Pregnancy Outcomes after Frozen Embryo Transfer and Fresh Embryo Transfer in Women of Advanced Maternal Age: Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
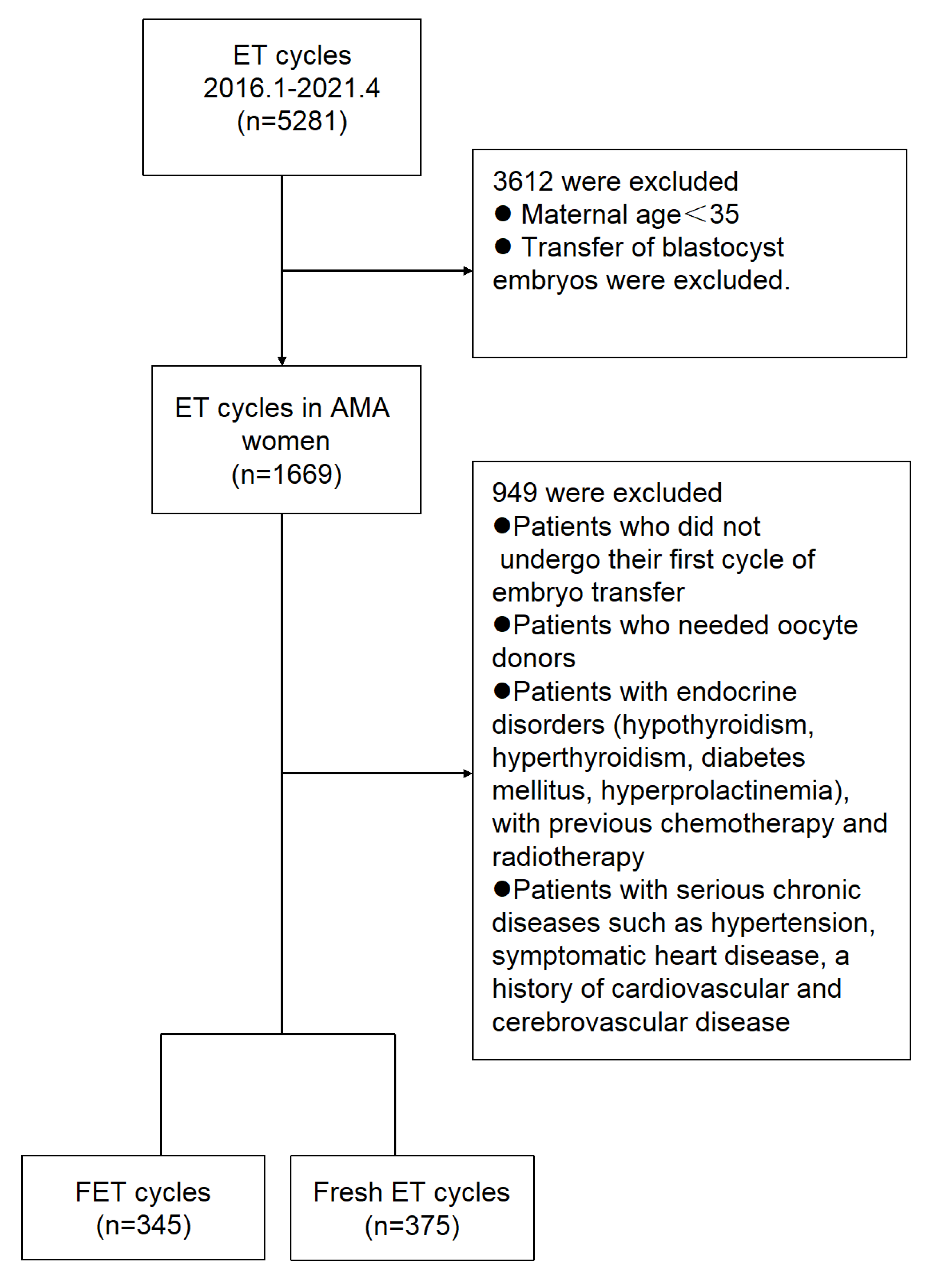
2.1. Study Design and Participants
2.2. IVF Treatment
2.2.1. Depot GnRHa Protocol
2.2.2. Flexible Short Protocol
2.3. Measurement Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Groups
3.2. Clinical Features of the Study Groups
3.3. Fertility and Neonatal Outcomes of the Study Groups
3.4. Maternal Outcomes of the Study Groups
4. Discussion
4.1. Neonatal Outcomes
4.2. Maternal Outcome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ubaldi, F.M.; Cimadomo, D.; Vaiarelli, A.; Fabozzi, G.; Venturella, R.; Maggiulli, R.; Mazzilli, R.; Ferrero, S.; Palagiano, A.; Rienzi, L. Advanced Maternal Age in IVF: Still a Challenge? The Present and the Future of Its Treatment. Front. Endocrinol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.; Rindfuss, R.R.; McDonald, P.; te Velde, E.; Reproduction, E.; Society Task, F. Why do people postpone parenthood? Reasons and social policy incentives. Hum. Reprod. Update 2011, 17, 848–860. [Google Scholar] [CrossRef]
- Schmidt, L.; Sobotka, T.; Bentzen, J.G.; Nyboe Andersen, A.; Reproduction, E.; Society Task, F. Demographic and medical consequences of the postponement of parenthood. Hum. Reprod. Update 2012, 18, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Leridon, H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum. Reprod. 2004, 19, 1548–1553. [Google Scholar] [CrossRef]
- Pantazis, A.; Clark, S.J. A parsimonious characterization of change in global age-specific and total fertility rates. PLoS ONE 2018, 13, e0190574. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.; Bellver, J.; Remohi, J.; Simon, C.; Pellicer, A. Cumulative live-birth rates per total number of embryos needed to reach newborn in consecutive in vitro fertilization (IVF) cycles: A new approach to measuring the likelihood of IVF success. Fertil. Steril. 2011, 96, 40–46. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Hudson, C.; Thomas, S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil. Steril. 2011, 96, 344–348. [Google Scholar] [CrossRef]
- Chen, Z.J.; Shi, Y.; Sun, Y.; Zhang, B.; Liang, X.; Cao, Y.; Yang, J.; Liu, J.; Wei, D.; Weng, N.; et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 523–533. [Google Scholar] [CrossRef]
- Coates, A.; Kung, A.; Mounts, E.; Hesla, J.; Bankowski, B.; Barbieri, E.; Ata, B.; Cohen, J.; Munne, S. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: A randomized controlled trial. Fertil. Steril. 2017, 107, 723–730.e3. [Google Scholar] [CrossRef]
