Use of Levosimendan in Patients with Advanced Heart Failure: An Update
Abstract
1. Introduction
2. Pharmacology of Levosimendan
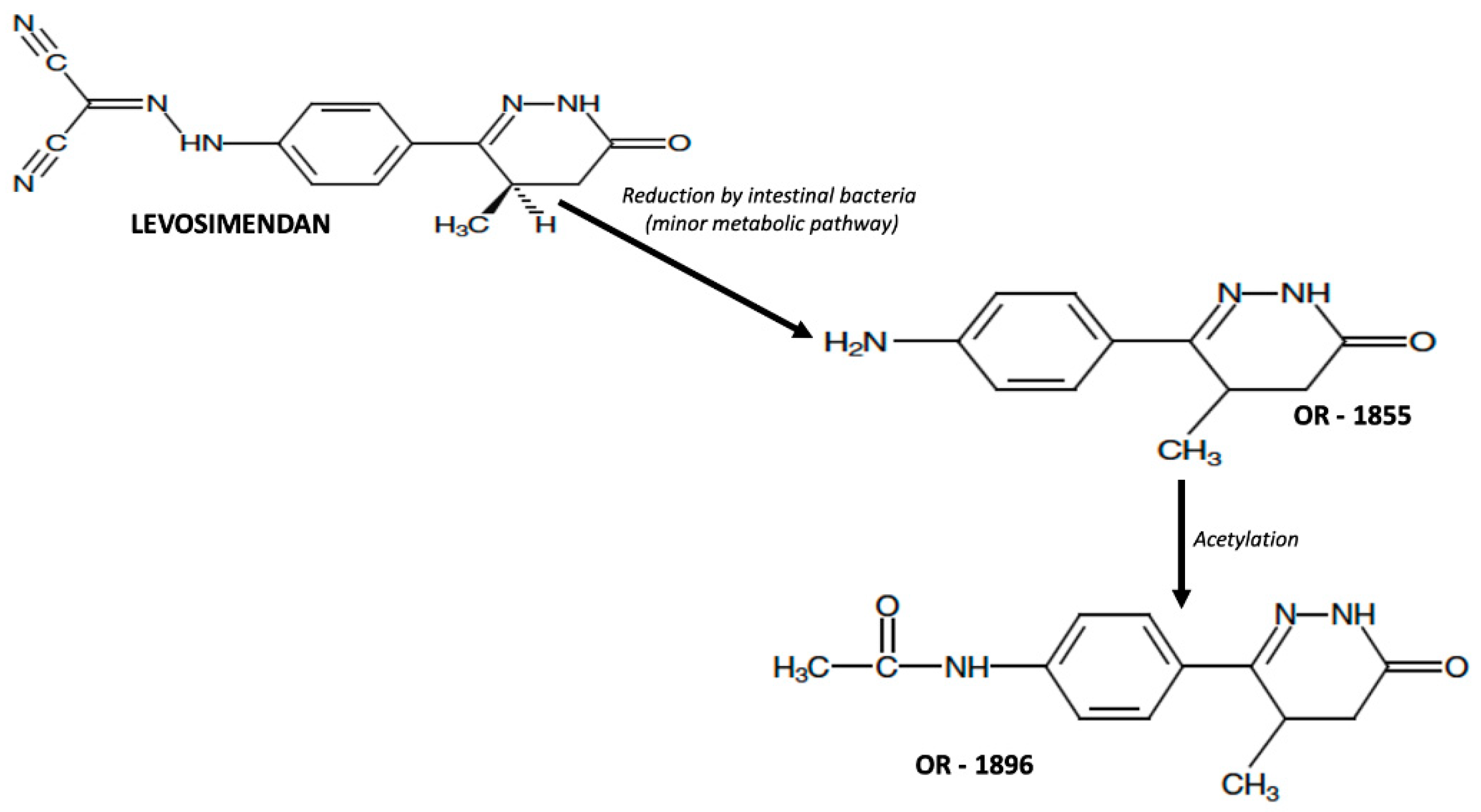
2.1. Pharmacokinetic of Levosimendan
2.2. Pharmacodynamics of Levosimendan
2.3. Side Effects and Contraindications of Levosimendan
3. Intermittent Levosimendan Infusion in Patients with advHFrEF
4. Levosimendan in Patients with advHFrEF Undergoing LVAD Implantation
5. Levosimendan in Patients with advHFrEF on the Waiting List for a Heart Transplant
6. Levosimendan in Patients with advHFpEF as a Future Perspective
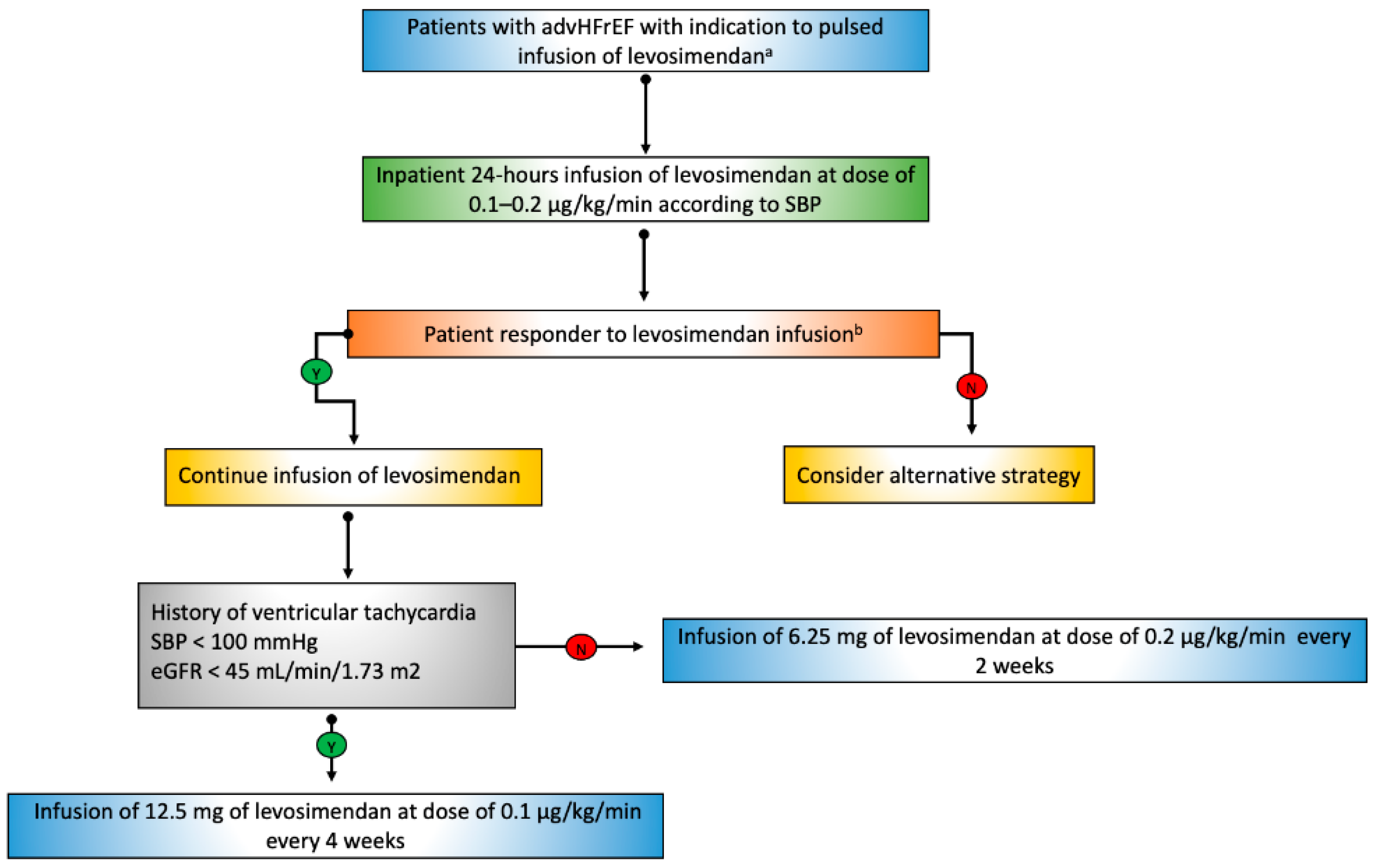
7. Tips and Tricks for the Use of Levosimendan in Clinical Practice
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Kępińska, K.; Adamczak, D.M.; Kałużna-Oleksy, M. Advanced heart failure: A review. Adv. Clin. Exp. Med. 2019, 28, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Kittleson, M.; Petraio, A.; Pacileo, G. Advanced heart failure: State of the art and future directions. Rev. Cardiovasc. Med. 2022, 23, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.C.; Ewald, G.A.; Allen, L.A.; Butler, J.; Canary, C.A.W.; Colvin-Adams, M.; Dickinson, M.G.; Levy, P.; Stough, W.G.; Sweitzer, N.K.; et al. Advanced (Stage D) Heart Failure: A Statement from the Heart Failure Society of America Guidelines Committee. J. Card. Fail. 2015, 21, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Vishram-Nielsen, J.K.K.; Tomasoni, D.; Gustafsson, F.; Metra, M. Contemporary Drug Treatment of Advanced Heart Failure with Reduced Ejection Fraction. Drugs 2022, 82, 375–405. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Melillo, E.; Gravino, R.; Errigo, V.; Martucci, M.L.; Caiazzo, A.; Petraio, A.; Pölzl, G.; Pacileo, G. Inotropes in Patients with Advanced Heart Failure: Not only palliative care. Heart Fail. Clin. 2021, 17, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Antila, S.; Sundberg, S.; Lehtonen, L.A. Clinical pharmacology of levosimendan. Clin. Pharm. 2007, 46, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, L.A.; Antila, S.; Pentikäinen, P.J. Pharmacokinetics and pharmacodynamics of intravenous inotropic agents. Clin. Pharm. 2004, 43, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Nikitin, N.; James, M. Levosimendan: First in a new class of inodilator for acute and chronic severe heart failure. Expert Rev. Cardiovasc. Ther. 2004, 2, 9–19. [Google Scholar] [CrossRef]
- Figgitt, D.P.; Gillies, P.S.; Goa, K.L. Levosimendan. Drugs 2001, 61, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Papp, Z.; Agostoni, P.; Alvarez, J.; Bettex, D.; Bouchez, S.; Brito, D.; Černý, V.; Comin-Colet, J.; Crespo-Leiro, M.G.; Delgado, J.F.; et al. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. Card Fail Rev. 2020, 6, e19. [Google Scholar] [CrossRef]
