Prevalence and Severity of Lower Gastrointestinal Symptoms amongst Non-Dialysis Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection, and Data Collection Process
2.4. Study Risk of Bias and Reporting Bias Assessment
2.5. Synthesis Methods and Certainty Assessment
3. Results
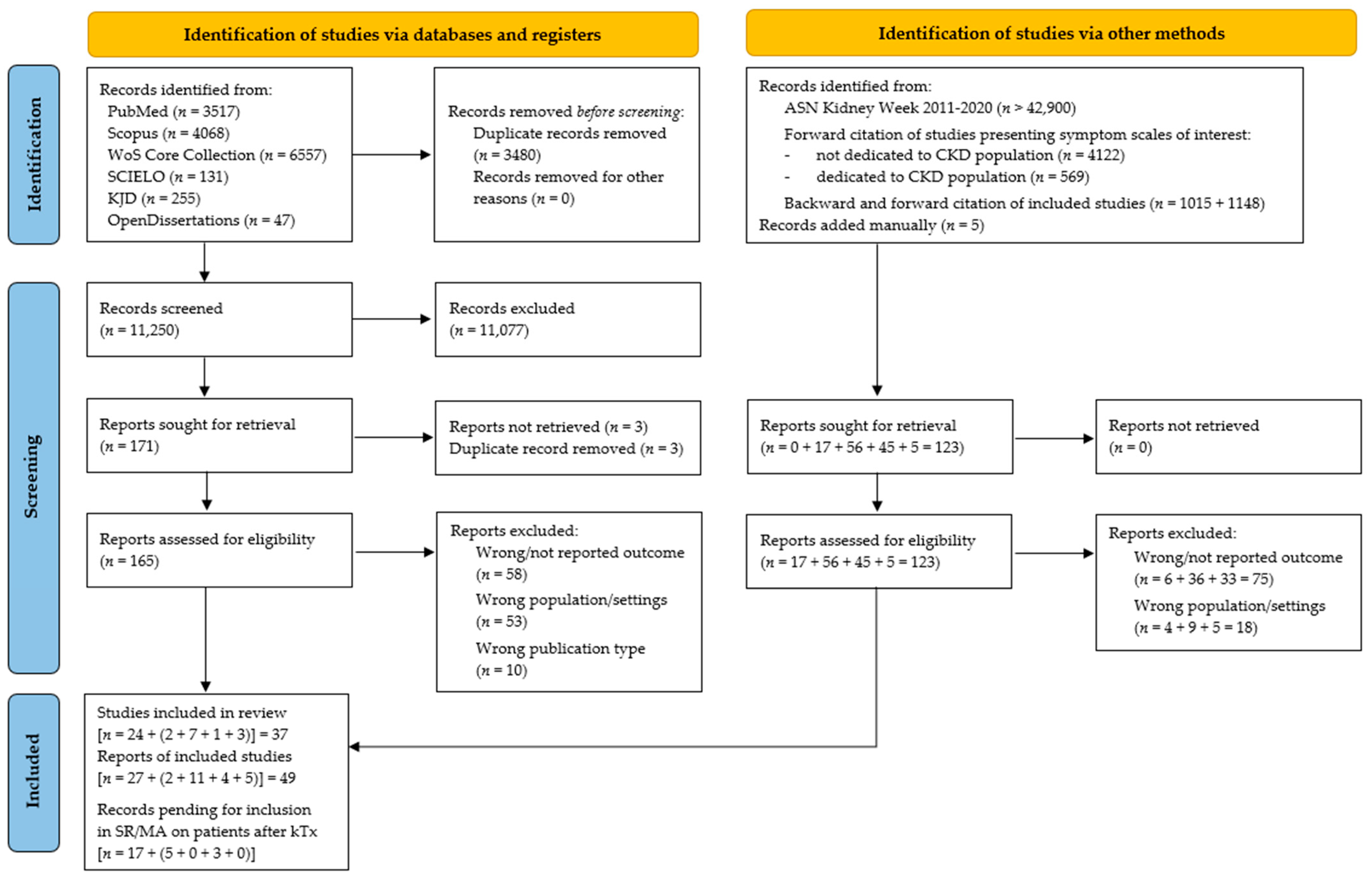
3.1. Study Selection
3.2. Study Characteristics and Risk of Bias
| Author, Reference | Extracted Populations | Outcomes | Study Design | Country | Study Period | ||
|---|---|---|---|---|---|---|---|
| CKD Stage (N) | Age | Male (%) | |||||
| Gordon, S.J.; et al. [41] | G4–5 (4) | 50 | 75.0% | D a | cross-sectional | USA | (1976) |
| Yapa, H.E.; et al. [39] | G3b (224) | 55.96 | 60.7% | C, C-s, D, D-s | cross-sectional | Sri Lanka | 2018–2019 |
| G4–5 (443) | 61.34 | 65.5% | |||||
| Muhd Ariffin, N.F.; et al. [42] | G5 (50) | NR | NR | C, AP, B, D | cross-sectional | Brunei | (2016) |
| Ramos, C.I.; et al. [40] | G3 (6) | 55.83 | 83.3% | BSFS, FC | cross-sectional | Brazil | 2015–2016 |
| G4–5 (36) | 59.58 | 52.8% | |||||
| Trimingham, C.; et al. [12] | General population of non-dialysis CKD patients (95) | NR | 47.4% | BSFS, AP, B, BM | cross-sectional | Australia | (2018) |
| Sanya, E.O. and Ogunniyi, A. [43] | General population of non-dialysis CKD patients (60) | 39 | NR | C, D | cross-sectional | Nigeria | 2000–2000 |
| Saini, T.; et al. [44] | G4–5 (11) | 67 | 72.7% | C, B, D | cross-sectional | UK | 2005–2005 |
| Ohkuma, T.; et al. [45] | General population of non-dialysis patients with diabetic kidney disease (2245) | NR | NR | BM | cross-sectional | Japan | 2008–2010 |
| Ruszkowski, J.; et al. [9,46] | G1–2 (16) | 49.6 | 37.5% | BSFS, FC, AP, AP-s, B, B-s, BM | cross-sectional | Poland | 2018–2019 |
| G3 (69) | 66.8 | 52.2% | |||||
| G4–5 (26) | 63.5 | 76.9% | |||||
| Quintal-Medina, I.A.; et al. [27] | G5 (70) | 59 | 55.7% | C, C-s, D, D-s | prospective cohort | Mexico | 2018–2018 |
| Meade, A.; et al. [47] | G4–5 (134) | 64.6 | 64.9% | BSFS, B, BM, RP, FI | cross-sectional | Australia | 2017–2018 |
| Wizemann, V. and Benz, U. [30] | G5 (20) | NR | NR | C, AP, B, D | cross-sectional | Germany | (1978) |
| Zhang, X.; et al. [32] | G1–2 (370) | 51.6 | 57.0% | B | cross-sectional | USA | 2003–2008 |
| G3 (2541) | 59.2 | 56.7% | |||||
| G4–5 (688) | 58.8 | 47.8% | |||||
| Gryp, T.; et al. [33,48] | G1–2 (37) | 51.3 | 54.05% | BSFS | cross-sectional | Belgium | (2020) |
| G3 (44) | 64 | 65.9% | |||||
| G4–5 (33) | 69.5 | 72.7% | |||||
| Miskulin, D.C. and the HALT-PKD studies investigators [49,50] | ADPKD G1–4 (1043) | 41.8 | 50.1% | AP, AP-s | cross-sectional | USA | 2006–2009 |
| Windahl, K. and the EQUAL study inves-tigators [23,51,52,53] | G4–5 (1205) | 76 b | 64.6% b | C, D, C-s, D-s | prospective cohort | Germany, Italy, the Netherlands, Poland, Sweden, UK | 2012–2018 |
| Ducharlet, K.; et al. [24] | G4 (31) | 71 | 74% | C, D, C-s, D-s | prospective cohort | Australia | 2014–2014 |
| Grove, B.E.; et al. [38] | G3 (141) | 65 | 63.1% | C, D, C-s, D-s | cross-sectional | Denmark | 2019–2019 |
| G4–5 (92) | 68 | 72.8% | |||||
| Onodugo, O.D.; et al. [54] | G5 (80) | 41.5 | 48.8% | C a, D a | cross-sectional | Nigeria | 2015–2015 |
| Allawi, A.A. [29] | G5 (35) | 55.4 | 62.9% | C | case-control | Iraq | 2016–2016 |
| Lee, A.; et al. [55] | G5 (21) | 64.2 | 47.6% | C, BSFS, FC | cross-sectional | Australia | (2016) |
| Dawson, J.; et al. [37] | G3 (8) | 80.6 | 75% | C, D, C-s, D-s | cross-sectional | Australia | 2016–2019 |
| G4–5 (92) | 82.4 | 64.1% | |||||
| Abeywickrama, H.M.; et al. [34] | G1–2 (2) | 66 | 100% | D, D-s | cross-sectional | Sri Lanka | 2019–2019 |
| G3 (45) | 60.5 | 73.3% | |||||
| G4–5 (73) | 62.6 | 65.8% | |||||
| Lee, S.J. and Jeon, J.H. [35] | G1–2 (22) | 61.7 | 95.5% | C, C-s, D, D-s | cross-sectional | South Korea | 2013–2013 |
| G3 (83) | 68.8 | 62.7% | |||||
| G4–5 (38) | 63.7 | 42.1% | |||||
