Dapsone-Associated Anemia in Heart Transplant Recipients with Normal Glucose-6-Phosphate Dehydrogenase Activity
Abstract
1. Introduction
2. Materials and Methods
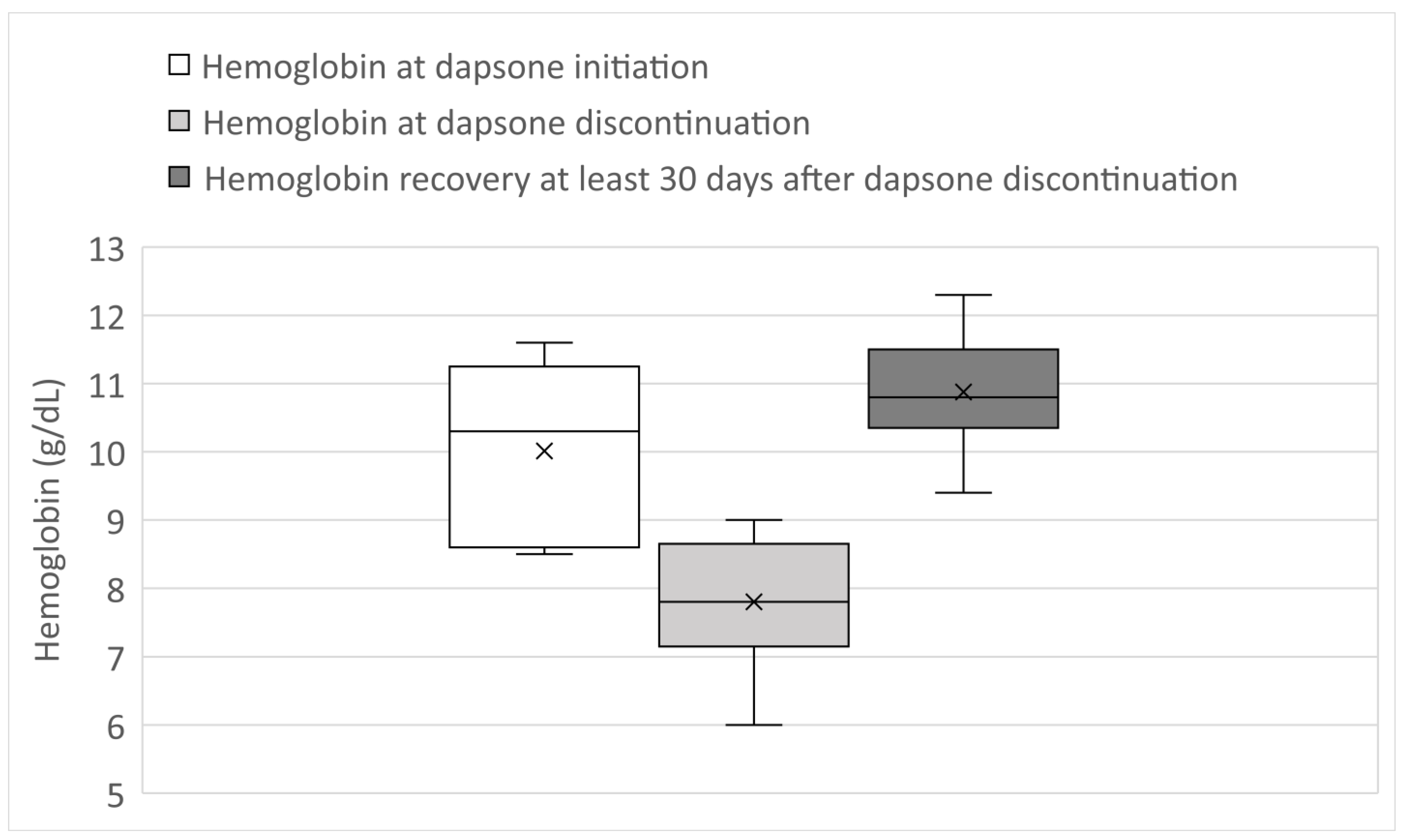
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, S.I.; Fishman, J.A. The AST Infectious Disease Community of Practice. Pneumocystis pneumonia in solid organ transplantation. Am. J. Transplant. 2013, 13, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Bigby, M.; Jick, S.; Jick, H.; Arndt, K. Drug-induced cutaneous reactions: A report from the Boston collaborative drug surveillance program on 15438 consecutive inpatients, 1975 to 1982. JAMA 1986, 256, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Deps, P.; Guerra, P.; Nasser, S.; Simon, M. Hemolytic anemia in patients receiving daily dapsone for the treatment of leprosy. Lepr Rev. 2012, 83, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Belfield, K.D.; Tichy, E.M. Review of drug therapy implications of glucose-6-phosphate dehydrogenase deficiency. Am. J. Health-Syst. Pharm. 2018, 75, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014, 306, 103–124. [Google Scholar] [CrossRef]
- Luzzatto, L.; Seneca, E. G6PD deficiency: A classic example of pharmacogenetics with on-going clinical implications. Br. J. Haematol. 2014, 164, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.T. Use of dapsone in the prevention and treatment of Pneumocystis carinii pneumonia: A review. Clin. Infect. Dis. 1998, 27, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Esbenshade, A.J.; Ho, R.H.; Shintani, A.; Zhao, Z.; Smith, L.A.; Friedman, D.L. Dapsone-induced methemoglobinemia: A dose-related occurrence? Cancer 2011, 117, 3485–3492. [Google Scholar] [CrossRef]
- Lee, I.; Barton, T.D.; Goral, S.; Doyle, A.M.; Bloom, R.D.; Chojnowski, D.; Korenda, K.; Blumberg, E.A. Complications related to dapsone use for Pneumocystis Jirovecii pneumonia prophylaxis in solid organ transplant recipients. Am. J. Transplant. 2005, 5, 2791–2795. [Google Scholar] [CrossRef]
- Olteanu, H.; Harrington, A.M.; George, B.; Hari, P.N.; Bredeson, C.; Kroft, S.H. High prevalence of dapsone-induced oxidant hemolysis in North American SCT recipients without glucose-6-phosphate-dehydrogenase deficiency. Bone Marrow Transplant. 2012, 47, 399–403. [Google Scholar] [CrossRef]