- Puttonen, J.; Kantele, S.; Ruck, A.; Ramela, M.; Hakkinen, S.; Kivikko, M.; Pentikainen, P.J. Pharmacokinetics of intravenous levosimendan and its metabolites in subjects with hepatic impairment. J. Clin. Pharmacol. 2008, 48, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, J.; Kantele, S.; Kivikko, M.; Häkkinen, S.; Harjola, V.-P.; Koskinen, P.; Pentikäinen, P.J. Effect of severe renal failure and haemodialysis on the pharmacokinetics of levosimendan and its metabolites. Clin Pharm. 2007, 46, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Agostoni, P.; Baholli, L.; Bautin, A.; Comin-Colet, J.; Crespo-Leiro, M.G.; Fedele, F.; García-Pinilla, J.M.; Giannakoulas, G.; Grigioni, F.; et al. A pragmatic approach to the use of inotropes for the management of acute and advanced heart failure: An expert panel consensus. Int. J. Cardiol. 2019, 297, 83–90. [Google Scholar] [CrossRef]
- Papp, Z.; Csapo, K.; Pollesello, P.; Haikala, H.; Edes, I. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc. Drug Rev. 2005, 23, 71–98. [Google Scholar] [CrossRef] [PubMed]
- Haikala, H.; Kaivola, J.; Nissinen, E.; Wall, P.; Levijoki, J.; Linden, I.B. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J. Mol. Cell Cardiol. 1995, 27, 1859–1866. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Pollesello, P.; Vajda, G.; Papp, Z. Effects of levosimendan on the energy balance: Preclinical and clinical evidence. J. Cardiovasc. Pharmacol. 2009, 53, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Pollesello, P.; Saris, N.E. Levosimendan is a mitochondrial K (ATP) channel opener. Eur. J. Pharmacol. 2011, 428, 311–314. [Google Scholar] [CrossRef]
- Burkhoff, D.; Rich, S.; Pollesello, P.; Papp, Z. Levosimendan-induced venodilation is mediated by opening of potassium channels. ESC Heart Fail. 2021, 8, 4454–4464. [Google Scholar] [CrossRef] [PubMed]
- Milligan, D.J.; Fields, A.M. Levosimendan: Calcium sensitizer and inodilator. Anesthesiol. Clin. 2010, 28, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.M.; Akhter, M.W. Levosimendan: Dual mechanisms for acute heart failure and beyond? Minerva Cardioangiol. 2005, 53, 565–584. [Google Scholar] [PubMed]
- Trikas, A.; Antoniades, C.; Latsios, G.; Vasiliadou, K.; Karamitros, I.; Tousoulis, D.; Tentolouris, C.; Stefanadis, C. Long-term effects of levosimendan infusion on inflammatory processes and sFas in patients with severe heart failure. Eur. J. Heart Fail. 2006, 8, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Parissis, J.T.; Adamopoulos, S.; Antoniades, C.; Kostakis, G.; Rigas, A.; Kyrzopoulos, S.; Iliodromitis, E.; Kremastinos, D. Effects of levosimendan on circulating pro-inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am. J. Cardiol. 2004, 93, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Erhardt, L. Levosimendan: A new dual-action drug in the treatment of acute heart failure. Int. J. Clin. Pract. 2003, 57, 410–416. [Google Scholar]
