Response to Initial Anti-Vascular Endothelial Growth Factor for Diabetic Macular Edema Is Significantly Correlated with Response to Third Consecutive Monthly Injection
Abstract
1. Introduction
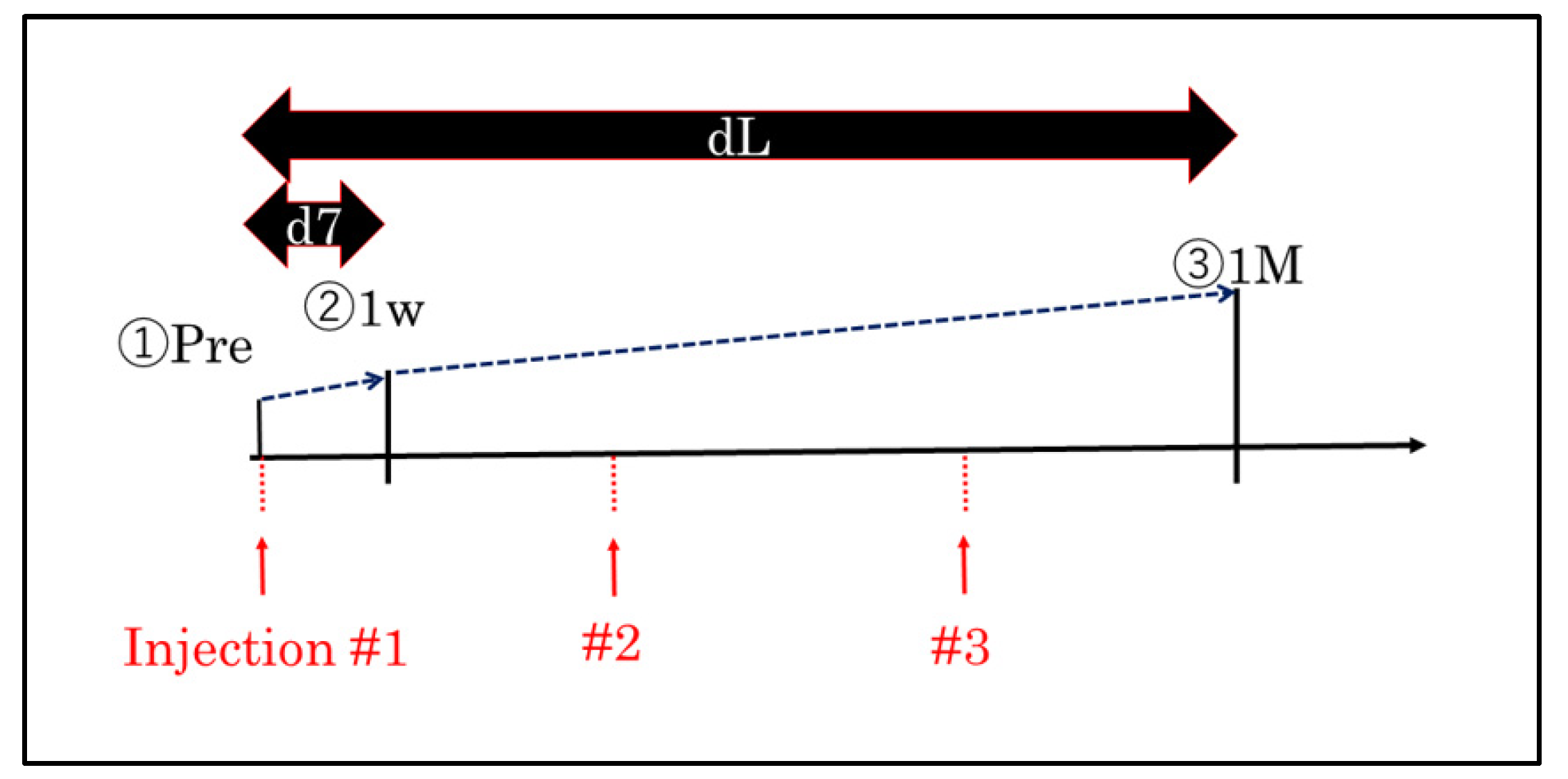
2. Patients and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Procedures
2.3. Measurement of BCVA and CMT
2.4. Intravitreal Anti-VEGF Agent Injections
2.5. Statistical Analyses
3. Results
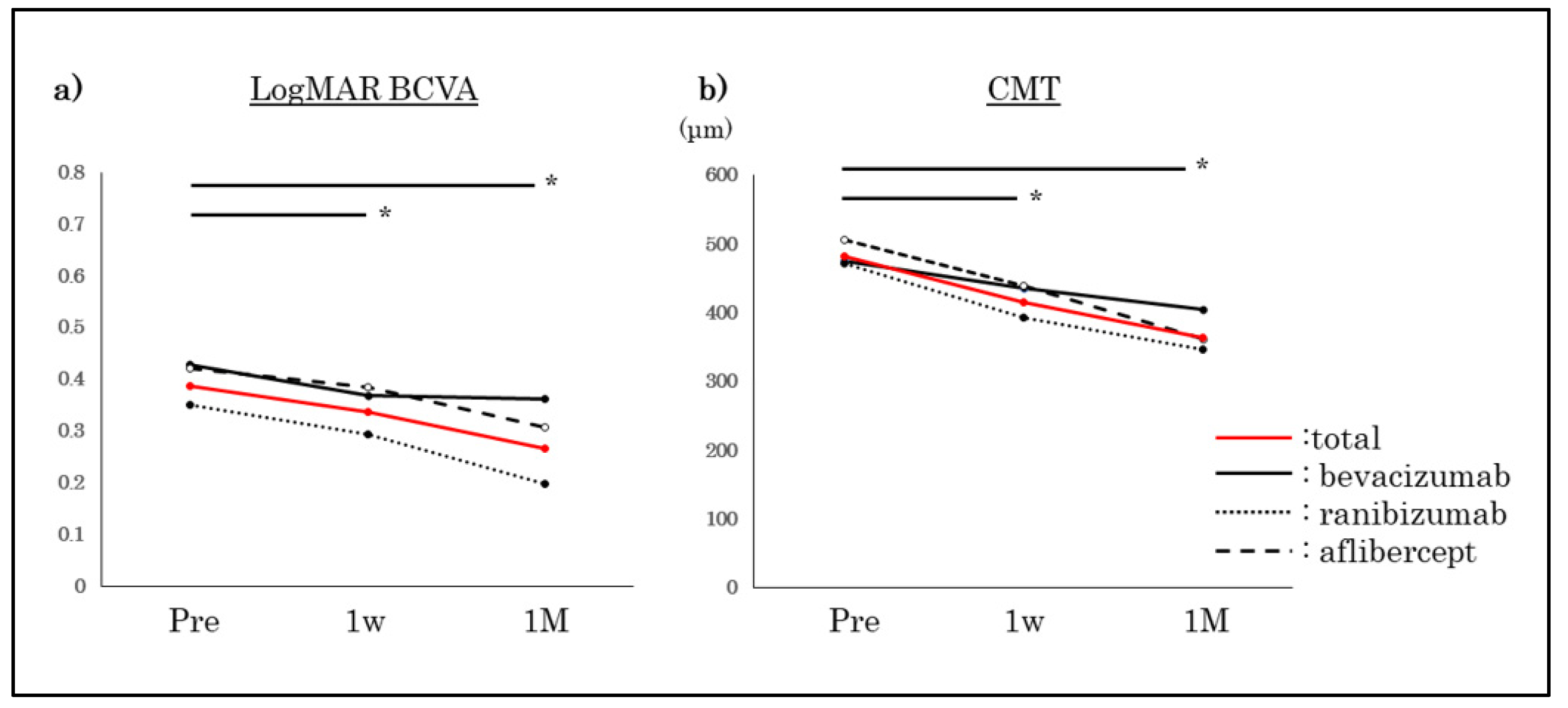
3.1. BCVA and CMT Improved Significantly for All Agents (Figure 2)
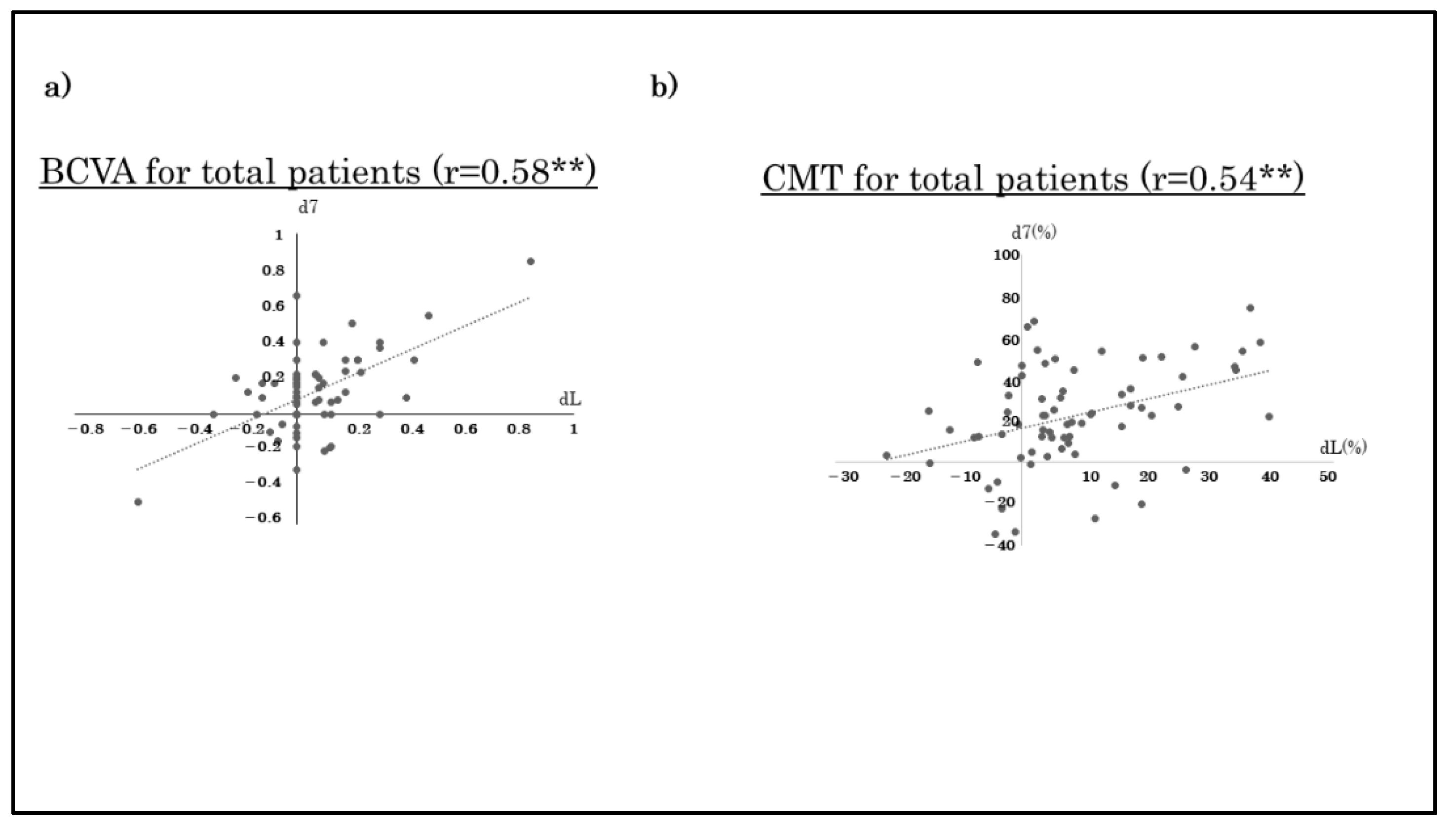
3.2. Correlations between BCVA and CMT at Day 7 and 1 Month after the Third Monthly Injection of Anti-VEGF Agent(Table 2)
| (r-Value) | Total | Bevacizumab | Ranibizumab | Aflibercept |
|---|---|---|---|---|
| BCVA (logMAR) | 0.58 ** | 0.09 | 0.42 * | 0.83 ** |
| CMT | 0.54 ** | 0.68 * | 0.41 ** | 0.53 ** |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, S.E.; Klein, R.; Klein, B.E.K. The incidence of visual loss in a diabetic population. Ophthalmology 1988, 95, 1340–1348. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Fromow-Guerra, J.; Quiroz-Mercado, H.; Sanchez, J.G.; Wu, L.; Maia, M.; Berrocal, M.H.; Solis-Vivanco, A.; Farah, M.E.; Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema. Ophthalmology 2007, 114, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Haritoglou, C.; Kook, D.; Neubauer, A.; Wolf, A.; Priglinger, S.; Strauss, R.; Gandorfer, A.; Ulbig, M.; Kampik, A. