVEF.
3.2. Course of Myocardial Parameter Development between CMR1 and CMR2
3.3. CRPgrad and Prognosis
3.3.1. CMR1
3.3.2. CMR2
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banai, A.; Levit, D.; Morgan, S.; Loewenstein, I.; Merdler, I.; Hochstadt, A.; Szekely, Y.; Topilsky, Y.; Banai, S.; Shacham, Y. Association between C-Reactive Protein Velocity and Left Ventricular Function in Patients with ST-Elevated Myocardial Infarction. J. Clin. Med. 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, C.; Sheriff, A.; Zimmermann, S.; Schaefauer, L.; Schlundt, C.; Raaz, D.; Garlichs, C.D.; Achenbach, S. C-reactive protein levels predict systolic heart failure and outcome in patients with first ST-elevation myocardial infarction treated with coronary angioplasty. Arch. Med. Sci. 2017, 13, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Holzknecht, M.; Tiller, C.; Reindl, M.; Lechner, I.; Fink, P.; Lunger, P.; Mayr, A.; Henninger, B.; Brenner, C.; Klug, G.; et al. Association of C-Reactive Protein Velocity with Early Left Ventricular Dysfunction in Patients with First ST-Elevation Myocardial Infarction. J. Clin. Med. 2021, 10, 5494. [Google Scholar] [CrossRef] [PubMed]
- Ries, W.; Torzewski, J.; Heigl, F.; Pfluecke, C.; Kelle, S.; Darius, H.; Ince, H.; Mitzner, S.; Nordbeck, P.; Butter, C.; et al. C-Reactive Protein Apheresis as Anti-inflammatory Therapy in Acute Myocardial Infarction: Results of the CAMI-1 Study. Front. Cardiovasc. Med. 2021, 8, 591714. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair after Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Buerke, M.; Sheriff, A.; Garlichs, C.D. CRP apheresis in acute myocardial infarction and COVID-19. Med. Klin. Intensivmed. Notfmed. 2022, 117, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, A.; Kayser, S.; Brunner, P.; Vogt, B. C-Reactive Protein Triggers Cell Death in Ischemic Cells. Front. Immunol. 2021, 12, 630430. [Google Scholar] [CrossRef] [PubMed]
- Torzewski, J.; Brunner, P.; Ries, W.; Garlichs, C.D.; Kayser, S.; Heigl, F.; Sheriff, A. Targeting C-Reactive Protein by Selective Apheresis in Humans: Pros and Cons. J. Clin. Med. 2022, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.E. Healing After Myocardial Infarction: A Loosely Defined Process. JACC Cardiovasc. Imaging 2015, 8, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Pokorney, S.D.; Rodriguez, J.F.; Ortiz, J.T.; Lee, D.C.; Bonow, R.O.; Wu, E. Infarct healing is a dynamic process following acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2012, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis from 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Tiller, C.; Reindl, M.; Holzknecht, M.; Lechner, I.; Simma, F.; Schwaiger, J.; Mayr, A.; Klug, G.; Bauer, A.; Reinstadler, S.J.; et al. High sensitivity C-reactive protein is associated with worse infarct healing after revascularized ST-elevation myocardial infarction. Int. J. Cardiol. 2021, 328, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, M.; Rakowski, T. Circulating biomarkers as predictors of left ventricular remodeling after myocardial infarction. Postep. Kardiol. Interwencyjnej 2021, 17, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, M.; Wojtasik-Bakalarz, J.; Malinowski, K.; Surmiak, M.; Dziewierz, A.; Sorysz, D.; Tokarek, T.; Dudek, D.; Bartus, S.; Surdacki, A.; et al. Mid-regional pro-adrenomedullin and lactate dehydrogenase as predictors of left ventricular remodeling in patients with myocardial infarction treated with percutaneous coronary intervention. Pol. Arch. Intern. Med. 2022, 132, 16150. [Google Scholar] [CrossRef]
