Characteristics and Cost of Unscheduled Hospitalizations in Patients Treated with New Oral Anticancer Drugs in Germany: Evidence from the Randomized AMBORA Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Assessment of Drug-Related Problems
2.4. Assessment of Hospitalizations
2.5. Economic Data Collection and Analysis
2.6. Data Analysis SHI Collective (AOK Bayern)
2.7. Estimating the Potential Savings of Direct Hospital Cost by an Intensified Care Program as Provided in AMBORA
2.8. Statistical Analysis
3. Results
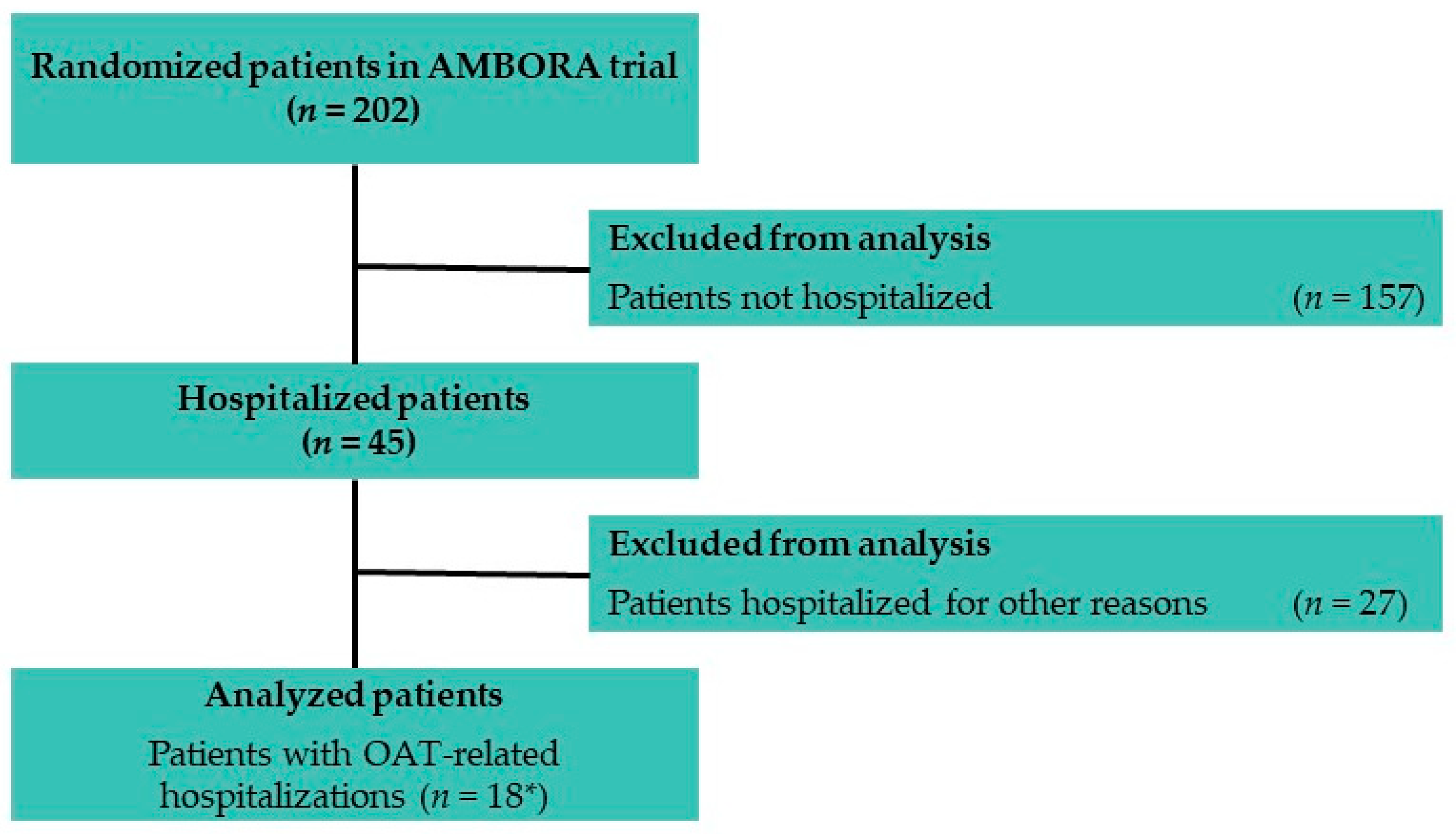
3.1. Patients within AMBORA
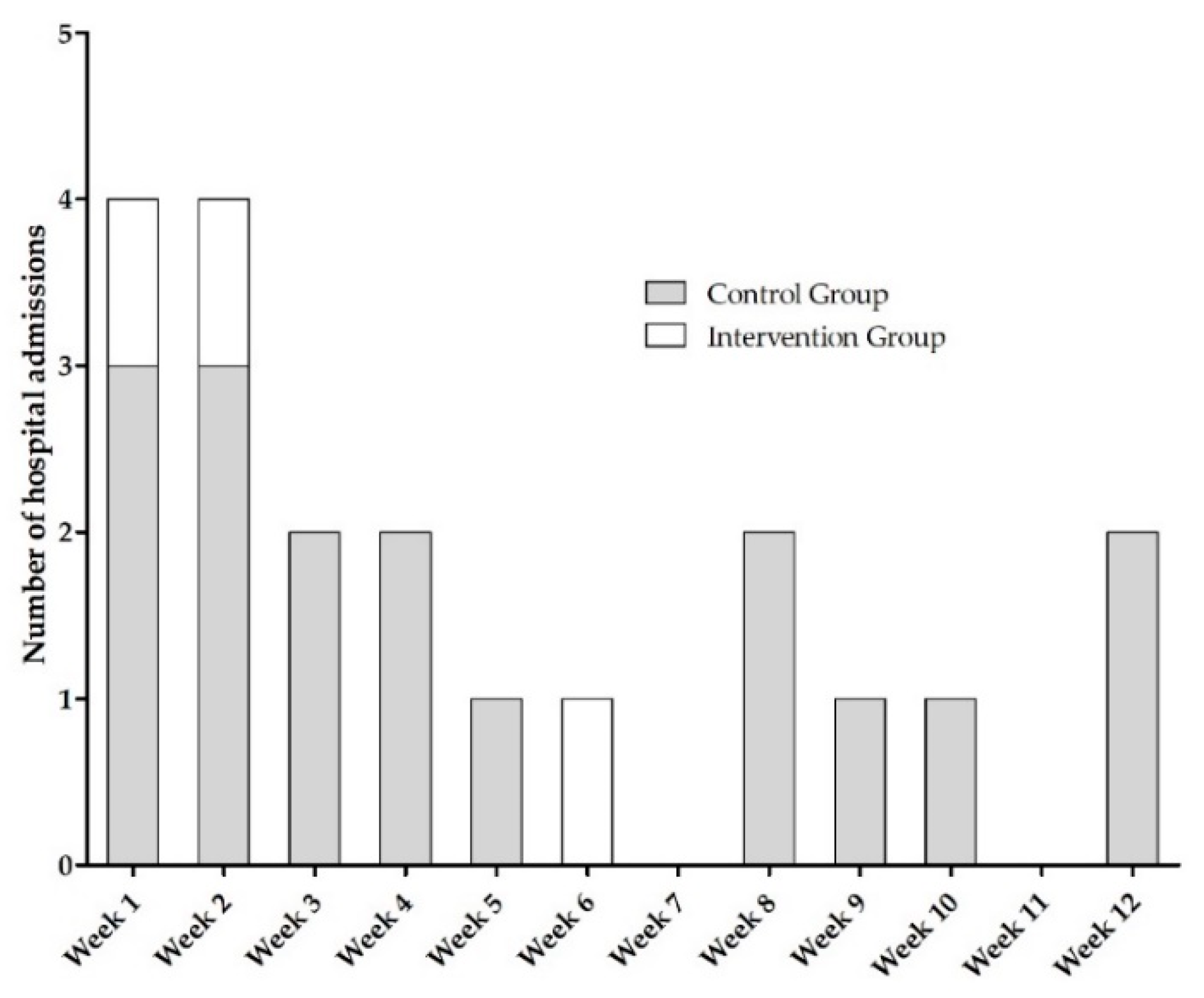
3.1.1. Characteristics of Hospitalizations within AMBORA
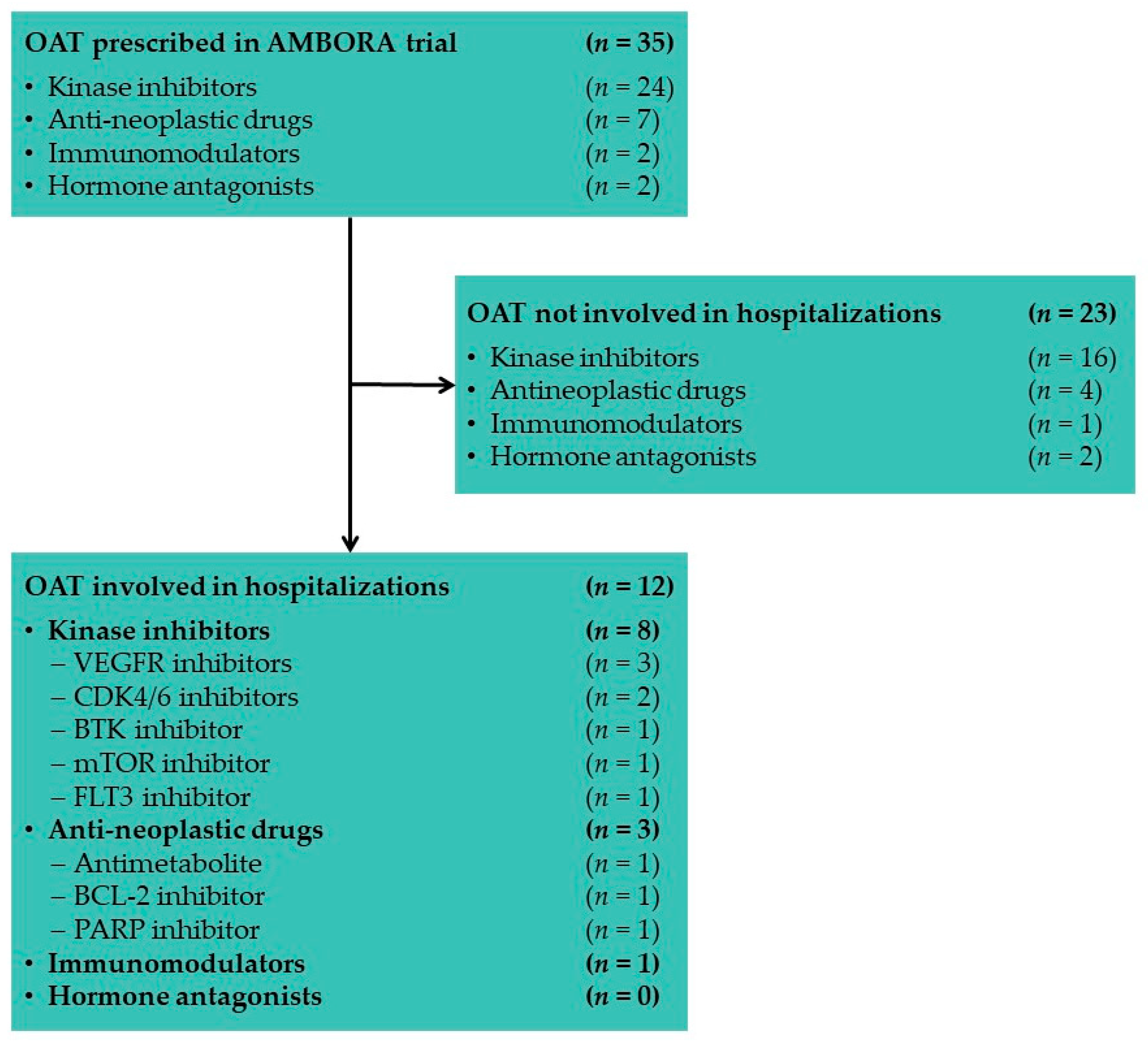
3.1.2. Oral Anticancer Drugs Associated with Hospitalizations in AMBORA
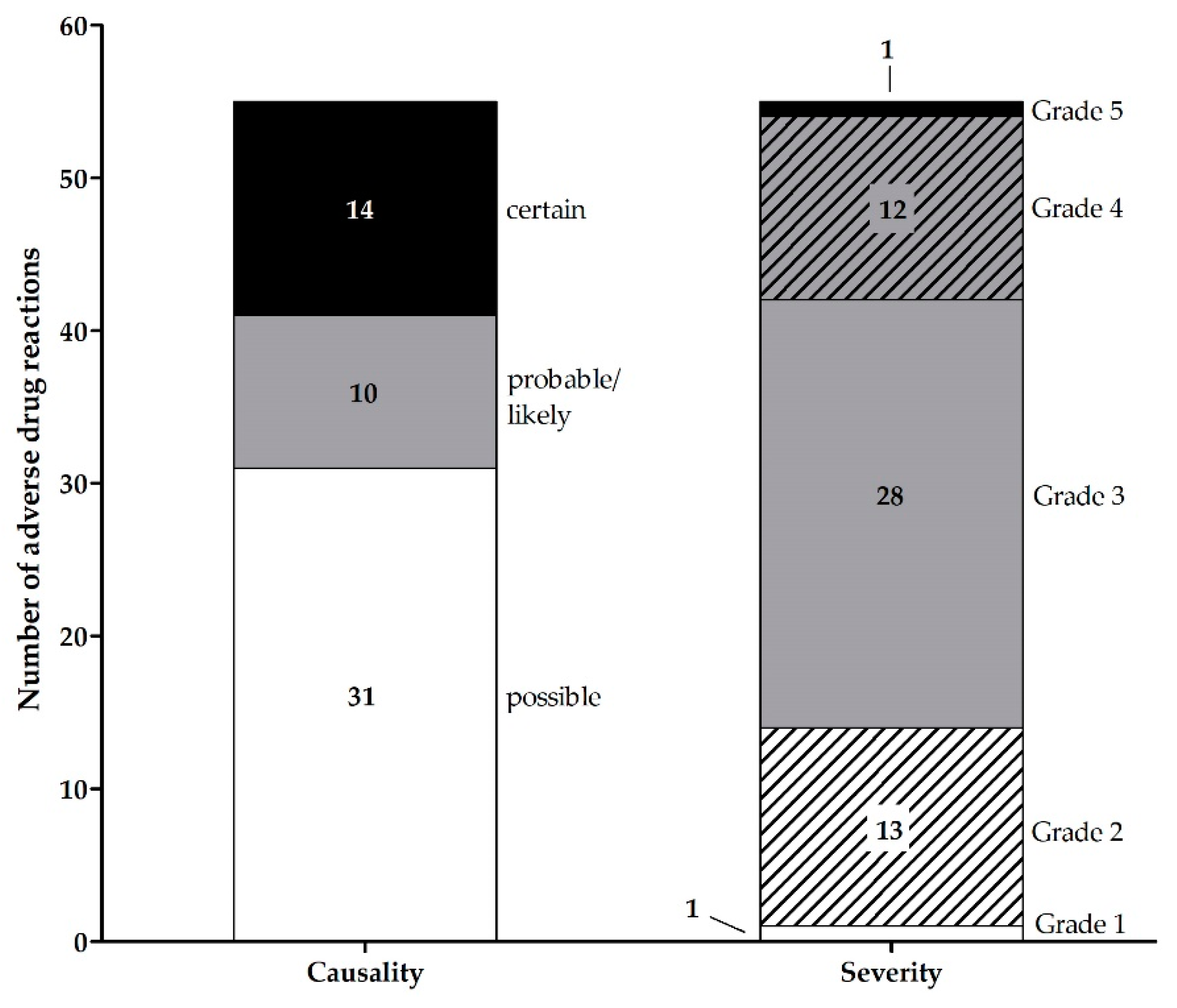
3.1.3. Adverse Drug Reactions Associated with Hospitalizations in AMBORA
3.2. Characteristics of Hospitalizations within the SHI Collective
3.3. Cost of OAT-Related Hospitalizations within AMBORA
3.4. Potential Savings within the SHI Collective
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weingart, S.N.; Brown, E.; Bach, P.B.; Eng, K.; Johnson, S.A.; Kuzel, T.M.; Langbaum, T.S.; Leedy, R.D.; Muller, R.J.; Newcomer, L.N.; et al. NCCN task force report: Oral chemotherapy. J. Natl. Compr. Cancer Netw. 2008, 6 (Suppl. S3), S1–S14. [Google Scholar] [CrossRef]
- van Leeuwen, R.W.; Brundel, D.H.; Neef, C.; van Gelder, T.; Mathijssen, R.H.; Burger, D.M.; Jansman, F.G. Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br. J. Cancer 2013, 108, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Weingart, S.N.; Zhang, L.; Sweeney, M.; Hassett, M. Chemotherapy medication errors. Lancet Oncol. 2018, 19, e191–e199. [Google Scholar] [CrossRef]
- Schlichtig, K.; Dürr, P.; Dörje, F.; Fromm, M.F. New oral anti-cancer drugs and medication safety. Dtsch. Ärzteblatt Int. 2019, 116, 775–782. [Google Scholar] [CrossRef]
- Schlichtig, K.; Dürr, P.; Dörje, F.; Fromm, M.F. Medication errors during treatment with new oral anticancer agents: Consequences for clinical practice based on the AMBORA study. Clin. Pharmacol. Ther. 2021, 110, 1075–1086. [Google Scholar] [CrossRef]
- Dürr, P.; Schlichtig, K.; Kelz, C.; Deutsch, B.; Maas, R.; Eckart, M.J.; Wilke, J.; Wagner, H.; Wolff, K.; Preuß, C.; et al. The randomized AMBORA trial: Impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J. Clin. Oncol. 2021, 39, 1983–1994. [Google Scholar] [CrossRef]