- Mebazaa, A.; Nieminen, M.S.; Packer, M.; Cohen-Solal, A.; Kleber, F.X.; Pocock, S.J.; Thakkar, R.; Padley, R.J.; Põder, P.; Kivikko, M.; et al. Levosimendan vs. dobutamine for patients with acute decompensated heart failure: The SURVIVE randomized trial. JAMA 2007, 297, 1883–1891. [Google Scholar] [CrossRef]
- Packer, M.; Colucci, W.; Fisher, L.; Massie, B.M.; Teerlink, J.R.; Young, J.; Padley, R.J.; Thakkar, R.; Delgado-Herrera, L.; Salon, J.; et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013, 1, 103–111. [Google Scholar] [CrossRef]
- Silva-Cardoso, J.; Ferreira, J.; Oliveira-Soares, A.; Martins-de-Campos, J.; Fonseca, C.; Lousada, N.; Ilídio-Moreira, J.; Rabaçal, C.; Damasceno, A.; Amorim, S.; et al. Effectiveness and safety of levosimendan in clinical practice. Rev. Port. Cardiol. 2009, 28, 143–154. [Google Scholar]
- Moreno, N.; Tavares-Silva, M.; Lourenço, A.P.; Oliveira-Pinto, J.; Henriques-Coelho, T.; Leite-Moreira, A.F. Levosimendan: The current situation and new prospects. Rev. Port. Cardiol. 2014, 33, 795–800. [Google Scholar] [CrossRef]
- Masarone, D.; Valente, F.; Verrengia, M.; Ammendola, E.; Gravino, R.; D’Alterio, G.; Petraio, A.; Pacileo, G. Efficacy and safety of repeated infusion of levosimendan in outpatients with advanced heart failure: A real-world experience. J. Cardiovasc. Med. 2020, 21, 919–921. [Google Scholar] [CrossRef]
- Masarone, D.; Melillo, E.; Errigo, V.; Martucci, M.L.; Pacileo, R.; Pollesello, P.; Petraio, A.; Pacileo, G. Hemodynamic Effects of Levosimendan in Outpatients with Advanced Heart Failure: An Echocardiographic Pilot Study. J. Cardiovasc. Pharmacol. 2022, 79, e36–e40. [Google Scholar] [CrossRef]
- Mushtaq, S.; Andreini, D.; Farina, S.; Salvioni, E.; Pontone, G.; Sciomer, S.; Volpato, V.; Agostoni, P. Levosimendan improves exercise performance in patients with advanced chronic heart failure. ESC Heart Fail. 2015, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Apostolo, A.; Vignati, C.; Della Rocca, M.; De Martino, F.; Berna, G.; Campodonico, J.; Contini, M.; Muratori, M.; Palermo, P.; Mapelli, M.; et al. Why Levosimendan Improves the Clinical Condition of Patients with Advanced Heart Failure: A Holistic Approach. J. Card. Fail. 2022, 28, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Perna, E.; Marini, M.; Nassiacos, D.; Cirò, A.; Malfatto, G.; Morandi, F.; Caico, I.; Perna, G.; Meloni, S.; et al. Scheduled intermittent inotropes for Ambulatory Advanced Heart Failure. The RELEVANT-HF multicentre collaboration. Int. J. Cardiol. 