| Yong, D.S.P.; et al. [56] | G5 (45) | 73.1 | 46.7% | C, C-s, B, B-s | cross-sectional | China | 2006–2007 |
| Senanayake, S.; et al. [36] | G1–2 (96) | 52.5 | 32.3% | D, D-s | cross-sectional | Sri Lanka | 2016–2016 |
| G3 (163) | 57.9 | 51.5% | |||||
| G4–5 (782) | 59.7 | 68.3% | |||||
| Abdel-Kader, K.; et al. [57] | G4–5 (87) | 51 | 65.5% | C, C-s, D, D-s | cross-sectional | USA | 2004–2006 |
| Wan Zukiman, W.Z.H.; et al. [58] | G5 (100) | 61.0 | 48% | C, C-s, D, D-s | cross-sectional | Malaysia | 2015–2016 |
| Gutiérrez Sánchez, D.; et al. [31,59,60,61] | G4–5 (124) | 69.8 | 70.2% | C, C-s, D, D-s | cross-sectional | Spain | 2015–2015 |
| Murtagh, F.E.; et al. [62,63] | G5 (66) | 82 | 48.5% | C, C-s, B, B-s, D, D-s | cross-sectional | England | 2005–2006 |
| Turkmen, K.; et al. [25] | Fabry nephropathy (11) | 41.3 | 63.6% | AP | prospective cohort | Turkey | 2014–2016 |
| Brennan, F.; et al. [64] | G5 (42) | 83 | 42.9% | C, C-s, D, D-s | cross-sectional | Australia | 2010–2012 |
| Murphy, E.L.; et al. [65] | G4–5 (55) | 82 | 47.3% | C, C-s, D, D-s | cross-sectional | UK | 2005–2006 |
| Taira, K.; et al. [66] | General population of non-dialysis CKD patients (15) | 54.9 | 73.3% | C, D | cross-sectional | Sri Lanka | 2015–2015 |
| Purtell, L.; et al. [26,67] | G4–5 (46) | 78.3 | 39.1% | C, C-s, D, D-s | prospective cohort | Australia | 2016–2017 |
| de Miguel, C.; et al. [28] | G4–5 (102) | 79.6 | 59.8% | C | retrospective cohort | Spain | 1997–2009 |
| Almutary, H.; et al. [68,69] | G4–5 (107) | 51.6 | 55.1% | C, C-s, D, D-s | cross-sectional | Saudi Arabia | 2013–2014 |
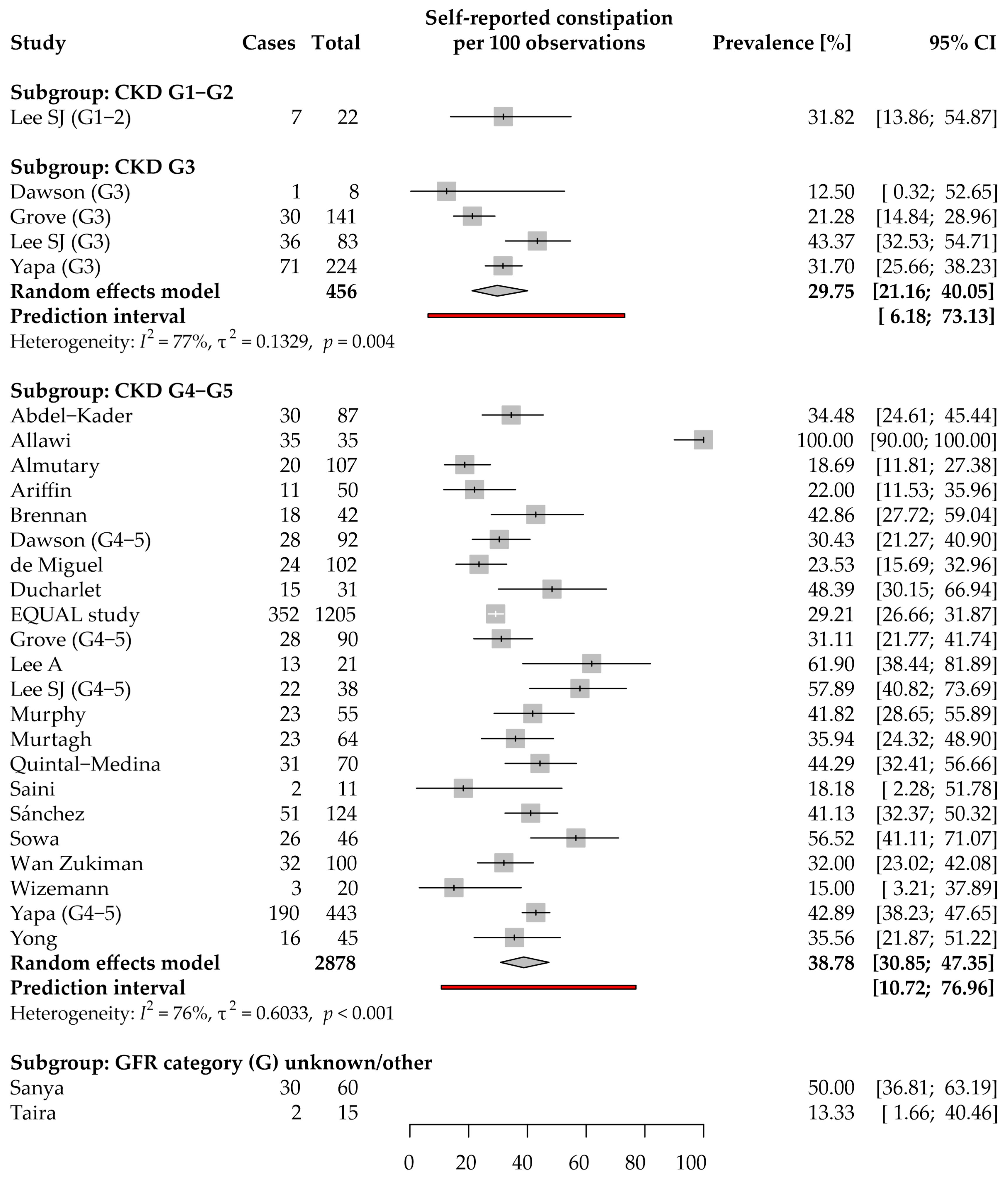
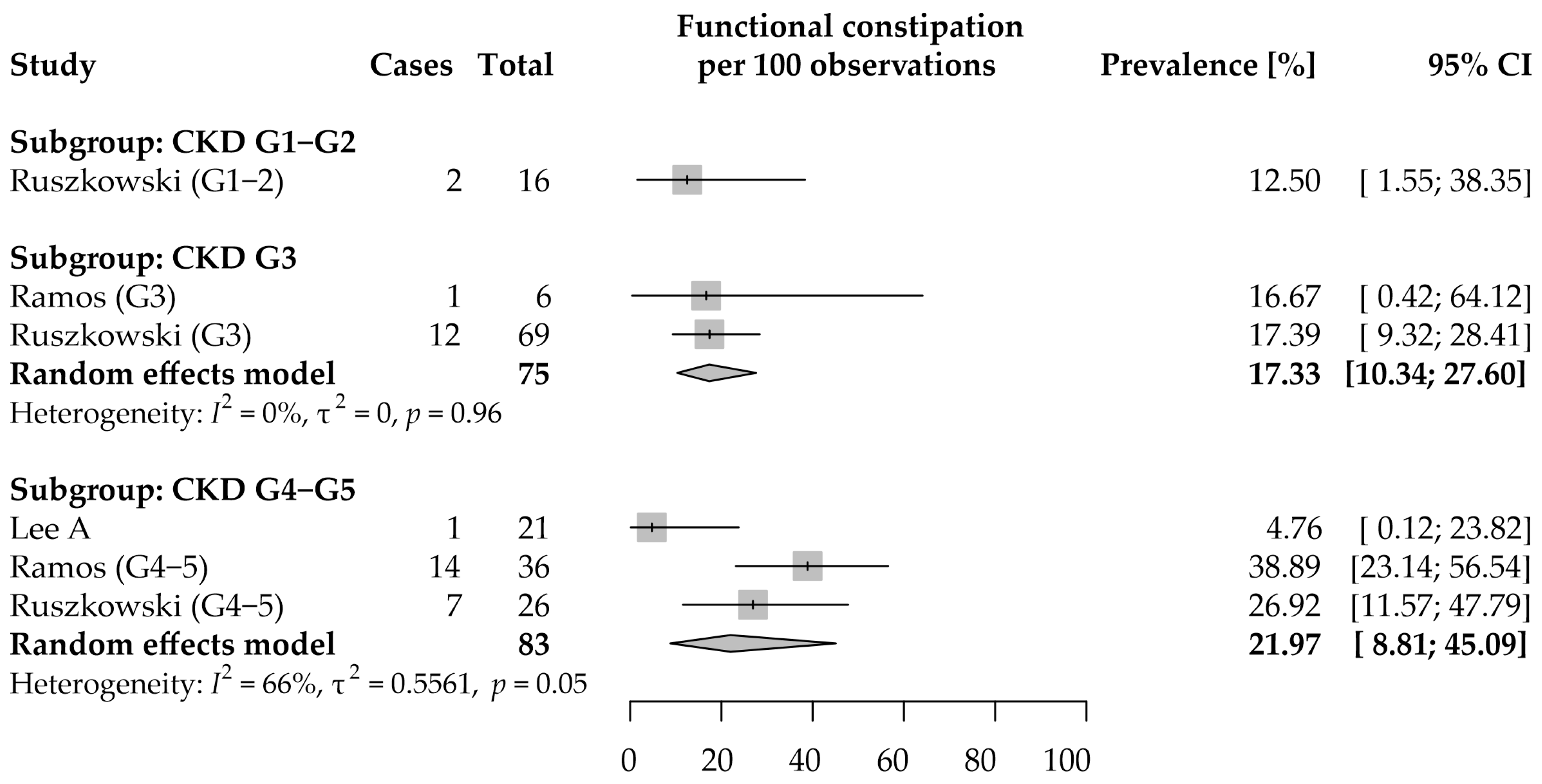
3.3. Constipation: Prevalence and Severity
3.3.1. Self-Reported Constipation
3.3.2. Functional Constipation
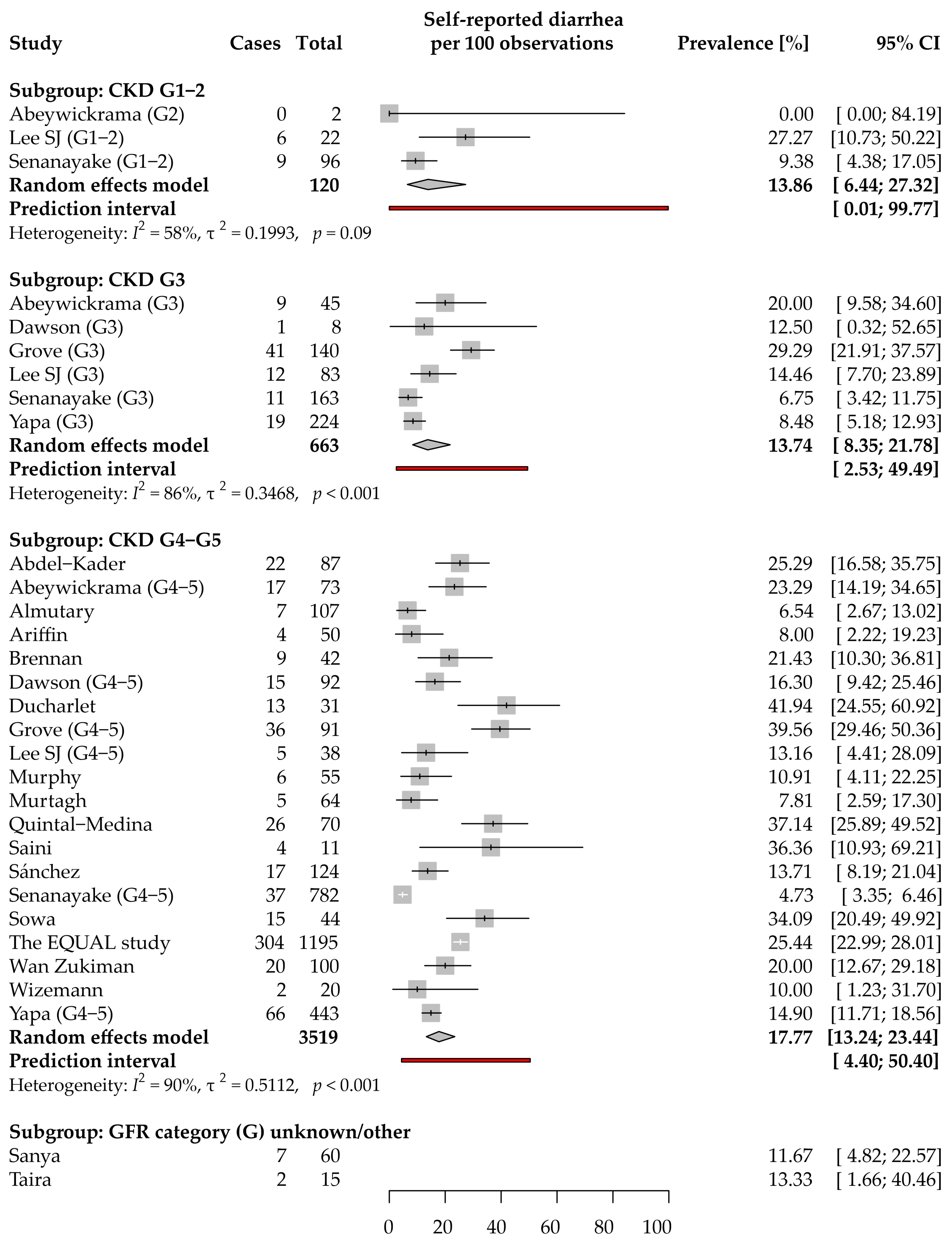
3.4. Diarrhea: Prevalence and Severity
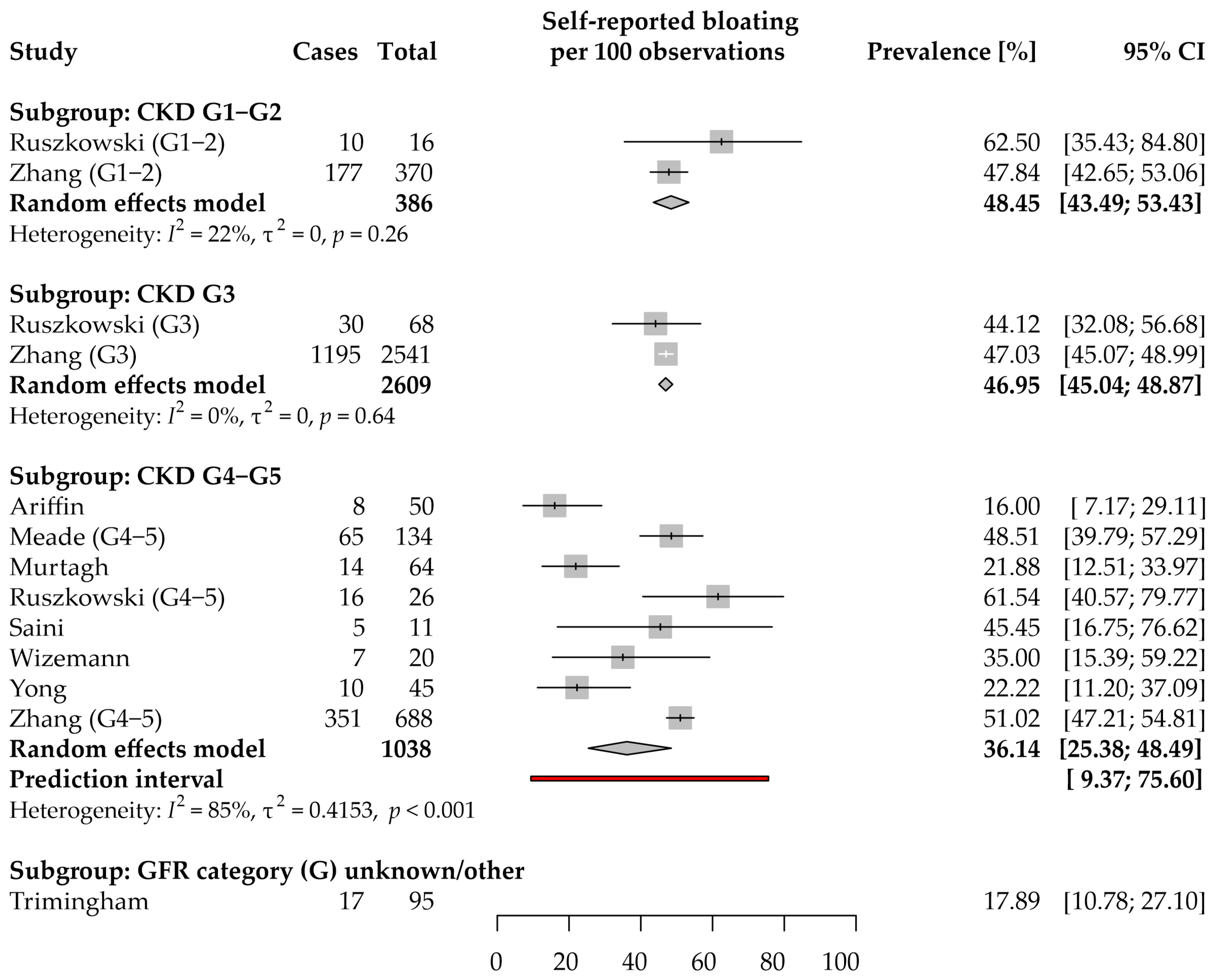
3.5. Bloating: Prevalence and Severity
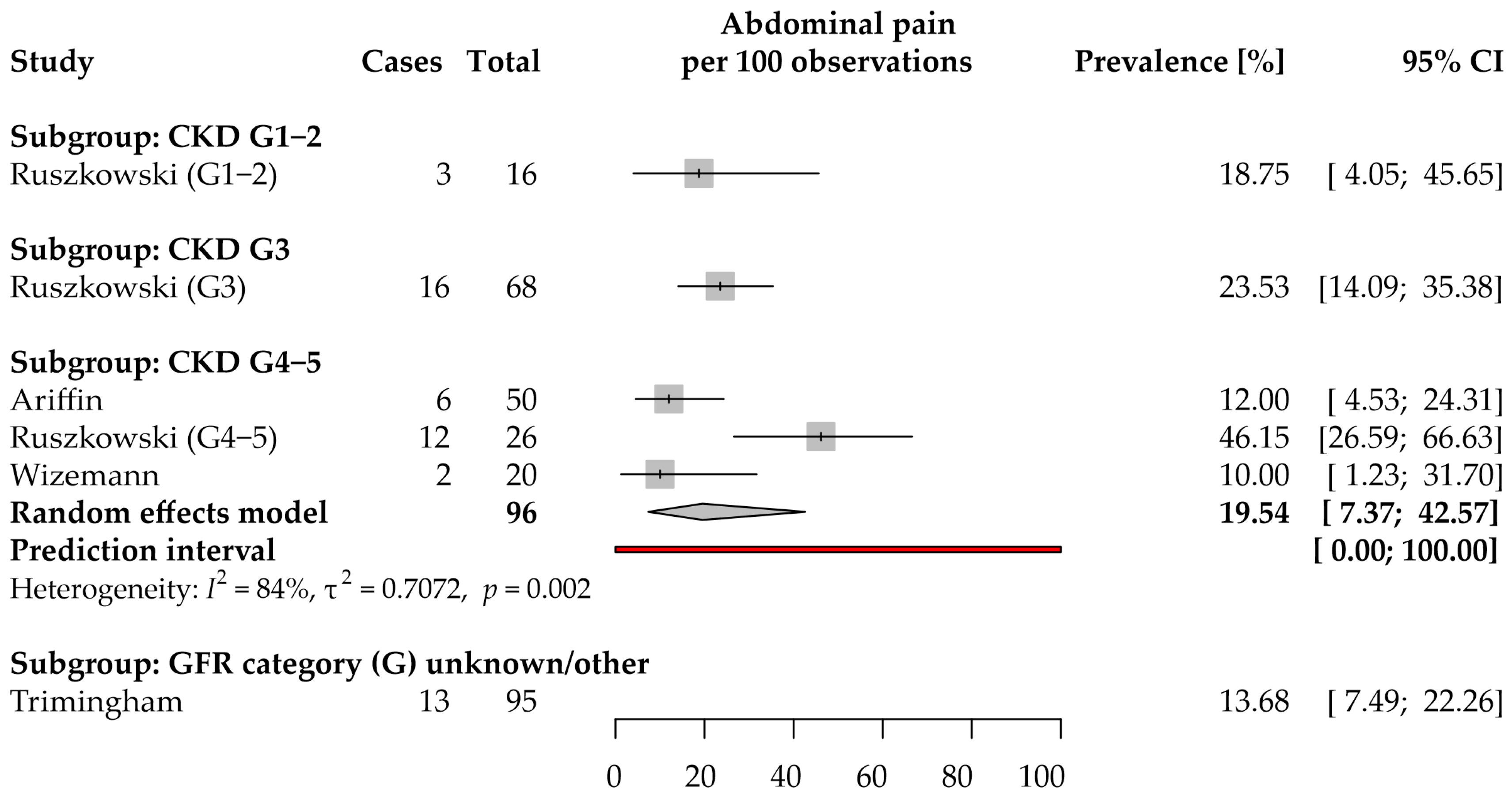
3.6. Abdominal Pain: Prevalence and Severity
3.7. Stool Consistency (Bristol Stool Scale)
3.8. Number of Bowel Movements per Week
3.9. Fecal Incontinence and Rectal Pain
3.10. Sensitivity Analyses
4. Discussion
4.1. Limitations of the Evidence Included and the Review Processes Used
4.2. Implications of the Results for Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The Systemic Nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Almutary, H.; Bonner, A.; Douglas, C. Symptom Burden in Chronic Kidney Disease: A Review of Recent Literature. J. Ren. Care 2013, 39, 140–150. [Google Scholar] [CrossRef]
- Yapa, H.E.; Purtell, L.; Chambers, S.; Bonner, A. The Relationship Between Chronic Kidney Disease, Symptoms and Health-Related Quality of Life: A Systematic Review. J. Ren. Care 2020, 46, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and Incident CKD. J. Am. Soc. Nephrol. 2017, 28, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Chen, Y.C.; Lu, Y.W.; Muo, C.H.; Chang, R.E. Association of Constipation with Risk of End-Stage Renal Disease in Patients with Chronic Kidney Disease. BMC Nephrol. 2019, 20, 304. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Ruszkowski, J.; Heleniak, Z.; Król, E.; Tarasewicz, A.; Gałgowska, J.; Witkowski, J.M.; Dębska-Ślizień, A. Constipation and the Quality of Life in Conservatively Treated Chronic Kidney Disease Patients: A Cross-Sectional Study. Int. J. Med. Sci. 2020, 17, 2954–2963. [Google Scholar] [CrossRef]
- Zuvela, J.; Trimingham, C.; Le Leu, R.; Faull, R.; Clayton, P.; Jesudason, S.; Meade, A. Gastrointestinal Symptoms in Patients Receiving Dialysis: A Systematic Review. Nephrology 2018, 23, 718–727. [Google Scholar] [CrossRef]