- Wong, C.I.; Zerillo, J.A.; Stuver, S.O.; Siegel, J.H.; Jacobson, J.O.; McNiff, K.K. Role of adverse events in unscheduled hospitalizations among patients with solid tumors who receive medical oncology treatment. J. Oncol. Pract. 2019, 15, e39–e45. [Google Scholar] [CrossRef]
- Brooks, G.A.; Kansagra, A.J.; Rao, S.R.; Weitzman, J.I.; Linden, E.A.; Jacobson, J.O. A clinical prediction model to assess risk for chemotherapy-related hospitalization in patients initiating palliative chemotherapy. JAMA Oncol. 2015, 1, 441–447. [Google Scholar] [CrossRef]
- Hassett, M.J.; O’Malley, A.J.; Pakes, J.R.; Newhouse, J.P.; Earle, C.C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J. Natl. Cancer Inst. 2006, 98, 1108–1117. [Google Scholar] [CrossRef]
- Krzyzanowska, M.K.; Treacy, J.; Maloney, B.; Lavino, A.; Jacobson, J.O. Development of a patient registry to evaluate hospital admissions related to chemotherapy toxicity in a community cancer center. J. Oncol. Pract. 2005, 1, 15–19. [Google Scholar] [CrossRef]
- McKenzie, H.; Hayes, L.; White, K.; Cox, K.; Fethney, J.; Boughton, M.; Dunn, J. Chemotherapy outpatients’ unplanned presentations to hospital: A retrospective study. Support Care Cancer 2011, 19, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Duncan, I.; Ahmed, T.; Rubinstein, E.; Pegus, C. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admissions. J. Natl. Compr. Cancer Netw. 2012, 10, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Kawasumi, K.; Kujirai, A.; Matsui, R.; Kawano, Y.; Yamaguchi, M.; Aoyama, T. Survey of serious adverse events and safety evaluation of oral anticancer drug treatment in Japan: A retrospective study. Mol. Clin. Oncol. 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Kabadi, S.M.; Near, A.; Wada, K.; Burudpakdee, C. Treatment patterns, adverse events, healthcare resource use and costs among commercially insured patients with mantle cell lymphoma in the United States. Cancer Med. 2019, 8, 7174–7185. [Google Scholar] [CrossRef]
- Gonzalez-McQuire, S.; Yong, K.; Leleu, H.; Mennini, F.S.; Flinois, A.; Gazzola, C.; Schoen, P.; Campioni, M.; DeCosta, L.; Fink, L. Healthcare resource utilization among patients with relapsed multiple myeloma in the UK, France, and Italy. J. Med. Econ. 2018, 21, 450–467. [Google Scholar] [CrossRef]