2018, 272, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Kittleson, M.M.; Martucci, M.L.; Valente, F.; Gravino, R.; Verrengia, M.; Ammendola, E.; Contaldi, C.; Di Palma, V.; Caiazzo, A.; et al. Levosimendan as a “Bridge to Optimization” in Patients with Advanced Heart Failure with Reduced Ejection-A Single-Center Study. J. Clin. Med. 2022, 11, 4227. [Google Scholar] [CrossRef] [PubMed]
- Nanas, J.N.; Papazoglou, P.; Tsagalou, E.P.; Ntalianis, A.; Tsolakis, E.; Terrovitis, J.V.; Kanakakis, J.; Nanas, S.N.; Alexopoulos, G.P.; Anastasiou-Nana, M.I. Efficacy and safety of intermittent, long-term, concomitant dobutamine and levosimendan infusions in severe heart failure refractory to dobutamine alone. Am. J. Cardiol. 2005, 95, 768–771. [Google Scholar] [CrossRef]
- Parissis, J.T.; Adamopoulos, S.; Farmakis, D.; Filippatos, G.; Paraskevaidis, I.; Panou, F.; Iliodromitis, E.; Kremastinos, D.T. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart 2006, 92, 1768–1772. [Google Scholar] [CrossRef][Green Version]
- Mavrogeni, S.; Giamouzis, G.; Papadopoulou, E.; Thomopoulou, S.; Dritsas, A.; Athanasopoulos, G.; Adreanides, E.; Vassiliadis, I.; Spargias, K.; Panagiotakos, D.; et al. A 6-month follow-up of intermittent levosimendan administration effect on systolic function, specific activity questionnaire, and arrhythmia in advanced heart failure. J. Card. Fail. 2007, 13, 556–559. [Google Scholar] [CrossRef]
- Papadopoulou, E.F.; Mavrogeni, S.I.; Dritsas, A.; Cokkinos, D.V. Assessment of quality of life using three activity questionnaires in heart failure patients after monthly, intermittent administration of levosimendan during a six-month period. Hellenic. J. Cardiol. 2009, 50, 269–274. [Google Scholar]
- Malfatto, G.; Della Rosa, F.; Villani, A.; Rella, V.; Branzi, G.; Facchini, M.; Parati, G. Intermittent levosimendan infusions in advanced heart failure: Favourable effects on left ventricular function, neurohormonal balance, and one-year survival. J. Cardiovasc. Pharmacol. 2012, 60, 450–455. [Google Scholar] [CrossRef]
- Altenberger, J.; Parissis, J.T.; Costard-Jaeckle, A.; Winter, A.; Ebner, C.; Karavidas, A.; Sihorsch, K.; Avgeropoulou, E.; Weber, T.; Dimopoulos, L.; et al. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: A multicentre randomized trial. Eur. J. Heart Fail. 2014, 16, 898–906. [Google Scholar] [CrossRef]