- Werth, B.L.; Williams, K.A.; Fisher, M.J.; Pont, L.G. Defining Constipation to Estimate Its Prevalence in the Community: Results from a National Survey. BMC Gastroenterol. 2019, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Trimingham, C.; McDonald, S.; Dansie, K.; Jesudason, S.; Faull, R.; Clayton, P.; Liew, G.; Le Leu, R.; Meade, A. Bowel Health in Chronic Kidney Disease: Patient Perceptions Differ from Clinical Definitions. J. Ren. Care 2018, 44, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Iansavichus, A.V.; Hildebrand, A.M.; Haynes, R.B.; Wilczynski, N.L.; Levin, A.; Hemmelgarn, B.R.; Tu, K.; Nesrallah, G.E.; Nash, D.M.; Garg, A.X. High-Performance Information Search Filters for CKD Content in PubMed, Ovid MEDLINE, and EMBASE. Am. J. Kidney Dis. 2015, 65, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: An R Package for Forward and Backward Citations Chasing in Academic Searching. Zenodo 2021. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid. Based. Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Lockwood, M.B.; Rhee, C.M.; Tantisattamo, E.; Andreoli, S.; Balducci, A.; Laffin, P.; Harris, T.; Knight, R.; Kumaraswami, L.; et al. Patient-Centred Approaches for the Management of Unpleasant Symptoms in Kidney Disease. Nat. Rev. Nephrol. 2022, 18, 185–198. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based. Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A Re-Evaluation of Random-Effects Meta-Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-Analysis of Prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Windahl, K.; Irving, G.F.; Almquist, T.; Lidén, M.K.; Stenvinkel, P.; Chesnaye, N.C.; Drechsler, C.; Szymczak, M.; Krajewska, M.; Fu, E.L.; et al. Patient-Reported Measures and Lifestyle Are Associated with Deterioration in Nutritional Status in CKD Stage 4-5: The EQUAL Cohort Study. J. Ren. Nutr. 2022, 32, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ducharlet, K.; Sundararajan, V.; Philip, J.; Weil, J.; Barker, N.; Langham, R.G.; Burchell, J.; Gock, H. Patient-Reported Outcome Measures and Their Utility in the Management of Patients with Advanced Chronic Kidney Disease. Nephrology (Carlton) 2019, 24, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Guclu, A.; Sahin, G.; Kocyigit, I.; Demirtas, L.; Erdur, F.M.; Sengül, E.; Ozkan, O.; Emre, H.; Turgut, F.; et al. The Prevalence of Fabry Disease in Patients with Chronic Kidney Disease in Turkey: The TURKFAB Study. Kidney Blood Press. Res. 2016, 41, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Purtell, L.; Sowa, P.M.; Berquier, I.; Scuderi, C.; Douglas, C.; Taylor, B.; Kramer, K.; Hoy, W.; Healy, H.; Bonner, A. The Kidney Supportive Care Programme: Characteristics of Patients Referred to a New Model of Care. BMJ Support. Palliat. Care 2018. [Google Scholar] [CrossRef]
- Quintal-Medina, I.A.; Rocha-González, H.I.; Noyola-García, M.E.; Flores-Padilla, M.G. Factores Asociados a Carga Sintomática Residual En Pacientes Con Diálisis Peritoneal: Estudio de Cohorte. Rev. Med. Inst. Mex. Seguro Soc. 2020, 58, 174–180. [Google Scholar] [CrossRef]
- de Miguel, C.; Morán, C.; Rumayor, M.; Fernández, B.; del Cura, I.; Sanz, E.M.; Gutiérrez, B. Cómo Son Los Últimos Meses de Vida de Los Pacientes Con Insuficiencia Renal Crónica Avanzada En Los Que Se Desestima El Tratamiento Con Hemodiálisis o Con Diálisis Peritoneal? Med. Paliativa 2018, 25, 83–94. [Google Scholar] [CrossRef]
- Allawi, A.A. dyab Prediction of Autonomic Neuropathy in Chronic Kidney Disease (Stage 5) Iraqi Patients (Case Control Study). Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S969–S973. [Google Scholar] [CrossRef]
- Wizemann, V.; Benz, U. Gastrointestinale Befunde Bei Chronisch Niereninsuffizienten Patienten. Med. Welt 1978, 29, 1025–1029. [Google Scholar]
- Sánchez, D.G.; Leiva-Santos, J.P.; López, M.J.M.; Cuesta Vargas, A.I. Perfil Sintomático de Los Pacientes Con Enfermedad Renal Crónica Estudio 4 y 5. Enferm. Nefrol. 2017, 20, 259–266. [Google Scholar] [CrossRef]