- Schultz, N.M.; Flanders, S.C.; Wilson, S.; Brown, B.A.; Song, Y.; Yang, H.; Lechpammer, S.; Kassabian, V. Treatment duration, healthcare resource utilization, and costs among chemotherapy-naïve patients with metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate: A retrospective claims analysis. Adv. Ther. 2018, 35, 1639–1655. [Google Scholar] [CrossRef]
- Phuar, H.L.; Begley, C.E.; Chan, W.; Krause, T.M. Tyrosine kinase inhibitors initiation, cost sharing, and health care utilization in patients with newly diagnosed chronic myeloid leukemia: A retrospective claims-based study. J. Manag. Care Spec. Pharm. 2019, 25, 1140–1150. [Google Scholar] [CrossRef]
- Jairam, V.; Lee, V.; Park, H.S.; Thomas, C.R., Jr.; Melnick, E.R.; Gross, C.P.; Presley, C.J.; Adelson, K.B.; Yu, J.B. Treatment-Related Complications of Systemic Therapy and Radiotherapy. JAMA Oncol. 2019, 5, 1028–1035. [Google Scholar] [CrossRef]
- Ihbe-Heffinger, A.; Paessens, B.; Berger, K.; Shlaen, M.; Bernard, R.; von Schilling, C.; Peschel, C. The impact of chemotherapy-induced side effects on medical care usage and cost in German hospital care−an observational analysis on non-small-cell lung cancer patients. Support Care Cancer 2013, 21, 1665–1675. [Google Scholar] [CrossRef]
- O’Neill, C.B.; Atoria, C.L.; O’Reilly, E.M.; Henman, M.C.; Bach, P.B.; Elkin, E.B.; O’Neill, C.B.; Atoria, C.L.; O’Reilly, E.M.; Henman, M.C.; et al. ReCAP: Hospitalizations in older adults with advanced cancer: The role of chemotherapy. J. Oncol. Pract. 2016, 12, 151–152. [Google Scholar] [CrossRef]
- Hassett, M.J.; Rao, S.R.; Brozovic, S.; Stahl, J.E.; Schwartz, J.H.; Maloney, B.; Jacobson, J.O. Chemotherapy-related hospitalization among community cancer center patients. Oncologist 2011, 16, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pittman, N.M.; Hopman, W.M.; Mates, M. Emergency room visits and hospital admission rates after curative chemotherapy for breast cancer. J. Oncol. Pract. 2015, 11, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Dürr, P.; Schlichtig, K.; Krebs, S.; Schramm, A.; Schötz, L.; Fromm, M.F.; Dörje, F. Ökonomische Aspekte bei der Versorgung von Patient*innen mit neuen oralen Tumortherapeutika: Erkenntnisse aus der AMBORA-Studie. Z. Für Evidenz Fortbild. Und Qual. Im Gesundh. 2022, 169, 84–93. [Google Scholar] [CrossRef]
- National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. 2010. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 5 March 2022).