- Comín-Colet, J.; Manito, N.; Segovia-Cubero, J.; Delgado, J.; Pinilla, J.M.G.; Almenar, L.; Crespo-Leiro, M.G.; Sionis, A.; Blasco, T.; Pascual-Figal, D.; et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: The LION-HEART multicentre randomised trial. Eur. J. Heart Fail. 2018, 20, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.J.; Perona, A.A.; Padron, A.L.; Rull, J.L.M.; Martínez-Sellés, M.; Martin, M.D.M.; Díaz, J.L.; Fernandez, S.L.; Oficialdegui, P.O.; Sosa, A.J. Efficacy and safety of intermittent repeated levosimendan infusions in advanced heart failure patients: The LAICA study. ESC Heart Fail. 2021, 8, 4820–4831. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, H.; Soliman, O.; Zijderhand, C.; Lenzen, M.; Hoeks, S.E.; Kaddoura, R.; Izham, M.; Alkhulaifi, A.; Omar, A.S.; Caliskan, K. Intermittent levosimendan infusion in ambulatory patients with end-stage heart failure: A systematic review and meta-analysis of 984 patients. Heart Fail. Rev. 2022, 27, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Liao, Y.; Li, G.; Chen, Y. Levosimendan Can Improve the Level of B-Type Natriuretic Peptide and the Left Ventricular Ejection Fraction of Patients with Advanced Heart Failure: A Meta-analysis of Randomized Controlled Trials. Am. J. Cardiovasc. Drugs 2021, 21, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Silvetti, S.; Belletti, A.; Fontana, A.; Pollesello, P. Rehospitalization after intermittent levosimendan treatment in advanced heart failure patients: A meta-analysis of randomized trials. ESC Heart Fail. 2017, 4, 595–604. [Google Scholar] [CrossRef]
- Pölzl, G.; Birgani, S.A.; Comín-Colet, J.; Delgado, J.F.; Fedele, F.; García-Gonzáles, M.J.; Gustafsson, F.; Masip, J.; Papp, Z.; Störk, S.; et al. Repetitive levosimendan infusions for patients with advanced chronic heart failure in the vulnerable post-discharge period. ESC Heart Fail. 2019, 6, 174–181. [Google Scholar] [CrossRef]
- Tycińska, A.; Gierlotka, M.; Bartuś, S.; Gąsior, M.; Główczyńska, R.; Grześk, G.; Jaguszewski, M.; Kasprzak, J.D.; Kubica, J.; Legutko, J.; et al. Repetitive use of LEvosimendan in Ambulatory Heart Failure patients (LEIA-HF)—The rationale and study design. Adv. Med. Sci. 2022, 67, 18–22. [Google Scholar] [CrossRef]
- Jefferson, H.L.; Kent, W.D.T.; MacQueen, K.T.; Miller, R.J.H.; Holloway, D.D.; Hassanabad, A.F. Left ventricular assist devices: A comprehensive review of major clinical trials, devices, and future directions. J. Card. Surg. 2021, 36, 1480–1491. [Google Scholar] [CrossRef]
- McNamara, N.; Narroway, H.; Williams, M.; Brookes, J.; Farag, J.; Cistulli, D.; Bannon, P.; Marasco, S.; Potapov, E.; Loforte, A. Contemporary outcomes of continuous-flow left ventricular assist devices-a systematic review. Ann. Cardiothorac. Surg. 2021, 10, 186–208. [Google Scholar] [CrossRef]
- Kapelios, C.J.; Lund, L.H.; Wever-Pinzon, O.; Selzman, C.H.; Myers, S.L.; Cantor, R.S.; Stehlik, J.; Chamogeorgakis, T.; McKellar, S.H.; Koliopoulou, A.; et al. Right Heart Failure Following Left Ventricular Device Implantation: Natural History, Risk Factors, and Outcomes: An Analysis of the STS INTERMACS Database. Circ. Heart Fail. 2022, 15, e008706. [Google Scholar] [CrossRef]