- Zhang, X.; Bansal, N.; Go, A.S.; Hsu, C. Gastrointestinal Symptoms, Inflammation and Hypoalbuminemia in Chronic Kidney Disease Patients: A Cross-Sectional Study. BMC Nephrol. 2015, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; De Paepe, K.; Vanholder, R.; Kerckhof, F.M.; Van Biesen, W.; Van de Wiele, T.; Verbeke, F.; Speeckaert, M.; Joossens, M.; Couttenye, M.M.; et al. Gut Microbiota Generation of Protein-Bound Uremic Toxins and Related Metabolites Is Not Altered at Different Stages of Chronic Kidney Disease. Kidney Int. 2020, 97, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Abeywickrama, H.M.; Wimalasiri, S.; Koyama, Y.; Uchiyama, M.; Shimizu, U.; Kakihara, N.; Chandrajith, R.; Nanayakkara, N. Quality of Life and Symptom Burden among Chronic Kidney Disease of Uncertain Etiology (CKDU) Patients in Girandurukotte, Sri Lanka. Int. J. Environ. Res. Public Health 2020, 17, 4041. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeon, J.H. Relationship between Symptom Clusters and Quality of Life in Patients at Stages 2 to 4 Chronic Kidney Disease in Korea. Appl. Nurs. Res. 2015, 28, e13–e19. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, S.; Gunawardena, N.; Palihawadana, P.; Bandara, P.; Haniffa, R.; Karunarathna, R.; Kumara, P. Symptom Burden in Chronic Kidney Disease; a Population Based Cross Sectional Study. BMC Nephrol. 2017, 18, 228. [Google Scholar] [CrossRef]
- Dawson, J.; Brennan, F.P.; Hoffman, A.; Josland, E.; Li, K.C.; Smyth, A.; Brown, M.A. Prevalence of Taste Changes and Association with Other Nutrition-Related Symptoms in End-Stage Kidney Disease Patients. J. Ren. Nutr. 2021, 31, 80–84. [Google Scholar] [CrossRef]
- Grove, B.E.; Schougaard, L.M.V.; Ivarsen, P.R.; Kyte, D.; Hjollund, N.H.; de Thurah, A. Patient-Reported Outcome Measures for Clinical Decision-Making in Outpatient Follow-up: Validity and Reliability of a Renal Disease Questionnaire. J. Patient-Reported Outcomes 2021, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Yapa, H.E.; Purtell, L.; Chambers, S.; Bonner, A. Alterations in Symptoms and Health-Related Quality of Life as Kidney Function Deteriorates: A Cross-Sectional Study. J. Clin. Nurs. 2021, 30, 1787–1796. [Google Scholar] [CrossRef]
- Ramos, C.I.; Armani, R.G.; Canziani, M.E.; Ribeiro Dolenga, C.J.; Nakao, L.S.; Campbell, K.L.; Cuppari, L. Bowel Habits and the Association with Uremic Toxins in Non–Dialysis-Dependent Chronic Kidney Disease Patients. J. Ren. Nutr. 2020, 30, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.J.; Miller, L.J.; Haeffner, L.J.; Kinsey, M.D.; Kowlessar, O.D. Abnormal Intestinal Bile Acid Distribution in Azotaemic Man: A Possible Role in the Pathogenesis of Uraemic Diarrhoea. Gut 1976, 17, 58–67. [Google Scholar] [CrossRef]
- Muhd Ariffin, N.F.; Naing, L.; Pisharam, J.; Maboud Khalil, M.A.; Tamin, N.; Chong, V.H.; Tan, J. Appetite and Gastrointestinal Symptoms in End Stage Renal Disease Patients. J. Clin. Exp. Nephrol. 2016, 1, 1–6. [Google Scholar] [CrossRef][Green Version]
- Sanya, E.O.; Ogunniyi, A. Cardiovascular Autonomic Neuropathy in Non-Diabetic Nigerian Patients with Chronic Renal Failure. West Afr. J. Med. 2004, 23, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Saini, T.; Murtagh, F.E.M.; Dupont, P.J.; McKinnon, P.M.; Hatfield, P.; Saunders, Y. Comparative Pilot Study of Symptoms and Quality of Life in Cancer Patients and Patients with End Stage Renal Disease. Palliat. Med. 2006, 20, 631–636. [Google Scholar] [CrossRef]
- Ohkuma, T.; Iwase, M.; Fujii, H.; Ide, H.; Komorita, Y.; Yoshinari, M.; Oku, Y.; Higashi, T.; Oshiro, A.; Nakamura, U.; et al. Constipation and Diabetic Kidney Disease: The Fukuoka Diabetes Registry. Clin. Exp. Nephrol. 2021, 25, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowski, J.; Heleniak, Z.; Król, E.; Tarasewicz, A.; Witkowski, J.M.; Dębska-Ślizień, A. Associations between Symptoms of Constipation and Sleep Quality in Patients with Nondialysis Chronic Kidney Disease: A Cross-sectional Study. Polish Arch. Intern. Med. 2021, 131, 512–519. [Google Scholar] [CrossRef]