- The Uppsala Monitoring Center. The Use of the WHO-UMC System for Standardised Case Causality Assessment. Available online: https://cdn.who.int/media/docs/default-source/medicines/pharmacovigilance/whocausality-assessment.pdf?sfvrsn=5d8130bb_2&download=true (accessed on 5 June 2022).
- Barber, J.A.; Thompson, S.G. Analysis of cost data in randomized trials: An application of the non-parametric bootstrap. Stat Med. 2000, 19, 3219–3236. [Google Scholar] [CrossRef]
- Thompson, S.G.; Barber, J.A. How should cost data in pragmatic randomised trials be analysed? BMJ 2000, 320, 1197–1200. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1986, 1, 54–75. [Google Scholar] [CrossRef]
- Klaeger, S.; Heinzlmeir, S.; Wilhelm, M.; Polzer, H.; Vick, B.; Koenig, P.-A.; Reinecke, M.; Ruprecht, B.; Petzoldt, S.; Meng, C.; et al. The target landscape of clinical kinase drugs. Science 2017, 358, eaan4368. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef]
- Meier, F.; Maas, R.; Sonst, A.; Patapovas, A.; Müller, F.; Plank-Kiegele, B.; Pfistermeister, B.; Schöffski, O.; Bürkle, T.; Dormann, H. Adverse drug events in patients admitted to an emergency department: An analysis of direct costs. Pharmacoepidemiol. Drug Saf. 2015, 24, 176–186. [Google Scholar] [CrossRef]
- Schulz, C.; Fischer, A.; Vogt, W.; Leichenberg, K.; Warnke, U.; Liekweg, A.; Georgi, U.; Langebrake, C.; Hoppe-Tichy, T.; Dörje, F.; et al. Clinical pharmacy services in Germany: A national survey. Eur. J. Hosp. Pharm. 2021, 28, 301–305. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Silva, M.P.; Miranda, Í.K.; Calumby, R.T.; de Araújo-Calumby, R.F. Impact of clinical pharmacy in oncology and hematology centers: A systematic review. J. Oncol. Pharm. Pract. 2021, 27, 679–692. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | No. (%) Total (n = 18) |
|---|---|
| Age, years (mean, range) | 67.8 (47–91) |
| Female sex | 11 (61.1) |
| Cancer type | |
| Solid tumors | |
| Breast | 3 (16.7) |
| Soft tissue sarcoma | 3 (16.7) |
| Small intestine | 2 (11.1) |
| Others * | 4 (22.2) |
| Hematologic malignancies | |
| Acute myeloid leukemia | 2 (11.1) |
| Mantle cell lymphoma | 2 (11.1) |
| Multiple myeloma | 2 (11.1) |
| ECOG performance status | |
| 0 | 4 (22.2) |
| 1 | 11 (61.1) |
| 2 | 2 (11.1) |
| 3 | 1 (5.6) |
| Number of drugs + (median, range) | 9 (3–24) |