- Hansen, M.S.; Andersen, A.; Nielsen-Kudsk, J.E. Levosimendan in pulmonary hypertension and right heart failure. Pulm. Circ. 2018, 8, 2045894018790905. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wei, Z.; Zhang, C.; Lu, C.; Zeng, Z. The effect of levosimendan on right ventricular function in patients with heart dysfunction: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 24097. [Google Scholar] [CrossRef]
- Sponga, S.; Ivanitskaia, E.; Potapov, E.; Krabatsch, T.; Hetzer, R.; Lehmkuhl, H. Preoperative treatment with levosimendan in candidates for mechanical circulatory support. ASAIO J. 2012, 58, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Theiss, H.D.; Grabmaier, U.; Kreissl, N.; Hagl, C.; Steinbeck, G.; Sodian, R.; Franz, W.-M.; Kaczmarek, I. Preconditioning with levosimendan before implantation of left ventricular assist devices. Artif. Organs. 2014, 38, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Kocabeyoglu, S.S.; Kervan, U.; Sert, D.E.; Karahan, M.; Aygun, E.; Beyazal, O.F.; Unal, E.U.; Akin, Y.; Demirkan, B.; Pac, M. Optimization with levosimendan improves outcomes after left ventricular assist device implantation. Eur. J. Cardio-Thoracic Surg. 2020, 57, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Lavee, J.; Kassif, Y.; Shemesh, Y.; Kogan, A.; Freimark, D.; Morgan, A.; Lotan, D.; Itelman, E.; Grupper, A. Effect of levosimendan infusion prior to left ventricular assist device implantation on right ventricular failure. J. Cardiothorac. Surg. 2022, 17, 158–164. [Google Scholar] [CrossRef]
- Abdelshafy, M.; Elsherbini, H.; Elkoumy, A.; Simpkin, A.J.; Elzomor, H.; Caliskan, K.; Soliman, O. Perioperative Levosimendan Infusion in Patients with End-Stage Heart Failure Undergoing Left Ventricular Assist Device Implantation. Front. Cardiovasc. Med. 2022, 9, 888136. [Google Scholar] [CrossRef]
- Shah, K.S.; Kittleson, M.M.; Kobashigawa, J.A. Updates on Heart Transplantation. Curr. Heart Fail. Rep. 2019, 16, 150–156. [Google Scholar] [CrossRef]
- Bastani, B. The present and future of transplant organ shortage: Some potential remedies. J. Nephrol. 2020, 33, 277–288. [Google Scholar] [CrossRef]
- Ponz de Antonio, I.; de Juan Bagudá, J.S.; Rodríguez Chaverri, A.; García-Cosío, C.; Arribas Ynsaurriaga, F.; Delgado Jiménez, J.F. Levosimendan as bridge to transplant in patients with advanced heart failure. Rev. Esp. Cardiol. 2020, 73, 422–424. [Google Scholar] [CrossRef]
