Cricothyroid Dysfunction in Unilateral Vocal Fold Paralysis Females Impairs Lexical Tone Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Procedures
2.3. Real-Time Mandarin Fundamental Frequency Assessment
2.4. Functional Laryngeal EMG
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crumley, R.L. Unilateral recurrent laryngeal nerve paralysis. J. Voice 1994, 8, 79–83. [Google Scholar] [CrossRef]
- Ko, H.-C.; Lee, L.-A.; Li, H.-Y.; Fang, T.-J. Etiologic features in patients with unilateral vocal fold paralysis in Taiwan. Chang Gung Med. J. 2009, 32, 290–296. [Google Scholar] [PubMed]
- Rosenthal, L.H.S.; Benninger, M.S.; Deeb, R.H. Vocal fold immobility: A longitudinal analysis of etiology over 20 years. Laryngoscope 2007, 117, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, E.; Minoda, R.; Hyodo, M.; Yamagata, T. Causes of recurrent laryngeal nerve paralysis. Auris Nasus Larynx 2002, 29, 41–45. [Google Scholar] [CrossRef]
- Faaborg-Andersen, K. The position of paretic vocal cords. Acta Oto-Laryngol. 1964, 57, 50–54. [Google Scholar] [CrossRef]
- Koufinan, J.A.; Walker, F.O.; Joharji, G.M. The cricothyroid muscle does not influence vocal fold position in laryngeal paralysis. Laryngoscope 1995, 105, 368–372. [Google Scholar] [CrossRef]
- Pei, Y.C.; Fang, T.J.; Li, H.Y.; Wong, A.M. Cricothyroid muscle dysfunction impairs vocal fold vibration in unilateral vocal fold paralysis. Laryngoscope 2014, 124, 201–206. [Google Scholar] [CrossRef]
- Tseng, W.-C.; Pei, Y.-C.; Wong, A.M.; Li, H.-Y.; Fang, T.-J. Distinct disease and functional characteristics of thyroid surgery–related vocal fold palsy. Thyroid 2016, 26, 943–950. [Google Scholar] [CrossRef]
- Bao, Y.; Szymaszek, A.; Wang, X.; Oron, A.; Pöppel, E.; Szelag, E. Temporal order perception of auditory stimuli is selectively modified by tonal and non-tonal language environments. Cognition 2013, 129, 579–585. [Google Scholar] [CrossRef]