- Naik, P.M.; Lyon, G.M., III; Ramirez, A.; Lawrence, E.C.; Neujahr, D.C.; Force, S.; Pelaez, A. Dapsone-induced hemolytic anemia in lung allograft recipients. J. Heart Lung Transplant. 2008, 27, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Hedvat, J.; Poladi, N.; Salerno, D.M.; Dube, G.K.; Lange, N.W. An evaluation of PJP prophylaxis and anemia among renal transplant recipients. Transpl. Infect. Dis. 2021, 23, e13543. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Henderson, A.C. Hemolytic Anemia: Evaluation and differential diagnosis. Am. Fam. Physician 2018, 98, 354–361. [Google Scholar]
- Kaferle, J.; Strzoda, C.E. Evaluation of macrocytosis. Am. Fam. Physician 2009, 79, 203–208. [Google Scholar]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Beumont, M.G.; Graziani, A.; Ubel, P.A.; MacGregor, R.R. Safety of dapsone as Pneumocysitis carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with allergy to trimethoprim/sulfamethoxazole. Am. J. Med. 1996, 100, 611–616. [Google Scholar] [CrossRef]
- Bordin, L.; Fiore, C.; Zen, F.; Coleman, M.D.; Ragazzi, E.; Clari, G. Dapsone hydroxylamine induces premature removal of human erythrocytes by membrane reorganization and antibody binding. Br. J. Pharmacol. 2010, 16, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.P.; McMillan, D.C.; Crouch, R.K.; Jollow, D.J. Formation of free radicals and protein mixed disulfides in rat red cells exposed to dapsone hydroxylamine. Free Radic. Biol. Med. 1997, 22, 1183–1193. [Google Scholar] [CrossRef]
- May, D.G.; Porter, J.A.; Uetrecht, J.P.; Wilkinson, G.R.; Branch, R.A. The contribution of N-hydroxylation and acetylation to dapsone pharmacokinetics in normal subjects. Clin. Pharmacol. Ther. 1990, 48, 619–627. [Google Scholar] [CrossRef]
- Mitra, A.K.; Thummel, K.E.; Kalhorn, T.F.; Kharasch, E.D.; Unadkat, J.D.; Slattery, J.T. Metabolism of dapsone to its hydroxylamine by CYP2E1 in vitro and in vivo. Clin. Pharmacol. Ther. 1995, 58, 556–566. [Google Scholar] [CrossRef]
- Bluhm, R.E.; Adedoyin, A.; McCarver, D.G.; Branch, R.A. Development of dapsone toxicity in patients with inflammatory dermatoses: Activity of acetylation and hydroxylation of dapsone as risk factors. Clin. Pharmacol. Ther. 1999, 65, 598–605. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Shih, A.W.Y.; McFarlane, A.; Verhovsek, M. Haptoglobin testing in hemolysis: Measurement and interpretation. Am. J. Hematol. 2014, 89, 443–447. [Google Scholar] [CrossRef] [PubMed]