- Meade, A.; Le Leu, R.; Watson, N.; Jesudason, S.; Clayton, P.; Faull, R.; McDonald, S.; Trimingham, C. Gastrointestinal Symptom Burden and Dietary Intake in Patients with Chronic Kidney Disease. J. Ren. Care 2021, 47, 234–241. [Google Scholar] [CrossRef]
- Gryp, T.; Faust, K.; Van Biesen, W.; Huys, G.R.B.; Verbeke, F.; Speeckaert, M.; Raes, J.; Vaneechoutte, M.; Joossens, M.; Glorieux, G. Gut Microbiome Profiling Uncovers a Lower Abundance of Butyricicoccus in Advanced Stages of Chronic Kidney Disease. J. Pers. Med. 2021, 11, 1118. [Google Scholar] [CrossRef]
- Miskulin, D.C.; Abebe, K.Z.; Chapman, A.B.; Perrone, R.D.; Steinman, T.I.; Torres, V.E.; Bae, K.T.; Braun, W.; Winklhofer, F.T.; Hogan, M.C.; et al. Health-Related Quality of Life in Patients with Autosomal Dominant Polycystic Kidney Disease and CKD Stages 1-4: A Cross-Sectional Study. Am. J. Kidney Dis. 2014, 63, 214–226. [Google Scholar] [CrossRef]
- Nowak, K.L.; Murray, K.; You, Z.; Gitomer, B.; Brosnahan, G.; Abebe, K.Z.; Braun, W.; Chapman, A.; Harris, P.C.; Miskulin, D.; et al. Pain and Obesity in Autosomal Dominant Polycystic Kidney Disease: A Post Hoc Analysis of the Halt Progression of Polycystic Kidney Disease (HALT-PKD) Studies. Kidney Med. 2021, 3, 536–545.e1. [Google Scholar] [CrossRef]
- van de Luijtgaarden, M.W.M.; Caskey, F.J.; Wanner, C.; Chesnaye, N.C.; Postorino, M.; Janmaat, C.J.; Rao, A.; Torino, C.; Klinger, M.; Drechsler, C.; et al. Uraemic Symptom Burden and Clinical Condition in Women and Men of ≥65 Years of Age with Advanced Chronic Kidney Disease: Results from the EQUAL Study. Nephrol. Dial. Transplant. 2019, 34, 1189–1196. [Google Scholar] [CrossRef]
- Janmaat, C.J.; van Diepen, M.; Meuleman, Y.; Chesnaye, N.C.; Drechsler, C.; Torino, C.; Wanner, C.; Postorino, M.; Szymczak, M.; Evans, M.; et al. Kidney Function and Symptom Development over Time in Elderly Patients with Advanced Chronic Kidney Disease: Results of the EQUAL Cohort Study. Nephrol. Dial. Transplant. 2021, 36, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Voskamp, P.W.M.; Van Diepen, M.; Evans, M.; Caskey, F.J.; Torino, C.; Postorino, M.; Szymczak, M.; Klinger, M.; Wallquist, C.; Van De Luijtgaarden, M.W.M.; et al. The Impact of Symptoms on Health-Related Quality of Life in Elderly Pre-Dialysis Patients: Effect and Importance in the EQUAL Study. Nephrol. Dial. Transplant 2019, 34, 1707–1715. [Google Scholar] [CrossRef]
- Onodugo, O.D.; Ulasi, I.I.; Ijoma, C.K.; Arodiwe, E.B.; Okoye, J.U.; Ezeala-Adikaibe, B.A.; Onodugo, N.P.; Ugwu, E.O. Predictors of Autonomic Dysfunction among Predialysis Chronic Kidney Disease Patients in Nigeria. Niger. J. Clin. Pract. 2018, 21, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Lambert, K.; Byrne, P.; Lonergan, M. Prevalence of Constipation in Patients with Advanced Kidney Disease. J. Ren. Care 2016, 42, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.S.P.; Kwok, A.O.L.; Wong, D.M.L.; Suen, M.H.P.; Chen, W.T.; Tse, D.M.W. Symptom Burden and Quality of Life in End-Stage Renal Disease: A Study of 179 Patients on Dialysis and Palliative Care. Palliat. Med. 2009, 23, 111–119. [Google Scholar] [CrossRef]
- Abdel-Kader, K.; Unruh, M.L.; Weisbord, S.D. Symptom Burden, Depression, and Quality of Life in Chronic and End-Stage Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1057–1064. [Google Scholar] [CrossRef]
- Wan Zukiman, W.Z.H.; Yaakup, H.; Zakaria, N.F.; Shah, S.A. Bin Symptom Prevalence and the Negative Emotional States in End-Stage Renal Disease Patients with or without Renal Replacement Therapy: A Cross-Sectional Analysis. J. Palliat. Med. 2017, 20, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Sánchez, D.; Leiva-Santos, J.P. Symptom Burden, Comorbidity and Functional Status of Patients with Chronic Kidney Disease Stage 5 Managed Conservately. Enfermería Glob. 2021, 20, 44. [Google Scholar] [CrossRef]