- Agostoni, P.; Farmakis, D.T.; García-Pinilla, J.M.; Harjola, V.P.; Karason, K.; von Lewinski, D.; Parissis, J.; Pollesello, P.; Pölzl, G.; Recio-Mayoral, A.; et al. Haemodynamic Balance in Acute and Advanced Heart Failure: An Expert Perspective on the Role of Levosimendan. Card. Fail. Rev. 2019, 5, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Killian, J.M.; Weston, S.A.; Schulte, P.J.; Subramaniam, A.V.; Blecker, S.B.; Redfield, M.M. Advanced Heart Failure Epidemiology and Outcomes: A Population-Based Study. JACC Heart Fail. 2021, 9, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, A.B.; Boen, J.R.A.; Segers, V.F.; Van Craenenbroeck, E.M. Heart Failure with Preserved Ejection Fraction: A Review of Cardiac and Noncardiac Pathophysiology. Front. Physiol. 2019, 10, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Deis, T.; Wolsk, E.; Mujkanovic, J.; Komtebedde, J.; Burkhoff, D.; Kaye, D.; Hasenfuß, G.; Hayward, C.; Van der Heyden, J.; Petrie, M.; et al. Resting and exercise haemodynamic characteristics of patients with advanced heart failure and preserved ejection fraction. ESC Heart Fail. 2022, 9, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Borlaug, B.A.; Shah, S.J.; Zolty, R.; Tedford, R.J.; Thenappan, T.; Zamanian, R.T.; Mazurek, J.A.; Rich, J.D.; Simon, M.A.; et al. Levosimendan Improves Hemodynamics and Exercise Tolerance in PH-HFpEF: Results of the Randomized Placebo-Controlled HELP Trial. JACC Heart Fail. 2021, 9, 360–370. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
| Study | N° of Patients | Levosimendan Dose | Time of Infusion | Interval of Infusion | Results |
|---|---|---|---|---|---|
| Nanas 2005 [35] | 36 | Bolus dose (6 mg/kg) plus Infusion rate (0.2 mcg/Kg/min). Levosimendan was added to dobutamine infusion | 24 h | 2 weeks for 45 days | Improvement in survival (6% vs. 61% p = 0.0002) |
| Parissis 2006 [36] | 25 | Bolus dose (6 mg/kg) plus Infusion rate (0.1–0.4 mcg/Kg/min) | 24 h | 3 weeks for 114 days | Reduction of LVEDVi (120 vs. 156 mL/m2; p < 0.01), LVESVi (80 vs. 106 mL/m2; p < 0.01) and NT-proBNP plasma levels (966 vs. 1529 pg/mL; p < 0.01) increase of LVEF (26 vs. 22%, p < 0.01) |
| Mavrogeni 2007 [37] | 50 | Bolus dose (6 mg/kg) plus Infusion rate (0.1–0.2 mcg/Kg/min) | 24 h | 30 days for 6 months | Increase of LVEF (28 + 7 vs. 21 + 4%, p = 0.003) and LVFS (15 + vs. 11 + 3%, p = 0.006). |
| Papadopoulou [38] | 20 | No bolus dose Infusion rate (0.1 mcg/kg/min) | 24 h | 30 days for 6 months | Increase of LVEF (30.3 ± 6.9 vs. 32.1 ± 7.4%; p = 0.01) and quality of life (LIhFE score i 35.4 ± 18.6 vs. 22.2 ± 13.0; p < 0.0001). |
| Malfatto 2012 [39] | 33 | No bolus dose Infusion rate (0.1–0.4 mcg/kg/min) | 24 h | 30 days for 12 months | Increase of LVEF (25.9 + 5.1 vs. 28.7 ± 5.4%; p < 0.05) and CI (2.34 + 0.58 vs. 2.77 + 0.65 L/min/m2; p < 0.05). Reduction of PASP (51.8 ± 15.4 vs. 42.6 ± 13.0 mmHg; p < 0.05), E/e’ ratio (18.3 ± 8.9 vs. 13.8 ± 4.1; p < 0.05) |