- Ishihara, O.; Araki, R.; Kuwahara, A.; Itakura, A.; Saito, H.; Adamson, G.D. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: An analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil. Steril. 2014, 101, 128–133. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Y.; Hao, C.; Zhang, H.; Wei, D.; Zhang, Y.; Zhu, Y.; Deng, X.; Qi, X.; Li, H.; et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women. N. Engl. J. Med. 2018, 378, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Haahr, T.; Geber, S.; Esteves, S.C.; Humaidan, P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: A systematic review and meta-analysis of reproductive outcomes. Hum. Reprod. Update 2019, 25, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, A.; Kallen, K.; Thurin-Kjellberg, A.; Wennerholm, U.B.; Bergh, C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum. Reprod. 2012, 27, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Henningsen, A.A.; Loft, A.; Malchau, S.S.; Forman, J.; Andersen, A.N. Large baby syndrome in singletons born after frozen embryo transfer (FET): Is it due to maternal factors or the cryotechnique? Hum. Reprod. 2014, 29, 618–627. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, T.; Yang, J.; Hao, Y.; Li, S.; Zhang, Y.; Qian, Y. A flexible short protocol in women with poor ovarian response over 40 years old. J. Ovarian Res. 2021, 14, 3. [Google Scholar] [CrossRef]
- Alpha Scientists in Reproductive Medicine; ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef]
- Mukaida, T.; Nakamura, S.; Tomiyama, T.; Wada, S.; Kasai, M.; Takahashi, K. Successful birth after transfer of vitrified human blastocysts with use of a cryoloop containerless technique. Fertil. Steril. 2001, 76, 618–620. [Google Scholar] [CrossRef]
- Alpha Scientists in Reproductive Medicine. The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: Proceedings of an expert meeting. Reprod. Biomed. Online 2012, 25, 146–167. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Shapiro, D.; Chang, C.C. Vitrification of the human embryo: A more efficient and safer in vitro fertilization treatment. Fertil. Steril. 2020, 113, 241–247. [Google Scholar] [CrossRef]
- Cobo, A.; de los Santos, M.J.; Castello, D.; Gamiz, P.; Campos, P.; Remohi, J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: Evaluation of 3150 warming cycles. Fertil. Steril. 2012, 98, 1138–1146.e1131. [Google Scholar] [CrossRef]
- Fernandez-Shaw, S.; Cercas, R.; Brana, C.; Villas, C.; Pons, I. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: Impact of age on the results. J. Assist. Reprod. Genet. 2015, 32, 177–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, D.; Ding, J.; Smith, G.W.; Smith, G.D.; Takayama, S. Slow and steady cell shrinkage reduces osmotic stress in bovine and murine oocyte and zygote vitrification. Hum. Reprod. 2015, 30, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, K.; Berkkanoglu, M.; Bulut, H.; Humaidan, P.; Coetzee, K. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: Retrospective analysis. Fertil. Steril. 2015, 104, 899–907.e3. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Lattes, K.; Serra, S.; Sola, I.; Geber, S.; Carreras, R.; Checa, M.A. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 2013, 99, 156–162. [Google Scholar] [CrossRef]