- Jaisin, K.; Suphanchaimat, R.; Figueroa Candia, M.A.; Warren, J.D. The speech-to-song illusion is reduced in speakers of tonal (vs. non-tonal) languages. Front. Psychol. 2016, 7, 662. [Google Scholar] [CrossRef]
- Järvikivi, J.; Vainio, M.; Aalto, D. Real-time correlates of phonological quantity reveal unity of tonal and non-tonal languages. PLoS ONE 2010, 5, e12603. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, L.C.; Lunders, E.R. Resolution of lexical ambiguity by emotional tone of voice. Mem. Cogn. 2002, 30, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.K.M. The Prosody of Mandarin Chinese. J. Phon. 1993, 21, 343–347. [Google Scholar] [CrossRef]
- Chao, Y.R. Mandarin Primer; Harvard University Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Sereno, J.A.; Lee, H. The contribution of segmental and tonal information in Mandarin spoken word processing. Lang. Speech 2015, 58, 131–151. [Google Scholar] [CrossRef]
- Spataro, E.A.; Grindler, D.J.; Paniello, R.C. Etiology and time to presentation of unilateral vocal fold paralysis. Otolaryngol.-Head Neck Surg. 2014, 151, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.C.; Li, H.Y.; Chen, C.L.; Wong, A.M.; Huang, P.C.; Fang, T.J. Disease Characteristics and Electromyographic Findings of Nonsurgery-Related Unilateral Vocal Fold Paralysis. Laryngoscope 2017, 127, 1381–1387. [Google Scholar] [CrossRef]
- Fang, T.J.; Pei, Y.C.; Hsin, L.J.; Lin, W.N.; Lee, L.A.; Li, H.Y.; Wong, A.M. Quantitative laryngeal electromyography assessment of cricothyroid function in patients with unilateral vocal fold paralysis. Laryngoscope 2015, 125, 2530–2535. [Google Scholar] [CrossRef]
- Ohala, J.J. Production of tone. In Tone; Elsevier: Amsterdam, The Netherlands, 1978; pp. 5–39. [Google Scholar]