| Oliva (RELEVANT-HF) 2018 [33] | 185 | No bolus dose Infusion rate (0.2 mcg/Kg/min) | 24 h | 3–4 weeks for 6 months | Reduction of days in hospital (9.4 vs. 2.8 days; p < 0.0001) and length of HF admissions (17.4 vs. 21.6 days; p = 0.0001) |
| Masarone 2020 [29] | 15 | No bolus dose Infusion rate (0.2 mcg/Kg/min | 6 h | 2 weeks for 12 months | Reduction of HF-related hospitalizations (2 vs. 10; p < 0.05) and increase of distance walked at six-minute walking test (282 ± 52 vs. 248 ± 30 meters; p < 0.05) |
| Altenberger (LevoRep) 2014 [40] | 120 | No bolus dose Infusion rate (0.2 mcg/Kg/min) | 6 h | 2 weeks for 42 days | No increase in the distance walked on the 6-minute walking test and no increase in score on the Kansas City Cardiomyopathy Questionnaire (19% vs. 15%; OR.25; 95% CI 0.44–3.59; p = 0.810). |
| Comín-Colet (LION HEART) 2018 [41] | 69 | No bolus dose Infusion rate (0.2 mcg/Kg/min) | 6 h | 2 weeks for 6 months | Reduction of NT-proBNP plasma levels (mean change in NT-proBNP–1446 vs. –1320 pg/mL; p < 0.001) and of the rate of HF-related hospitalization (hazard ratio 0.25; 95% CI 0.11–0.56; p = 0.001) |
| García-González (LAICA) 2021 [42] | 97 | No bolus dose Infusion rate (0.1 mcg/Kg/min) | 24 h | 4 weeks for 12 months | No reduction in HF-related hospitalizations (HR 0.66; 95% CI, 0.32–1.32; p = 0.24). Reduction of cumulative incidence of HF-related hospitalizations and death at 1 month (5.7% vs. 25.9%; p = 0.004) and 3 months (17.1% vs. 48.1%; p = 0.001). Improvement in survival (log-rank: 4.06; p = 0.044). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masarone, D.; Kittleson, M.M.; Pollesello, P.; Marini, M.; Iacoviello, M.; Oliva, F.; Caiazzo, A.; Petraio, A.; Pacileo, G. Use of Levosimendan in Patients with Advanced Heart Failure: An Update. J. Clin. Med. 2022, 11, 6408. https://doi.org/10.3390/jcm11216408
Masarone D, Kittleson MM, Pollesello P, Marini M, Iacoviello M, Oliva F, Caiazzo A, Petraio A, Pacileo G. Use of Levosimendan in Patients with Advanced Heart Failure: An Update. Journal of Clinical Medicine. 2022; 11(21):6408. https://doi.org/10.3390/jcm11216408
Chicago/Turabian StyleMasarone, Daniele, Michelle M. Kittleson, Piero Pollesello, Marco Marini, Massimo Iacoviello, Fabrizio Oliva, Angelo Caiazzo, Andrea Petraio, and Giuseppe Pacileo. 2022. "Use of Levosimendan in Patients with Advanced Heart Failure: An Update" Journal of Clinical Medicine 11, no. 21: 6408. https://doi.org/10.3390/jcm11216408
APA StyleMasarone, D., Kittleson, M. M., Pollesello, P., Marini, M., Iacoviello, M., Oliva, F., Caiazzo, A., Petraio, A., & Pacileo, G. (2022). Use of Levosimendan in Patients with Advanced Heart Failure: An Update. Journal of Clinical Medicine, 11(21), 6408. https://doi.org/10.3390/jcm11216408