- Gutiérrez Sánchez, D.; Leiva-Santos, J.P.; Cuesta-Vargas, A.I. Symptom Burden Clustering in Chronic Kidney Disease Stage 5. Clin. Nurs. Res. 2019, 28, 583–601. [Google Scholar] [CrossRef]
- Gutiérrez Sánchez, D.; Leiva-Santos, J.P.; Macías López, M.J.; Cuesta-Vargas, A.I. Prevalencia de Síntomas En Enfermedad Renal Crónica Avanzada. Nefrología 2018, 38, 560–562. [Google Scholar] [CrossRef]
- Murtagh, F.E.M.; Addington-Hall, J.M.; Edmonds, P.M.; Donohoe, P.; Carey, I.; Jenkins, K.; Higginson, I.J. Symptoms in Advanced Renal Disease: A Cross-Sectional Survey of Symptom Prevalence in Stage 5 Chronic Kidney Disease Managed without Dialysis. J. Palliat. Med. 2007, 10, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, F.E.; Addington-Hall, J.; Edmonds, P.; Donohoe, P.; Carey, I.; Jenkins, K.; Higginson, I.J. Symptoms in the Month before Death for Stage 5 Chronic Kidney Disease Patients Managed without Dialysis. J. Pain Symptom Manag. 2010, 40, 342–352. [Google Scholar] [CrossRef]
- Brennan, F.; Collett, G.; Josland, E.A.; Brown, M.A. The Symptoms of Patients with CKD Stage 5 Managed without Dialysis. Prog. Palliat. Care 2015, 23, 267–273. [Google Scholar] [CrossRef]
- Murphy, E.L.; Murtagh, F.E.M.; Carey, I.; Sheerin, N.S. Understanding Symptoms in Patients with Advanced Chronic Kidney Disease Managed without Dialysis: Use of a Short Patient-Completed Assessment Tool. Nephron. Clin. Pract. 2009, 111, c74–c80. [Google Scholar] [CrossRef] [PubMed]
- Taira, K.; Kawakami, T.; Weragoda, S.K.; Herath, H.M.A.S.; Ikenaka, Y.; Fujioka, K.; Hemachandra, M.; Pallewatta, N.; Aoyama, Y.; Ishizuka, M.; et al. Urinary Concentrations of Neonicotinoid Insecticides Were Related to Renal Tubular Dysfunction and Neuropsychological Complaints in Dry-Zone of Sri Lanka. Sci. Rep. 2021, 11, 22484. [Google Scholar] [CrossRef]
- Sowa, P.M.; Purtell, L.; Hoy, W.E.; Healy, H.G.; Bonner, A.; Connelly, L.B. Utilization and Costs of Health Care in a Kidney Supportive Care Program. J. Palliat. Care 2020, 35, 176–184. [Google Scholar] [CrossRef]
- Almutary, H.; Bonner, A.; Douglas, C. Which Patients with Chronic Kidney Disease Have the Greatest Symptom Burden? A Comparative Study of Advanced Ckd Stage and Dialysis Modality. J. Ren. Care 2016, 42, 73–82. [Google Scholar] [CrossRef]
- Almutary, H.; Douglas, C.; Bonner, A. Multidimensional Symptom Clusters: An Exploratory Factor Analysis in Advanced Chronic Kidney Disease. J. Adv. Nurs. 2016, 72, 2389–2400. [Google Scholar] [CrossRef]
- Davison, S.N.; Levin, A.; Moss, A.H.; Jha, V.; Brown, E.A.; Brennan, F.; Murtagh, F.E.M.; Naicker, S.; Germain, M.J.; O’Donoghue, D.J.; et al. Executive Summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: Developing a Roadmap to Improving Quality Care. Kidney Int. 2015, 88, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Gwee, K.A.; Gonlachanvit, S.; Ghoshal, U.C.; Chua, A.S.B.; Miwa, H.; Wu, J.; Bak, Y.T.; Lee, O.Y.; Lu, C.L.; Park, H.; et al. Second Asian Consensus on Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2019, 25, 343–362. [Google Scholar] [CrossRef]
- Chuah, K.H.; Mahadeva, S. Cultural Factors Influencing Functional Gastrointestinal Disorders in the East. J. Neurogastroenterol. Motil. 2018, 24, 536–543. [Google Scholar] [CrossRef]
- Patimah, A.W.; Lee, Y.Y.; Dariah, M.Y. Frequency Patterns of Core Constipation Symptoms among the Asian Adults: A Systematic Review. BMC Gastroenterol. 2017, 17, 115. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to Estimate the Between-Study Variance and Its Uncertainty in Meta-Analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef]
- Avci, E. Bayesian Approach to the Meta-Analysis of Multi-Category Prevalence. Commun. Stat. Simul. Comput. 2021, 50, 1541–1559. [Google Scholar] [CrossRef]