- Sapir, S.; Larson, C.; Campbell, C. Effect of geniohyoid, cricothyroid and sternothyroid muscle stimulation on voice fundamental frequency of electrically elicited phonation in rhesus macaque. Laryngoscope 1981, 91, 457–468. [Google Scholar] [CrossRef]
- Hirano, M.; Ohala, J.; Vennard, W. The function of laryngeal muscles in regulating fundamental frequency and intensity of phonation. J. Speech Hear. Res. 1969, 12, 616–628. [Google Scholar] [CrossRef]
- Sawashima, M.; Hirose, H.; Yoshioka, H.; Kiritani, S. Interaction between articulatory movements and vocal pitch control in Japanese word accent. Phonetica 1982, 39, 188–198. [Google Scholar] [CrossRef]
- Simada, Z.; Hirose, H. Physiological correlates of Japanese accent patterns. Annu. Bull. 1971, 5, 41–49. [Google Scholar]
- Erickson, D.; Abramson, A.S. Electromyographic study of the tones in Thai. J. Acoust. Soc. Am. 1973, 53, 231–236. [Google Scholar] [CrossRef]
- Garding, E.; Fujimura, O.; Hirose, H. Laryngeal control of Swedish word tone: A pre. Annu. Bull. Res. Inst. Logop. Phoniatr. 1970, 4, 45–54. [Google Scholar]
- Collier, R. Physiological correlates of intonation patterns. J. Acoust. Soc. Am. 1975, 58, 249–255. [Google Scholar] [CrossRef]
- Fischer-Jørgensen, E. Electromyographic investigation of Danish consonants, stress, and stod. Annu. Rep. Inst. Phon. Univ. Copehagen 1974, 8, 203–206. [Google Scholar] [CrossRef]
- Barczyński, M.; Randolph, G.W.; Cernea, C.R.; Dralle, H.; Dionigi, G.; Alesina, P.F.; Mihai, R.; Finck, C.; Lombardi, D.; Hartl, D.M. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013, 123, S1–S14. [Google Scholar] [CrossRef]
- Shipp, T.; Doherty, E.T.; Morrissey, P. Predicting vocal frequency from selected physiologic measures. J. Acoust. Soc. Am. 1979, 66, 678–684. [Google Scholar] [CrossRef]