- Geven, C.; Blet, A.; Kox, M.; Hartmann, O.; Scigalla, P.; Zimmermann, J.; Marx, G.; Laterre, P.F.; Mebazaa, A.; Pickkers, P. A double-blind, placebo-controlled, randomised, multicentre, proof-of-concept and dose-finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS-2). BMJ Open 2019, 9, e024475. [Google Scholar] [CrossRef]
- Ries, W.; Sheriff, A.; Heigl, F.; Zimmermann, O.; Garlichs, C.D.; Torzewski, J. “First in Man”: Case Report of Selective C-Reactive Protein Apheresis in a Patient with Acute ST Segment Elevation Myocardial Infarction. Case Rep. Cardiol. 2018, 2018, 4767105. [Google Scholar] [CrossRef]
| Mean ± Se | CMR1 | |||
|---|---|---|---|---|
| Control (n = 21/34) | Apheresis (n = 23/32) | p-Value | Improved | |
| IS (%) | 30.49 ± 2.8 | 22.51 ± 2.5 | 0.04 | 26% |
| LVEF (%) | 47.58 ± 1.7 | 51.32 ± 1.5 | 0.1 | 7.8% |
| CS (%) | −22.72 ± 1.1 | −25.51 ± 0.88 | 0.05 | 12% |
| LS (%) | −19.18 ± 1.0 | −19.61 ± 1.0 | 0.78 | 2.2% |
| Mean ± Se | CMR2 | |||
|---|---|---|---|---|
| Control (n = 21/34) | Apheresis (n = 23/32) | p-Value | Improved | |
| IS (%) | 23.52 ± 1.7 | 18.10 ± 2.1 | 0.05 | 23% |
| LVEF (%) | 50.34 ± 1.7 | 56.73 ± 1.8 | 0.03 | 13% |
| CS (%) | −22.48 ± 1.3 | −28.61 ± 1.2 | 0.005 | 27% |
| LS (%) | −19.51 ± 1.3 | −22.41 ± 1.0 | 0.1 | 15% |
| Mean ± Se | CMR1 | |||
|---|---|---|---|---|
| Control | Apheresis | |||
| CRPgrad < 0.6 (n = 13) | CRPgrad > 0.6 (n = 21) | CRPgrad < 0.6 (n = 9) | CRPgrad > 0.6 (n = 23) | |
| IS (%) | 20.03 ± 3.23 | 30.49 ± 2.8 | 20.45 ± 3.30 | 22.51 ± 2.5 |
| LVEF (%) | 57.15 ± 1.59 | 47.58 ± 1.7 | 52.26 ± 2.22 | 51.32 ± 1.5 |
| CS (%) | −27.86 ± 1.16 | −22.72 ± 1.1 | −24.83 ± 1.15 | −25.51 ± 0.88 |
| LS (%) | −21.73 ± 0.94 | −19.18 ± 1.0 | −20.1 ± 1.38 | −19.61 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarabis, H.; Torzewski, J.; Ries, W.; Heigl, F.; Garlichs, C.D.; Kunze, R.; Sheriff, A. Sustainability of C-Reactive Protein Apheresis in Acute Myocardial Infarction—Results from a Supplementary Data Analysis of the Exploratory C-Reactive Protein in Acute Myocardial Infarction-1 Study. J. Clin. Med. 2022, 11, 6446. https://doi.org/10.3390/jcm11216446
Skarabis H, Torzewski J, Ries W, Heigl F, Garlichs CD, Kunze R, Sheriff A. Sustainability of C-Reactive Protein Apheresis in Acute Myocardial Infarction—Results from a Supplementary Data Analysis of the Exploratory C-Reactive Protein in Acute Myocardial Infarction-1 Study. Journal of Clinical Medicine. 2022; 11(21):6446. https://doi.org/10.3390/jcm11216446
Chicago/Turabian StyleSkarabis, Horst, Jan Torzewski, Wolfgang Ries, Franz Heigl, Christoph D. Garlichs, Rudolf Kunze, and Ahmed Sheriff. 2022. "Sustainability of C-Reactive Protein Apheresis in Acute Myocardial Infarction—Results from a Supplementary Data Analysis of the Exploratory C-Reactive Protein in Acute Myocardial Infarction-1 Study" Journal of Clinical Medicine 11, no. 21: 6446. https://doi.org/10.3390/jcm11216446
APA StyleSkarabis, H., Torzewski, J., Ries, W., Heigl, F., Garlichs, C. D., Kunze, R., & Sheriff, A. (2022). Sustainability of C-Reactive Protein Apheresis in Acute Myocardial Infarction—Results from a Supplementary Data Analysis of the Exploratory C-Reactive Protein in Acute Myocardial Infarction-1 Study. Journal of Clinical Medicine, 11(21), 6446. https://doi.org/10.3390/jcm11216446