| Oral anticancer drug | |
| Protein kinase inhibitors | 13 (72.2) |
| Pazopanib | 3 (16.7) |
| Everolimus | 2 (11.1) |
| Ibrutinib | 2 (11.1) |
| Palbociclib | 2 (11.1) |
| Cabozantinib | 1 (5.6) |
| Lenvatinib | 1 (5.6) |
| Midostaurin | 1 (5.6) |
| Ribociclib | 1 (5.6) |
| Antineoplastic agents | 3 (16.7) |
| Niraparib | 1 (5.6) |
| Tegafur, gimeracil, oteracil | 1 (5.6) |
| Venetoclax | 1 (5.6) |
| Immunomodulators | 2 (11.1) |
| Lenalidomide | 2 (11.1) |
| CTCAE Term | No. (%) Total (n = 18 Patients) | |
|---|---|---|
| Any Grade | Grade ≥ 3 | |
| Total | 55 (100) | 41 (74.5) |
| Blood count disorders | 19 (34.5) | 19 (46.3) |
| Lymphocyte count decreased | 6 (10.9) | 6 (14.6) |
| Neutrophil count decreased | 5 (9.1) | 5 (12.2) |
| White blood cells decreased | 5 (9.1) | 5 (12.2) |
| Anemia | 2 (3.6) | 2 (4.9) |
| Platelet count decreased | 1 (1.8) | 1 (2.4) |
| Gastrointestinal disorders | 17 (30.9) | 5 (12.2) |
| Nausea | 3 (5.5) | 1 (2.4) |
| Anorexia | 2 (3.6) | 0 (0) |
| Diarrhea | 2 (3.6) | 1 (2.4) |
| Mucositis oral | 2 (3.6) | 1 (2.4) |
| Vomiting | 2 (3.6) | 1 (2.4) |
| Anal mucositis | 1 (1.8) | 0 (0) |
| Bloating | 1 (1.8) | 0 (0) |
| Constipation | 1 (1.8) | 1 (2.4) |
| Dysgeusia | 1 (1.8) | 0 (0) |
| Gastroesophageal reflux disease | 1 (1.8) | 0 (0) |
| Laryngeal mucositis | 1 (1.8) | 0 (0) |
| Cardiovascular and lung disorders | 6 (10.9) | 6 (14.6) |
| Dyspnea | 2 (3.6) | 2 (4.9) |
| Hypertension | 2 (3.6) | 2 (4.9) |
| Pleural effusion | 1 (1.8) | 1 (2.4) |
| Pulmonary fibrosis | 1 (1.8) | 1 (2.4) |
| Infections | 5 (9.1) | 4 (9.8) |
| Anorectal infection | 1 (1.8) | 1 (2.4) |
| Bronchial infection | 1 (1.8) | 1 (2.4) |
| Fever | 1 (1.8) | 0 (0) |
| Infections, other | 1 (1.8) | 1 (2.4) |
| Sepsis | 1 (1.8) | 1 (2.4) |
| Bleeding disorders | 3 (5.4) | 2 (4.9) |
| Hematoma | 1 (1.8) | 1 (2.4) |
| Lower gastrointestinal hemorrhage | 1 (1.8) | 1 (2.4) |
| Oral hemorrhage # | 1 (1.8) | 0 (0) |
| Organ failure | 2 (3.6) | 2 (4.9) |
| Heart failure | 1 (1.8) | 1 (2.4) |
| Hepatic failure | 1 (1.8) | 1 (2.4) |
| Other adverse drug reactions | 3 (5.5) | 3 (7.3) |
| Fatigue | 1 (1.8) | 1 (2.4) |
| Hypokalemia | 1 (1.8) | 1 (2.4) |
| Retinal detachment | 1 (1.8) | 1 (2.4) |
| OAT * | No. | Hospitalization Rate, Normalized by Prescription Numbers (%) | |
|---|---|---|---|
| First Prescriptions | Emergency Hospitalizations | ||
| Total (n = 8102) | Total (n = 2761) | 34.1 | |