- Malins, J.G.; Joanisse, M.F. The roles of tonal and segmental information in Mandarin spoken word recognition: An eyetracking study. J. Mem. Lang. 2010, 62, 407–420. [Google Scholar] [CrossRef]
- Zou, Y.; Lui, M.; Tsang, Y.-K. The roles of lexical tone and rime during Mandarin sentence comprehension: An event-related potential study. Neuropsychologia 2020, 147, 107578. [Google Scholar] [CrossRef]
- Kochis-Jennings, K.A.; Finnegan, E.M.; Hoffman, H.T.; Jaiswal, S.; Hull, D. Cricothyroid muscle and thyroarytenoid muscle dominance in vocal register control: Preliminary results. J. Voice 2014, 28, 652.e621–652.e629. [Google Scholar] [CrossRef]
- McGarr, N.S.; Osberger, M.J. Pitch deviancy and intelligibility of deaf speech. J. Commun. Disord. 1978, 11, 237–247. [Google Scholar] [CrossRef]
- Kent, R.D.; Rosenbek, J.C. Prosodic disturbance and neurologic lesion. Brain Lang. 1982, 15, 259–291. [Google Scholar] [CrossRef]
- Laures, J.S.; Bunton, K. Perceptual effects of a flattened fundamental frequency at the sentence level under different listening conditions. J. Commun. Disord. 2003, 36, 449–464. [Google Scholar] [CrossRef]
- Laures, J.S.; Weismer, G. The effects of a flattened fundamental frequency on intelligibility at the sentence level. J. Speech Lang. Hear. Res. 1999, 42, 1148–1156. [Google Scholar] [CrossRef]
- Watson, P.J.; Schlauch, R.S. The effect of fundamental frequency on the intelligibility of speech with flattened intonation contours. Am. J. Speech-Lang. Pathol. 2008, 17, 348–355. [Google Scholar] [CrossRef]
- Wingfield, A.; Lombardi, L.; Sokol, S. Prosodic features and the intelligibility of accelerated speech: Syntactic versus periodic segmentation. J. Speech Lang. Hear. Res. 1984, 27, 128–134. [Google Scholar] [CrossRef]