- Vuong, L.N.; Dang, V.Q.; Ho, T.M.; Huynh, B.G.; Ha, D.T.; Pham, T.D.; Nguyen, L.K.; Norman, R.J.; Mol, B.W. IVF Transfer of Fresh or Frozen Embryos in Women without Polycystic Ovaries. N. Engl. J. Med. 2018, 378, 137–147. [Google Scholar] [CrossRef]
- Bosdou, J.K.; Venetis, C.A.; Tarlatzis, B.C.; Grimbizis, G.F.; Kolibianakis, E.M. Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: A meta-analysis. Hum. Reprod. 2019, 34, 491–505. [Google Scholar] [CrossRef]
- Roque, M.; Valle, M.; Guimaraes, F.; Sampaio, M.; Geber, S. Freeze-all cycle for all normal responders? J. Assist. Reprod. Genet. 2017, 34, 179–185. [Google Scholar] [CrossRef]
- Acharya, K.S.; Acharya, C.R.; Bishop, K.; Harris, B.; Raburn, D.; Muasher, S.J. Freezing of all embryos in in vitro fertilization is beneficial in high responders, but not intermediate and low responders: An analysis of 82,935 cycles from the Society for Assisted Reproductive Technology registry. Fertil. Steril. 2018, 110, 880–887. [Google Scholar] [CrossRef]
- Maheshwari, A.; Pandey, S.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: A systematic review and meta-analysis. Fertil. Steril. 2012, 98, 368–377.e9. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Daneshmand, S.T.; Bedient, C.E.; Garner, F.C. Comparison of birth weights in patients randomly assigned to fresh or frozen-thawed embryo transfer. Fertil. Steril. 2016, 106, 317–321. [Google Scholar] [CrossRef]
- Maheshwari, A.; Raja, E.A.; Bhattacharya, S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: An analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil. Steril. 2016, 106, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Sha, T.; Yin, X.; Cheng, W.; Massey, I.Y. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: A meta-analysis. Fertil. Steril. 2018, 109, 330–342.e9. [Google Scholar] [CrossRef]
- Maheshwari, A.; Pandey, S.; Amalraj Raja, E.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum. Reprod. Update 2018, 24, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, S.; Hartikainen, A.L.; Ritvanen, A.; Koivunen, R.; Martikainen, H.; Gissler, M.; Tiitinen, A. Major congenital anomalies in children born after frozen embryo transfer: A cohort study 1995–2006. Hum. Reprod. 2014, 29, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Wennerholm, U.B.; Henningsen, A.K.; Romundstad, L.B.; Bergh, C.; Pinborg, A.; Skjaerven, R.; Forman, J.; Gissler, M.; Nygren, K.G.; Tiitinen, A. Perinatal outcomes of children born after frozen-thawed embryo transfer: A Nordic cohort study from the CoNARTaS group. Hum. Reprod. 2013, 28, 2545–2553. [Google Scholar] [CrossRef]