- Von Hippel, P.T. The Heterogeneity Statistic I2 Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Oh, S.J.; Fuller, G.; Patel, D.; Khalil, C.; Spalding, W.; Nag, A.; Spiegel, B.M.R.; Almario, C.V. Chronic Constipation in the United States: Results from a Population-Based Survey Assessing Healthcare Seeking and Use of Pharmacotherapy. Am. J. Gastroenterol. 2020, 115, 895–905. [Google Scholar] [CrossRef]
- Van Der Willik, E.M.; Meuleman, Y.; Prantl, K.; Van Rijn, G.; Bos, W.J.W.; Van Ittersum, F.J.; Bart, H.A.J.; Hemmelder, M.H.; Dekker, F.W. Patient-Reported Outcome Measures: Selection of a Valid Questionnaire for Routine Symptom Assessment in Patients with Advanced Chronic Kidney Disease—A Four-Phase Mixed Methods Study. BMC Nephrol. 2019, 20, 344. [Google Scholar] [CrossRef]
- Chassany, O.; Tugaut, B.; Marrel, A.; Guyonnet, D.; Arbuckle, R.; Duracinsky, M.; Whorwell, P.J.; Azpiroz, F. The Intestinal Gas Questionnaire: Development of a New Instrument for Measuring Gas-Related Symptoms and Their Impact on Daily Life. Neurogastroenterol. Motil. 2015, 27, 885–898. [Google Scholar] [CrossRef]
- Duracinsky, M.; Archbold, S.; Lobo, B.; Bessonneau, P.; Thonon, F.; Santos, J.; Guagnozzi, D.; Payakachat, N.; Coffin, B.; Azpiroz, F.; et al. The Intestinal Gas Questionnaire (IGQ): Psychometric Validation of a New Instrument for Measuring Gas-Related Symptoms and Their Impact on Daily Life among General Population and Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2022, 34, e14202. [Google Scholar] [CrossRef]
- Frank, L.; Kleinman, L.; Farup, C.; Taylor, L.; Miner, P. Psychometric Validation of a Constipation Symptom Assessment Questionnaire. Scand. J. Gastroenterol. 1999, 34, 870–877. [Google Scholar] [CrossRef]
- Lohia, S.; Vlahou, A.; Zoidakis, J. Microbiome in Chronic Kidney Disease (CKD): An Omics Perspective. Toxins (Basel) 2022, 14, 176. [Google Scholar] [CrossRef]
- Lund, H.; Juhl, C.B.; Nørgaard, B.; Draborg, E.; Henriksen, M.; Andreasen, J.; Christensen, R.; Nasser, M.; Ciliska, D.; Clarke, M.; et al. Evidence-Based Research Series-Paper 2: Using an Evidence-Based Research Approach before a New Study Is Conducted to Ensure Value. J. Clin. Epidemiol. 2021, 129, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Vander Weele, T.J.; Mathur, M.B.; Chen, Y. Outcome-Wide Longitudinal Designs for Causal Inference: A New Template for Empirical Studies. Stat. Sci. 2020, 35, 437–466. [Google Scholar] [CrossRef]
- Werth, B.L.; Christopher, S.-A. Potential Risk Factors for Constipation in the Community. World, J. Gastroenterol. 2021, 27, 2795–2817. [Google Scholar] [CrossRef] [PubMed]
- Al-mansouri, A.; Al-Ali, F.S.; Hamad, A.I.; Mohamed Ibrahim, M.I.; Kheir, N.; Ibrahim, R.A.; AlBakri, M.; Awaisu, A. Assessment of Treatment Burden and Its Impact on Quality of Life in Dialysis-Dependent and Pre-Dialysis Chronic Kidney Disease Patients. Res. Soc. Adm. Pharm. 2021, 17, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific Alterations in Gut Microbiota in Patients with Chronic Kidney Disease: An Updated Systematic Review. Ren. Fail. 2021, 43, 102–112. [Google Scholar] [CrossRef]
- Bagasha, P.; Leng, M.; Katabira, E.; Petrova, M. Health-Related Quality of Life, Palliative Care Needs and 12-Month Survival among Patients with End Stage Renal Disease in Uganda: Protocol for a Mixed Methods Longitudinal Study. BMC Nephrol. 2020, 21, 531. [Google Scholar] [CrossRef]
- Cooper, T.E.; Dalton, A.; Kieu, A.; Howell, M.; Jayanti, S.; Khalid, R.; Lim, W.H.; Scholes-Robertson, N.; Craig, J.C.; Teixeira-Pinto, A.; et al. The CKD Bowel Health Study: Understanding the Bowel Health and Gastrointestinal Symptom Management in Patients with Chronic Kidney Disease: A Mixed-Methods Observational Longitudinal Study (Protocol). BMC Nephrol. 2021, 22, 388. [Google Scholar] [CrossRef]