- Bunton, K.; Kent, R.D.; Kent, J.F.; Duffy, J.R. The effects of flattening fundamental frequency contours on sentence intelligibility in speakers with dysarthria. Clin. Linguist. Phon. 2001, 15, 181–193. [Google Scholar]
- Ryalls, J.H.; Lieberman, P. Fundamental frequency and vowel perception. J. Acoust. Soc. Am. 1982, 72, 1631–1634. [Google Scholar] [CrossRef]
- de Cheveignè, A.; Kawahara, H. Missing-data model of vowel identification. J. Acoust. Soc. Am. 1999, 105, 3497–3508. [Google Scholar] [CrossRef]
- DiGiovanni, J.J.; Nelson, P.B.; Schlauch, R.S. A psychophysical evaluation of spectral enhancement. J. Speech Lang. Hear. Res. 2005, 48, 1121–1135. [Google Scholar] [CrossRef]
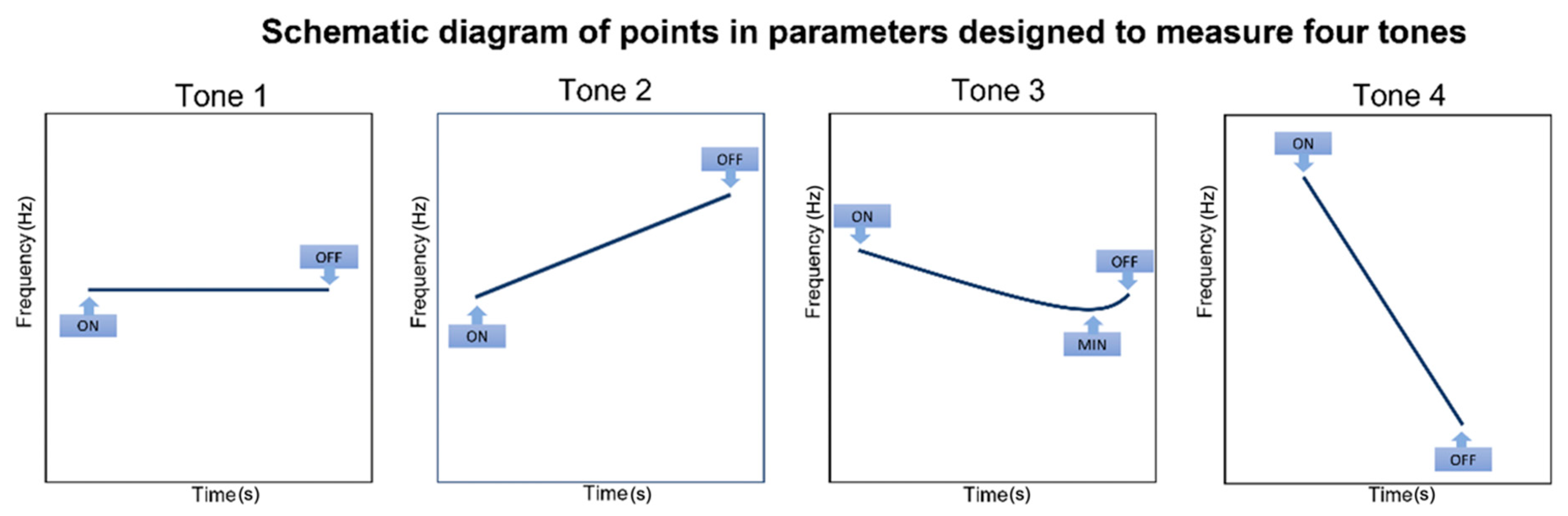
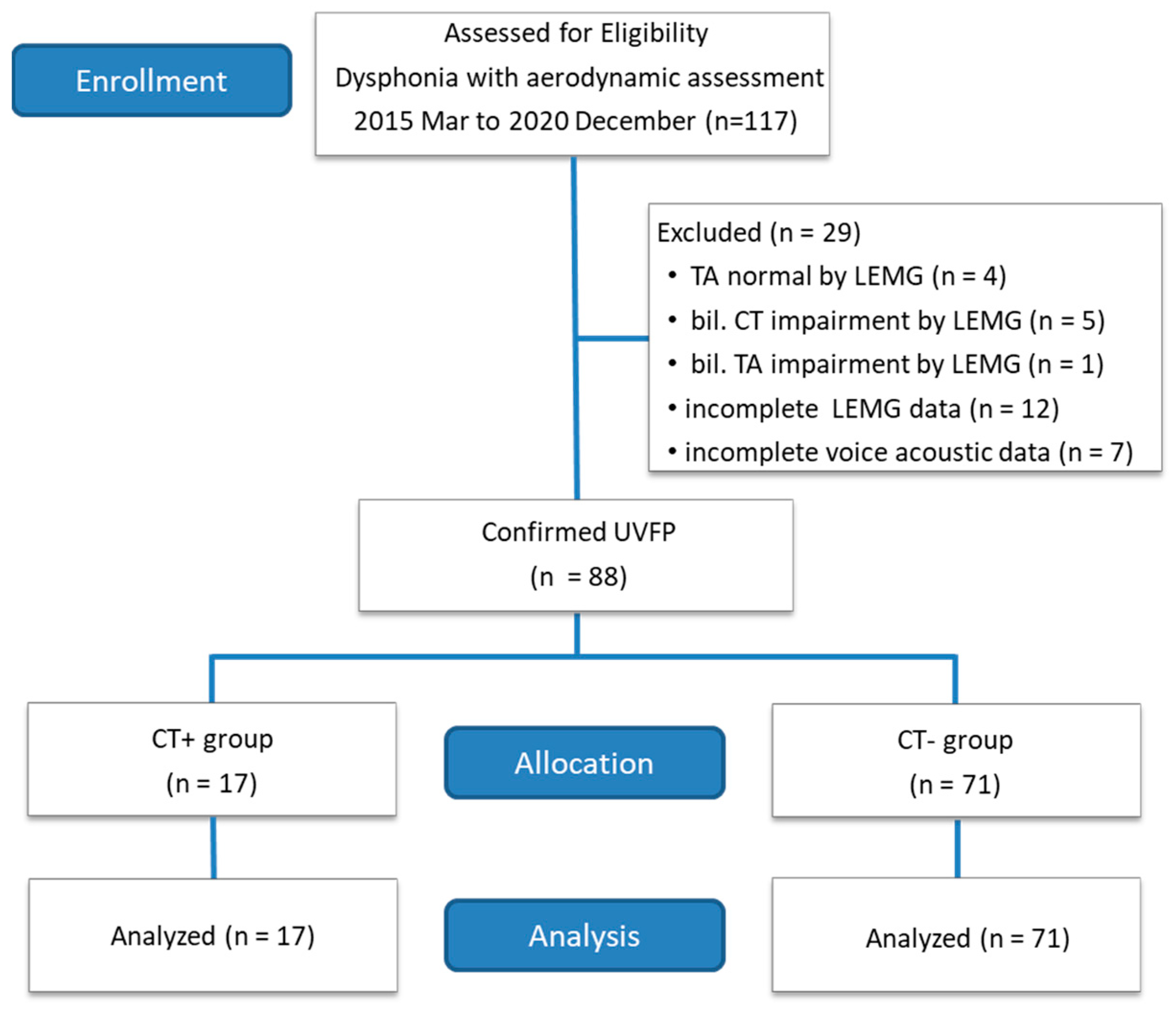
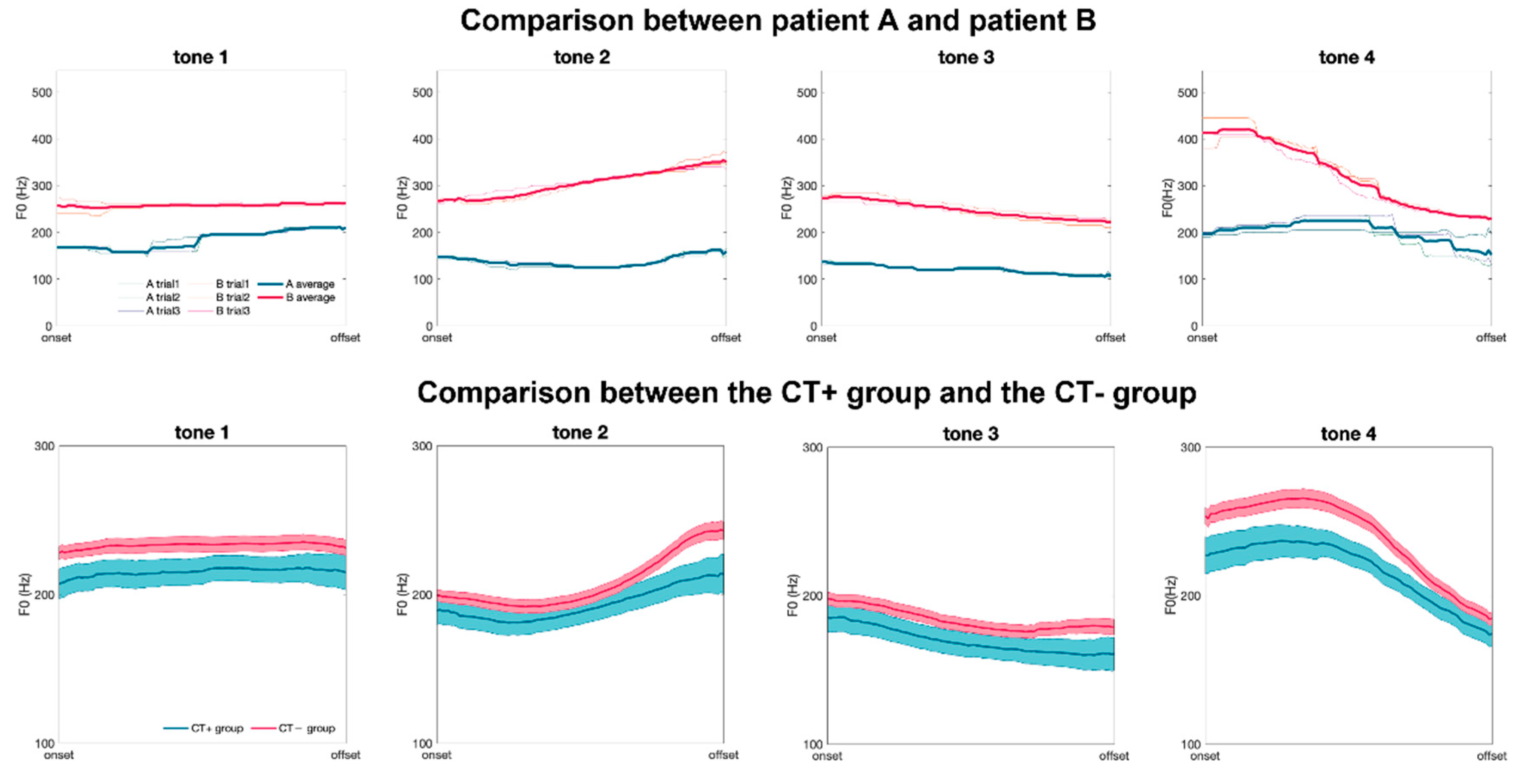
| Parameter | Total | Group | ||
|---|---|---|---|---|
| CT+ | CT− | p Value | ||
| n = 88 | n = 17 | n = 71 | ||
| Age (year) | 52.15 ± 14.05 | 50.76 ± 13.92 | 52.50 ± 14.06 | 0.659 |
| Paralysis side (left/right) | 49/33 | 7/10 | 47/24 | 0.099 |
| Time post-paralysis (month) | 9.56 ± 36.05 | 4.52 ± 3.10 | 10.88 ± 40.36 | 0.217 |
| Pathogenesis (n, %) | 0.112 | |||
| Thyroidectomy | 51 (62.2) | 13 (76.5) | 43 (60.6) | |
| Esophageal surgery | 5 (6.1) | 0 (0) | 5 (7.0) | |
| Lung surgery | 10 (12.2) | 0 (0) | 10(14.1) | |
| Skull base or brain surgery | 3 (3.7) | 2 (11.8) | 1 (1.4) | |
| Cervical spine surgery | 7 (8.5) | 2 (11.8) | 5 (7.0) | |
| Heart surgery | 4 (4.9) | 0 (0) | 4 (5.6) | |
| Other | 2 (2.4) | 0 (0) | 3 (4.2) | |
| F0(Hz) | Total | Group | p Value | Cohen’s d | |
|---|---|---|---|---|---|
| CT+ | CT− | ||||
| Tone 1 | |||||
| Onset | 229.3 ± 40.4 | 212.3 ± 41.5 | 233.4 ± 39.3 | 0.035 * | 0.53 |
| Offset | 233.3 ± 46.9 | 219.8 ± 48.6 | 236.5 ± 46.2 | 0.106 | 0.35 |
| 3.9 ± 17.1 | 7.5 ± 22.0 | 3.1 ± 15.8 | 0.219 | 0.26 | |