- Steward, R.G.; Lan, L.; Shah, A.A.; Yeh, J.S.; Price, T.M.; Goldfarb, J.M.; Muasher, S.J. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: An analysis of 256,381 in vitro fertilization cycles. Fertil. Steril. 2014, 101, 967–973. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, B.; Zhang, Q.; Li, Y.P. Which one has a better obstetric and perinatal outcome in singleton pregnancy, IVF/ICSI or FET? A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2016, 14, 51. [Google Scholar] [CrossRef]
- Weinerman, R.; Mainigi, M. Why we should transfer frozen instead of fresh embryos: The translational rationale. Fertil. Steril. 2014, 102, 10–18. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, L.; Ren, H.; Liu, X.; Guo, S.; Xu, P.; Zheng, J.; Zheng, L.; Tan, J. Perinatal and maternal outcomes after frozen versus fresh embryo transfer cycles in women of advanced maternal age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 257, 133–137. [Google Scholar] [CrossRef]
| Parameters | FET (n = 345) | Fresh ET (n = 375) | p-Value |
|---|---|---|---|
| Age, year | 39.48 ± 3.75 | 39.80 ± 3.72 | 0.258 |
| Duration of infertility, year | 4.39 ± 4.06 | 4.24 ± 3.82 | 0.633 |
| Infertility Type, n (%) | |||
| Primary | 64 (18.6) | 74 (19.7) | 0.687 |
| Secondary | 281 (81.4) | 301 (80.3) | 0.687 |
| Body mass index | 23.19 ± 2.96 | 23.02 ± 2.96 | 0.430 |
| Number of previous conceptions | 1.87 ± 1.50 | 1.98 ± 1.58 | 0.358 |
| Causes of Infertility, n (%) | |||
| Tubal factor | 175 (50.7) | 174 (46.4) | 0.246 |
| Diminished ovarian reserve | 162 (47.0) | 199 (53.1) | 0.101 |
| Antral follicle count in left ovary | 5.45 ± 3.17 | 5.08 ± 2.91 | 0.113 |
| Antral follicle count in right ovary | 5.59 ± 3.05 | 6.09 ± 2.73 | 0.022 |
| Anti-Müllerian hormone (AMH) | 2.25 ± 1.99 | 2.53 ± 2.14 | 0.076 |
| Baseline Sex Hormone | |||
| Follicle-Stimulating Hormone (FSH) | 9.26 ± 5.85 | 10.02 ± 8.48 | 0.177 |
| Luteinizing Hormone (LH) | 4.42 ± 5.06 | 4.95 ± 7.99 | 0.313 |
| Estradiol (pg/mL) | 63.11 ± 63.32 | 62.69 ± 46.50 | 0.922 |
| Total Testosterone (ng/mL) | 0.45 ± 0.32 | 0.46 ± 0.48 | 0.815 |
| Parameters | FET (n = 345) | Fresh ET (n = 375) | p-Value |
|---|---|---|---|
| Total amount of gonadotropins (IU) | 2452.70 ± 1054.21 | 2479.18 ± 1022.14 | 0.734 |
| Days of ovarian stimulation | 10.43 ± 3.59 | 10.59 ± 3.33 | 0.546 |
| Estradiol level on day of HCG (pg/mL) | 3088.90 ± 2625.68 | 2812.09 ± 1940.05 | 0.108 |
| Progesterone level on day of HCG (ng/mL) | 1.39 ± 1.44 | 1.26 ± 0.80 | 0.152 |
| Endometrial thickness before transfer (mm) | 9.67 ± 1.81 | 9.85 ± 2.12 | 0.226 |
| Treatment Protocols | |||
| Depot GnRHa protocol | 199 (57.7) | 197 (52.5) | 0.165 |
| Flexible short protocol | 146 (42.3) | 178 (47.5) | 0.165 |
| Number of oocytes retrieved | 8.12 ± 6.78 | 8.16 ± 4.79 | 0.919 |