| KINASE INHIBITORS | 4333 | 1536 | 35.4 |
| CDK4/6 inhibitors | 1058 | 255 | 24.1 |
| Palbociclib | 776 | 167 | 21.5 |
| Ribociclib | 229 | 69 | 30.1 |
| Abemaciclib | 53 | 19 | 35.8 |
| VEGFR inhibitors | 1016 | 509 | 50.1 |
| Sorafenib | 191 | 100 | 52.4 |
| Sunitinib | 170 | 83 | 48.8 |
| Cabozantinib | 159 | 71 | 44.7 |
| Other VEGFR inhibitors | 496 | 255 | 51.4 |
| BCR-ABL inhibitors | 388 | 84 | 21.6 |
| Imatinib | 197 | 47 | 23.9 |
| Dasatinib | 79 | 17 | 21.5 |
| Nilotinib | 74 | 14 | 18.9 |
| Other BCR-ABL inhibitors | 38 | 6 | 15.8 |
| EGFR inhibitors | 325 | 129 | 39.7 |
| Osimertinib | 165 | 57 | 34.5 |
| Afatinib | 84 | 41 | 48.8 |
| Erlotinib | 49 | 27 | 55.1 |
| Other EGFR inhibitors | 27 | 4 | 14.8 |
| BRAF inhibitors | 250 | 109 | 43.6 |
| Dabrafenib | 182 | 84 | 46.2 |
| Vemurafenib | 38 | 12 | 31.6 |
| Encorafenib | 30 | 13 | 43.3 |
| MEK inhibitors | 241 | 110 | 45.6 |
| Trametinib | 186 | 88 | 47.3 |
| Binimetinib | 33 | 15 | 45.5 |
| Cobimetinib | 22 | 7 | 31.8 |
| ALK inhibitors | 153 | 61 | 39.9 |
| Alectinib | 71 | 16 | 22.5 |
| Crizotinib | 44 | 19 | 43.2 |
| Lorlatinib | 15 | 11 | 73.3 |
| Other ALK inhibitors | 23 | 15 | 65.2 |
| Other kinase inhibitors | 902 | 279 | 30.9 |
| Ibrutinib | 369 | 120 | 32.5 |
| Ruxolitinib | 265 | 60 | 22.6 |
| Everolimus | 174 | 69 | 39.7 |
| Other kinase inhibitors | 94 | 30 | 31.9 |
| HORMONE ANTAGONISTS | 1733 | 495 | 28.6 |
| Abiraterone | 1048 | 291 | 27.8 |
| Enzalutamide | 652 | 197 | 30.2 |
| Apalutamide | 33 | 7 | 21.2 |
| ANTI-NEOPLASTIC DRUGS | 1343 | 481 | 35.8 |
| Antimetabolites | 262 | 123 | 46.9 |
| Trifluridine | 256 | 122 | 47.7 |
| Tegafur, gimeracil, oteracil | 6 | 1 | 16.7 |
| PARP inhibitors | 220 | 51 | 23.2 |
| Olaparib | 128 | 35 | 27.3 |
| Niraparib | 87 | 14 | 16.1 |
| Rucaparib | 5 | 2 | 40.0 |
| Other anti-neoplastic drugs | 861 | 307 | 35.7 |
| Temozolomide | 465 | 168 | 36.1 |
| Anagrelide | 139 | 22 | 15.8 |
| Venetoclax | 117 | 69 | 59.0 |
| Other anti-neoplastic drugs | 140 | 48 | 34.3 |
| IMMUNOMODULATORS | 693 | 249 | 35.9 |
| Lenalidomide | 569 | 181 | 31.8 |
| Pomalidomide | 107 | 57 | 53.3 |
| Thalidomide | 17 | 11 | 64.7 |
| Patient Characteristics | G-DRG Data | |||||
|---|---|---|---|---|---|---|
| Patient ID | OAT | Type of Adverse Drug Reaction(s) Related to Hospitalization | Length of Stay (Days) | G-DRG Code | G-DRG Name | G-DRG cost (EUR) |