| 5.1 ± 23.8 | 8.3 ± 28.7 | 4.3 ± 22.6 | 0.298 | 0.17 | |
| Tone 2 | |||||
| Onset | 204.3 ± 37.5 | 194.1 ± 38.6 | 206.7 ± 37.1 | 0.118 | 0.33 |
| Offset | 243.6 ± 55.8 | 218.7 ± 54.1 | 250.0 ± 54.9 | 0.023 * | 0.56 |
| 39.3 ± 32.4 | 24.6 ± 23.8 | 42.9 ± 33.3 | 0.007 ** | 0.57 | |
| 51.6 ± 43.2 | 30.7 ± 27.3 | 56.6 ± 44.9 | 0.002 ** | 0.61 | |
| Tone 3 | |||||
| Onset | 202.1 ± 38.6 | 190.5 ± 39.5 | 204.9 ± 38.0 | 0.093 | 0.37 |
| Offset | 184.2 ± 49.9 | 165.7 ± 46.2 | 188.7 ± 50.0 | 0.041 * | 0.46 |
| −17.9 ± 40.0 | −24.8 ± 31.5 | −16.2 ± 32.1 | 0.162 | 0.27 | |
| −36.5 ± 54.0 | −48.9 ± 70.0 | −33.5 ± 50.0 | 0.200 | 0.29 | |
| −34.3 ± 18.2 | −33.7 ± 21.6 | −34.5 ± 17.4 | 0.449 | 0.03 | |
| −100.3 ± 72.7 | −82.5 ± 62.2 | −105.9 ± 74.2 | 0.095 | 0.33 | |
| 16.4 ± 27.0 | 8.9 ± 16.3 | 18.2 ± 28.8 | 0.041 * | 0.35 | |
| 43.6 ± 62.2 | 20.4 ± 32.0 | 49.1 ± 66.5 | 0.006 ** | 0.47 | |
| Tone 4 | |||||
| Onset | 253.8 ± 50.9 | 231.0 ± 51.3 | 259.2 ± 49.6 | 0.025 * | 0.57 |
| Offset | 186.4 ± 37.2 | 177.8 ± 34.0 | 188.5 ± 37.9 | 0.131 | 0.29 |
| −67.4 ± 35.4 | −53.2 ± 28.3 | −70.8 ± 36.3 | 0.019 * | 0.50 | |
| −168.9 ± 85.9 | −125.9 ± 68.0 | −179.1 ± 87.0 | 0.005 ** | 0.64 | |
| Maximal drop in 0.005 s | 25.5 ± 14.3 | 16.1 ± 5.7 | 27.8 ± 14.8 | <0.001 *** | 0.86 |
| Parameters (Hz) | Mean ± STD | Cross-Trial STD | Intraclass Correlation Coefficient |
|---|---|---|---|
| Tone 1 | |||
| Onset | 229.3 ± 40.4 | 9.5 | 0.99 ** |
| Offset | 233.3 ± 46.9 | 10.9 | 0.98 ** |
| 3.9 ± 17.1 | 11.3 | 0.90 ** | |
| Time duration | 0.82 ± 0.18 | 0.08 | 0.95 ** |
| Tone 2 | |||
| Onset | 204.3 ± 37.5 | 8.7 | 0.99 ** |
| Offset | 243.6 ± 55.8 | 14.0 | 0.99 ** |
| 39.3 ± 32.4 | 15.1 | 0.94 ** | |
| Time duration | 0.84 ± 0.17 | 0.08 | 0.90 ** |
| Tone 3 | |||
| Onset | 202.1 ± 38.6 | 9.5 | 0.97 ** |