| Insemination Modes | |||
| IVF, n (%) | 285 (82.6) | 304 (81.1) | 0.592 |
| ICSI, n (%) | 60 (17.4) | 71 (18.9) | 0.592 |
| Number of good quality embryos | 1.9 | 1.92 | 0.873 |
| Number of embryos transferred | 1.83 ± 0.37 | 1.80 ± 0.39 | 0.321 |
| Parameters | FET (n = 345) | Fresh ET (n = 375) | p-Value |
|---|---|---|---|
| Fertility Outcome | |||
| Biochemical pregnancy, n (%) | 117 (33.9) | 143 (38.1) | 0.239 |
| Clinical pregnancy, n (%) | 91 (26.4) | 126 (33.6) | 0.035 |
| Implantation rate (%) | 15.2 (96/631) | 19.7 (133/675) | 0.265 |
| Ectopic pregnancy, n (%) | 3 (0.9) | 2 (0.5) | 0.587 |
| Miscarriage, n (%) | 20 (5.8) | 32 (8.5) | 0.156 |
| Live birth, n (%) | 68 (19.7) | 91 (24.3) | 0.141 |
| Neonatal Outcomes | |||
| Birth weight (g) | 3217.16 ± 734.44 | 3003.37 ± 635.00 | 0.037 |
| Low birth weight among live newborns, n (%) | 10 (12.3) | 17 (16.8) | 0.397 |
| Macrosomia, n (%) | 5 (6.2) | 5 (5.0) | 0.753 |
| Congenital anomalies among live newborns | 1 (1.2) | 0 | 0.445 |
| Perinatal mortality | 0 | 0 | |
| Maternal Outcome | FET (n = 345) | Fresh ET (n = 375) | p-Value |
|---|---|---|---|
| Moderate or severe ovarian hyperstimulation syndrome | 0 | 1 (0.3) | 1.000 |
| Gestational hypertension | 1 (1.1) | 2 (1.6) | 1.000 |
| preeclampsia | 3 (3.3) | 3 (2.4) | 0.697 |
| HELLP syndrome | 0 | 0 | |
| Gestational diabetes | 3 (3.3) | 5 (4.0) | 1.000 |
| PPROM | 4 (4.4) | 7 (5.6) | 0.765 |
| preterm birth, n (%) | 9 (2.6) | 21 (5.6) | 0.046 |
| Cesarean section, n (%) | 45 (13.0) | 62 (16.5) | 0.188 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhou, J.; Chen, Y.; Yang, J.; Hao, Y.; Feng, T.; Feng, R.; Qian, Y. Pregnancy Outcomes after Frozen Embryo Transfer and Fresh Embryo Transfer in Women of Advanced Maternal Age: Single-Center Experience. J. Clin. Med. 2022, 11, 6395. https://doi.org/10.3390/jcm11216395
Chen Y, Zhou J, Chen Y, Yang J, Hao Y, Feng T, Feng R, Qian Y. Pregnancy Outcomes after Frozen Embryo Transfer and Fresh Embryo Transfer in Women of Advanced Maternal Age: Single-Center Experience. Journal of Clinical Medicine. 2022; 11(21):6395. https://doi.org/10.3390/jcm11216395
Chicago/Turabian StyleChen, Yao, Jianbo Zhou, Yandong Chen, Jihong Yang, Yingying Hao, Ting Feng, Ruizhi Feng, and Yun Qian. 2022. "Pregnancy Outcomes after Frozen Embryo Transfer and Fresh Embryo Transfer in Women of Advanced Maternal Age: Single-Center Experience" Journal of Clinical Medicine 11, no. 21: 6395. https://doi.org/10.3390/jcm11216395
APA StyleChen, Y., Zhou, J., Chen, Y., Yang, J., Hao, Y., Feng, T., Feng, R., & Qian, Y. (2022). Pregnancy Outcomes after Frozen Embryo Transfer and Fresh Embryo Transfer in Women of Advanced Maternal Age: Single-Center Experience. Journal of Clinical Medicine, 11(21), 6395. https://doi.org/10.3390/jcm11216395