| Intervention Group | ||||||
| 1 | Palbociclib | Blood count disorder | 1 | Q63B | Aplastic anemia | 1176 |
| 2 | Ibrutinib | Bleeding disorder | 16 | R61A | Lymphoma and non-acute leukemia with sepsis or a certain complicating constellation | 17,024 |
| 3 | Lenali-domide | Infection, blood count disorders | 8 | I66B | Other connective tissue disorders | 7023 |
| Total | 25,223 | |||||
| Control group | ||||||
| 4 * | Niraparib | Gastrointestinal disorders | 3 | G67A | Esophagitis, gastroenteritis, gastrointestinal hemorrhage, ulcer disease, and various diseases of the digestive organs | 2500 |
| Other disorders (hypokalemia) | 2 | L71Z | Renal failure | 1621 | ||
| 5 | Pazopanib | Other disorders (retinal detachment) | 9 | C03B | Interventions on the retina with pars plana vitrectomy, with extracapsular extraction of the lens (ECCE) | 4008 |
| 6 | Lenali-domide | Lung disorders | 9 | E74Z | Interstitial lung disease | 2834 |
| 7 * | Lenvatinib | Cardiovascular disorder | 2 | F67D | Hypertension without a complicated diagnosis | 1808 |
| Bleeding disorder | 8 | D13B | Small operations on the nose, ears, mouth and throat without complicating the diagnosis | 2180 | ||
| 8 | Tegafur, gimeracil, oteracil | Gastrointestinal disorders | 9 | G60B | Malignant growth of the digestive organs | 2075 |
| 9 | Everolimus | Infection, gastrointestinal disorder | 2 | G71Z | Other moderately severe diseases of the digestive organs | 1990 |
| 10 | Cabozan-tinib | Organ failure | 9 | H61A | Malignant neoplasm of the hepatobiliary system and pancreas | 6409 |
| 11 | Ribociclib | Blood count disorders | 1 | J62B | Malignant neoplasms of the breast | 1491 |
| 12 | Palbociclib | Blood count disorders | 3 | Q60C | Diseases of the reticuloendothelial system, immune system and coagulation disorders with complex diagnosis | 2840 |
| 13 | Ibrutinib | Organ failure, lung disorders | 10 | R03Z | Lymphoma and leukemia with a specific OR procedure | 26,389 |
| 14 | Venetoclax | Infection, blood count disorders, gastrointestinal disorders | 12 | R60C | Acute myeloid leukemia with int. chemotherapy | 12,817 |
| 15 | Midostaurin | Infection, blood count disorders, gastrointestinal disorders | 9 | R60E | Acute myeloid leukemia with moderately complex chemotherapy | 5816 |
| 16 | Everolimus | Infection, blood count disorder | 2 | T60F | Sepsis, died <5 days after admission | 1951 |