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006, 26, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.J.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Bressler, S.B.; Edwards, A.R.; Ferris, I.I.I.F.L.; Friedman, S.M.; Glassman, A.R.; Miller, K.M.; et al. Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010, 117, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Shimura, M.; Kitano, S.; Ohji, M.; Ogura, Y.; Yamashita, H.; Suzaki, M.; Mori, K.; Ohashi, Y.; Yap, P.S.; et al. Impact on visual acuity and psychological outcomes of ranibizumab and subsequent treatment for diabetic macular oedema in Japan (MERCURY). Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Tsukitome, H.; Okamoto, F.; Oshika, T.; Ueda, T.; Niki, M.; Mitamura, Y.; Ishikawa, H.; Gomi, F.; Kitano, S.; et al. Clinical preferences and trends of anti-vascular endothelial growth factor treatments for diabetic macular edema in Japan. J. Diabetes Investig. 2019, 10, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Beaulieu, W.T.; Glassman, A.R.; Blinder, K.J.; Bressler, S.B.; Jampol, L.M.; Melia, M.; Wells, J.A., 3rd; Diabetic Retinopathy Clinical Research Network. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: A secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018, 136, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.H.; Campbell, J.; Holekamp, N.M.; Kiss, S.; Loewenstein, A.; Augustin, A.J.; Ma, J.; Ho, A.C.; Patel, V.; Whitcup, S.M.; et al. Early and Long-Term Responses to Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema: Analysis of Protocol I Data. Am. J. Ophthalmol. 2016, 172, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Nagaoka, T.; Ishibazawa, A.; Yoshida, A. Short-term effects of intravitreal ranibizumab therapy on diabetic macular edema. BMC Ophthalmol. 2017, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, Y.; Yoshida, S.; Kobayashi, Y.; Kubo, Y.; Nakama, T.; Ishikawa, K.; Nakao, S.; Hisatomi, T.; Ikeda, Y.; Oshima, Y.; et al. Visual Outcomes Based on Early Response to Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema. Ophthalmologica 2018, 239, 94–102. [Google Scholar] [CrossRef] [PubMed]
| Age (yrs) | Pre-BCVA (logMAR) | Pre-CMT (μm) | |
|---|---|---|---|
| Total (n = 70) | 63.6 ± 11.1 | 0.38 ± 0.22 | 481.9 ± 96.3 |
| Bevacizumab (n = 16) | 60.1 ± 15.2 | 0.43 ± 0.29 | 475.6 ± 90.4 |
| Ranibizumab (n = 35) | 64.2 ± 10.0 | 0.35 ± 0.19 | 471.9 ± 107.7 |
| Aflibercept (n = 19) | 65.5 ± 8.7 | 0.42 ± 0.29 | 505.7 ± 77.