| Hemolytic Anemia (n = 8) | No Hemolytic Anemia (n = 28) | p-Value | |
|---|---|---|---|
| Mean age +/− SD | 54.9 +/− 14.2 | 57.0 +/− 10.3 | 0.64 |
| Female (%) | 4 (50) | 5 (18) | 0.07 |
| Prior durable MCS device (%) | 2 (25) | 10 (36) | 0.57 |
| ATG induction (%) | 8 (100) | 22 (79) | 0.16 |
| Ethnicity White African American Hispanic Asian | 4 (50) 0 (0) 3 (37) 1 (13) | 18 (64) 7 (25) 2 (7) 1 (4) | 0.06 |
| Daily dapsone dose 100 mg 50 mg | 7 (87) 1 (13) | 25 (89) 3 (11) | 0.88 |
| Reason for dapsone initiation Sulfa intolerance Kidney injury Leukopenia Hyperkalemia Elevated alkaline phosphatase | 6 (75) 0 (0) 1 (13) 0 (0) 1 (13) | 15 (54) 8 (29) 3 (11) 2 (7) 0 (0) | 0.21 |
| Patient Number | G6PD Level | Baseline Hgb (g/dL) | Hgb Nadir (g/dL) | Hgb (g/dL) at Least 30 Days after Dapsone Discontinuation | Trans-Fusion (no. of PRBC Units) | Hapto-Globin (Normal: 36–195 mg/dL) | LDH (Normal: 125–220 U/L) | Reticul-ocyte % (Normal: 0.5–2%) | Schisto-Cytes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Normal | 8.5 | 7.5 | 9.4 | 0 | 85 | 244 | 10.7 | no |
| 2 | Normal | 8.5 | 7.4 | 12.3 | 0 | <8 | --- | 3.5 | --- |
| 3 | Normal | 11.4 | 6 | 10.6 | 0 | 138 | 468 | 7.4 | yes |
| 4 | Normal | 8.7 | 6.9 | 10.8 | 0 | 215 | 266 | 3.6 | no |
| 5 | Normal | 11.1 | 8.3 | 11.3 | 0 | <8 | --- | 11.2 | --- |
| 6 | Normal | 9.2/10.8 | 8.6/9 | 10.5/11.1 | 0 | --- | --- | --- | --- |
| 7 | Normal | 10.3 | 7.8 | 10.2 | 2 | 208 | 248 | 6.3 | no |
| 8 | Normal | 11.6 | 8.7 | 11.7 | 0 | --- | --- | --- | --- |
| Patient Number | Daily Dose (mg) | Days from Date of Transplant to Dapsone Initiation | Days from Dapsone Initiation to Onset of First Hgb Drop | Days from Dapsone Initiation to Hgb Nadir | Days from Date of Transplant to Dapsone Discontinuation | Days to Discontinuation of Dapsone after Initial Hgb Drop |
|---|---|---|---|---|---|---|
| 1 | 100 | 3 | 15 | 22 | 30 | 15 |
| 2 | 100 | 5 | 11 | 23 | 47 | 31 |
| 3 | 100 | 7 | 7 | 50 | 57 | 43 |
| 4 | 100 | 6 | 11 | 21 | 27 | 10 |
| 5 | 100 | 63 | 46 | 46 | 116 | 7 |
| 6 * | 100 | 25/166 | 90/45 | 90/101 | 115/267 | 0/56 |
| 7 | 50 | 18 | 13 | 76 | 94 | 63 |
| 8 | 100 | 58 | 59 | 59 | 117 | 0 |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Has this adverse event been documented before? (+1 Y, 0 N) | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 |
| Did the adverse reaction occur after suspected drug was given? (+2 Y, −1 N) | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 |