| 17 | Pazopanib | Cardiovascular disorder | 1 | X62Z | Poisoning/Toxic Effects of Drugs, Medicines and Other Substances | 957 |
| 18 | Pazopanib | Gastrointestinal disorders, other disorders (fatigue) | 3 | G67A | Esophagitis, gastroenteritis, gastrointestinal hemorrhage, ulcer disease, and various diseases of the digestive organs | 2815 |
| Total | 80,501 | |||||
| Mean per patient ± SD | 6.0 ± 4.3 | 5873 ± 6612 | ||||
| Number of Patients | Absolute Risk (%) | |||
|---|---|---|---|---|
| OAT-Related Hospitalization | No OAT-Related Hospitalization | Total | ||
| AMBORA collective | ||||
| Intervention group Intensified pharmacological/ pharmaceutical care program | 3 | 95 | 98 | 3.06 |
| Control group Standard of care | 15 | 89 | 104 | 14.42 |
| SHI collective AOK Bayern | ||||
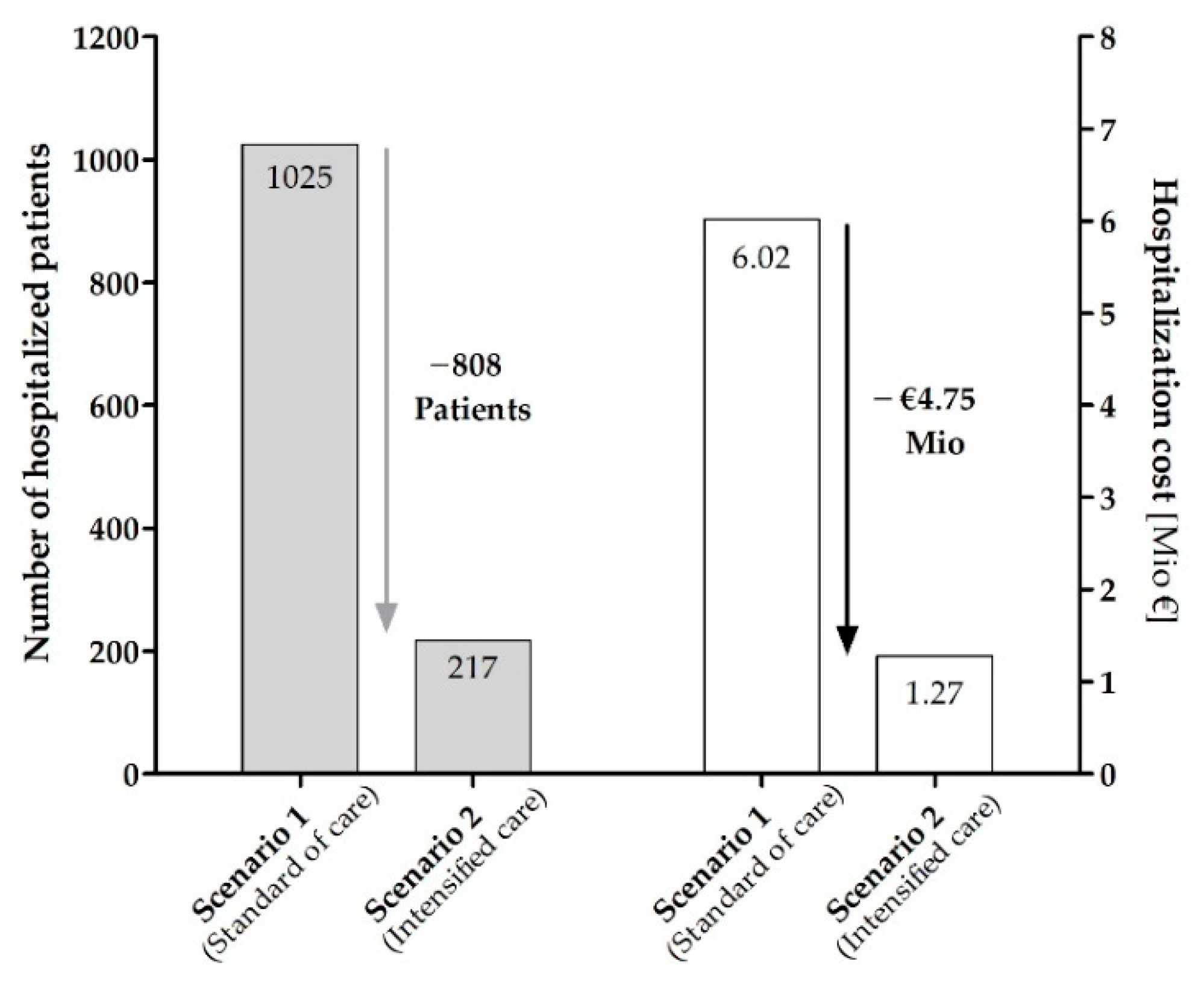
| Scenario 1 Standard of care for all patients | 1025 | 6081 | 7106 | 14.42 |
| Scenario 2 Intensified pharmacological/pharmaceutical care program for all patients | 217 | 6889 | 7106 | 3.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dürr, P.; Meier, F.; Schlichtig, K.; Schramm, A.; Schötz, L.; Fromm, M.F.; Dörje, F. Characteristics and Cost of Unscheduled Hospitalizations in Patients Treated with New Oral Anticancer Drugs in Germany: Evidence from the Randomized AMBORA Trial. J. Clin. Med. 2022, 11, 6392. https://doi.org/10.3390/jcm11216392
Dürr P, Meier F, Schlichtig K, Schramm A, Schötz L, Fromm MF, Dörje F. Characteristics and Cost of Unscheduled Hospitalizations in Patients Treated with New Oral Anticancer Drugs in Germany: Evidence from the Randomized AMBORA Trial. Journal of Clinical Medicine. 2022; 11(21):6392. https://doi.org/10.3390/jcm11216392
Chicago/Turabian StyleDürr, Pauline, Florian Meier, Katja Schlichtig, Anja Schramm, Lukas Schötz, Martin F. Fromm, and Frank Dörje. 2022. "Characteristics and Cost of Unscheduled Hospitalizations in Patients Treated with New Oral Anticancer Drugs in Germany: Evidence from the Randomized AMBORA Trial" Journal of Clinical Medicine 11, no. 21: 6392. https://doi.org/10.3390/jcm11216392
APA StyleDürr, P., Meier, F., Schlichtig, K., Schramm, A., Schötz, L., Fromm, M. F., & Dörje, F. (2022). Characteristics and Cost of Unscheduled Hospitalizations in Patients Treated with New Oral Anticancer Drugs in Germany: Evidence from the Randomized AMBORA Trial. Journal of Clinical Medicine, 11(21), 6392. https://doi.org/10.3390/jcm11216392