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, S.; Sugimoto, M.; Tenma, Y.; Tsukitome, H.; Kato, K.; Chujo, S.; Matsui, Y.; Matsubara, H.; Kondo, M. Response to Initial Anti-Vascular Endothelial Growth Factor for Diabetic Macular Edema Is Significantly Correlated with Response to Third Consecutive Monthly Injection. J. Clin. Med. 2022, 11, 6416. https://doi.org/10.3390/jcm11216416
Maeda S, Sugimoto M, Tenma Y, Tsukitome H, Kato K, Chujo S, Matsui Y, Matsubara H, Kondo M. Response to Initial Anti-Vascular Endothelial Growth Factor for Diabetic Macular Edema Is Significantly Correlated with Response to Third Consecutive Monthly Injection. Journal of Clinical Medicine. 2022; 11(21):6416. https://doi.org/10.3390/jcm11216416
Chicago/Turabian StyleMaeda, Satoshi, Masahiko Sugimoto, Yumiho Tenma, Hideyuki Tsukitome, Kumiko Kato, Shinichiro Chujo, Yoshitsugu Matsui, Hisashi Matsubara, and Mineo Kondo. 2022. "Response to Initial Anti-Vascular Endothelial Growth Factor for Diabetic Macular Edema Is Significantly Correlated with Response to Third Consecutive Monthly Injection" Journal of Clinical Medicine 11, no. 21: 6416. https://doi.org/10.3390/jcm11216416
APA StyleMaeda, S., Sugimoto, M., Tenma, Y., Tsukitome, H., Kato, K., Chujo, S., Matsui, Y., Matsubara, H., & Kondo, M. (2022). Response to Initial Anti-Vascular Endothelial Growth Factor for Diabetic Macular Edema Is Significantly Correlated with Response to Third Consecutive Monthly Injection. Journal of Clinical Medicine, 11(21), 6416. https://doi.org/10.3390/jcm11216416