| Offset | 184.2 ± 49.9 | 13.3 | 0.99 ** |
| −17.9 ± 40.0 | 16.5 | 0.93 ** | |
| Time duration | 0.71 ± 0.17 | 0.09 | 0.95 ** |
| Tone 4 | |||
| Onset | 253.8 ± 50.9 | 12.2 | 0.97 ** |
| Offset | 186.4 ± 37.2 | 14.0 | 0.95 ** |
| −67.4 ± 35.4 | 15.7 | 0.77 * | |
| Time duration | 0.45 ± 0.11 | 0.05 | 0.92 ** |
| Peak Turn Frequency in Lesion Site (Hz) | Total | Group | p Value | Cohen’s d | |
|---|---|---|---|---|---|
| CT+ | CT− | ||||
| Tone 1 | 674.9 ± 266.8 | 442.4 ± 285.0 | 730.6 ± 231.6 | <0.001 *** | 1.19 |
| Tone 2 | 622.9 ± 238.4 | 439.6 ± 252.5 | 666.7 ± 214.3 | 0.001 ** | 1.02 |
| Tone 3 | 553.3 ± 245.0 | 381.6 ± 263.1 | 594.4 ± 223.5 | 0.003 ** | 0.92 |
| Tone 4 | 736.3 ± 279.0 | 457.6 ± 287.5 | 803.0 ± 233.3 | <0.001 *** | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Fang, T.-J.; Chuang, H.-F.; Wong, A.M.K.; Pei, Y.-C. Cricothyroid Dysfunction in Unilateral Vocal Fold Paralysis Females Impairs Lexical Tone Production. J. Clin. Med. 2022, 11, 6442. https://doi.org/10.3390/jcm11216442
Wu Y-C, Fang T-J, Chuang H-F, Wong AMK, Pei Y-C. Cricothyroid Dysfunction in Unilateral Vocal Fold Paralysis Females Impairs Lexical Tone Production. Journal of Clinical Medicine. 2022; 11(21):6442. https://doi.org/10.3390/jcm11216442
Chicago/Turabian StyleWu, Yu-Cheng, Tuan-Jen Fang, Hsiu-Feng Chuang, Alice M. K. Wong, and Yu-Cheng Pei. 2022. "Cricothyroid Dysfunction in Unilateral Vocal Fold Paralysis Females Impairs Lexical Tone Production" Journal of Clinical Medicine 11, no. 21: 6442. https://doi.org/10.3390/jcm11216442
APA StyleWu, Y.-C., Fang, T.-J., Chuang, H.-F., Wong, A. M. K., & Pei, Y.-C. (2022). Cricothyroid Dysfunction in Unilateral Vocal Fold Paralysis Females Impairs Lexical Tone Production. Journal of Clinical Medicine, 11(21), 6442. https://doi.org/10.3390/jcm11216442