| Did the adverse reaction resolve after cessation of drug or was it reversible? (+1 Y, 0 N) | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 |
| Did the adverse reaction recur after re-challenge with suspected drug? (+2 Y, −1 N) | +2 | 0 | 0 | 0 | 0 | +2 | 0 | 0 |
| Have other causes been ruled out? (−1 Y, +2 N) * | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 |
| When an alternative was given, did the reaction occur? (−1 Y, +1 N) | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 |
| Was there any determination of toxic drug levels in the blood or other fluids? (+1 Y, 0 N) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Did changing the dose change the severity of the reaction? (+1 Y, 0 N) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| When the patient was given the drug or alternative previously, did they experience a reaction? (+1 Y, 0 N) | +1 | 0 | 0 | 0 | 0 | +1 | 0 | 0 |
| Was there any objective evidence to verify the adverse effect? (+1 Y, 0 N) | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 |
| Total (> +9: definite, +5–8 probable, possible +1–4, doubtful < +1 | +11 | +8 | +8 | +8 | +8 | +11 | +8 | +8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lor, K.W.; Kransdorf, E.P.; Patel, J.K.; Chang, D.H.; Kobashigawa, J.A.; Kittleson, M.M. Dapsone-Associated Anemia in Heart Transplant Recipients with Normal Glucose-6-Phosphate Dehydrogenase Activity. J. Clin. Med. 2022, 11, 6378. https://doi.org/10.3390/jcm11216378
Lor KW, Kransdorf EP, Patel JK, Chang DH, Kobashigawa JA, Kittleson MM. Dapsone-Associated Anemia in Heart Transplant Recipients with Normal Glucose-6-Phosphate Dehydrogenase Activity. Journal of Clinical Medicine. 2022; 11(21):6378. https://doi.org/10.3390/jcm11216378
Chicago/Turabian StyleLor, Kevin W., Evan P. Kransdorf, Jignesh K. Patel, David H. Chang, Jon A. Kobashigawa, and Michelle M. Kittleson. 2022. "Dapsone-Associated Anemia in Heart Transplant Recipients with Normal Glucose-6-Phosphate Dehydrogenase Activity" Journal of Clinical Medicine 11, no. 21: 6378. https://doi.org/10.3390/jcm11216378
APA StyleLor, K. W., Kransdorf, E. P., Patel, J. K., Chang, D. H., Kobashigawa, J. A., & Kittleson, M. M. (2022). Dapsone-Associated Anemia in Heart Transplant Recipients with Normal Glucose-6-Phosphate Dehydrogenase Activity. Journal of Clinical Medicine, 11(21), 6378. https://doi.org/10.3390/jcm11216378