- Nor, N.M.N.; Lee, Y.Y.; Adnan, A.S.; Nor, A.Y.; Hamid, N.A.A.H.; Hamid, N.; Abdullah, N.; Wong, M.S.; Jayaraman, T.; Hassan, W.H.H.W.; et al. OE-0461 (PE-0571) The Impact of Gastroesophageal Reflux Disease, Irritable Bowel Syndrome, and Functional Constipation on Health-Related Quality of Life in Patients with Chronic Kidney Disease. J. Gastroenterol. Hepatol. 2018, 33, 490. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A New Improved Graphical and Quantitative Method for Detecting Bias in Meta-Analysis. Int. J. Evid. Based. Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of Two Methods to Detect Publication Bias in Meta-Analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Metasens: Statistical Methods for Sensitivity Analysis in Meta-Analysis. 2022. Available online: https://github.com/guido-s/metasens (accessed on 18 September 2022).
- Sison, C.P.; Glaz, J. Simultaneous Confidence Intervals and Sample Size Determination for Multinomial Proportions. J. Am. Stat. Assoc. 1995, 90, 366–369. [Google Scholar] [CrossRef]
- Hilton Boon, M.; Thomson, H.; Shaw, B.; Akl, E.A.; Lhachimi, S.K.; López-Alcalde, J.; Klugar, M.; Choi, L.; Saz-Parkinson, Z.; Mustafa, R.A.; et al. Challenges in Applying the GRADE Approach in Public Health Guidelines and Systematic Reviews: A Concept Article from the GRADE Public Health Group. J. Clin. Epidemiol. 2021, 135, 42–53. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C.; Chu, H. Empirical Comparisons of 12 Meta-Analysis Methods for Synthesizing Proportions of Binary Outcomes. J. Gen. Intern. Med. 2022, 37, 308. [Google Scholar] [CrossRef]
- Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J.; Rücker, G. Seriously Misleading Results Using Inverse of Freeman-Tukey Double Arcsine Transformation in Meta-Analysis of Single Proportions. Res. Synth. Methods 2019, 10, 476–483. [Google Scholar] [CrossRef]
- Borges Migliavaca, C.; Stein, C.; Colpani, V.; Barker, T.H.; Munn, Z.; Falavigna, M. How Are Systematic Reviews of Prevalence Conducted? A Methodological Study. BMC Med. Res. Methodol. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Lash, J.P.; Go, A.S.; Appel, L.J.; He, J.; Ojo, A.; Rahman, M.; Townsend, R.R.; Xie, D.; Cifelli, D.; Cohan, J.; et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin. J. Am. Soc. Nephrol. 2009, 4, 1302–1311. [Google Scholar] [CrossRef]
- Senanayake, S.; Gunawardena, N.; Palihawadana, P.; Senanayake, S.; Karunarathna, R.; Kumara, P.; Kularatna, S. Health Related Quality of Life in Chronic Kidney Disease; A Descriptive Study in a Rural Sri Lankan Community Affected by Chronic Kidney Disease. Health Qual. Life Outcomes 2020, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peppas, G.; Alexiou, V.G.; Mourtzoukou, E.; Falagas, M.E. Epidemiology of Constipation in Europe and Oceania: A Systematic Review. BMC Gastroenterol. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Iraji, N.; Keshteli, A.H.; Sadeghpour, S.; Daneshpajouhnejad, P.; Fazel, M.; Adibi, P. Constipation in Iran: SEPAHAN Systematic Review No. 5. Int. J. Prev. Med. 2012, 3, S34. [Google Scholar] [PubMed]
- Higgins, P.D.R.; Johanson, J.F. Epidemiology of Constipation in North America: A Systematic Review. Am. J. Gastroenterol. 2004, 99, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Suares, N.C.; Ford, A.C. Prevalence of, and Risk Factors for, Chronic Idiopathic Constipation in the Community: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2011, 106, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.Q.; De Gouveia Santos, V.L.C. Prevalence of Constipation in the General Adult Population: An Integrative Review. J. Wound Ostomy Cont. Nurs. 2014, 41, 70–76. [Google Scholar] [CrossRef]
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global Prevalence of Functional Constipation According to the Rome Criteria: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef]
| Authors, Reference | Total (N Analyzed) | Bristol Stool Form Scale | ||
|---|---|---|---|---|
| Type 1–2 | Type 3–5 | Type 6–7 | ||
| CKD G1–2 | ||||
| Gryp, T.; et al. [33] | 37 | 4 | 28 | 5 |
| Ruszkowski, J.; et al. [9,46] | 13 | 3 | 10 | 0 |
| Prevalence (95% CI) a | 14.8% (0.8–37.5) | 77.2% (51.8–94.9) | 8.0% (0–24.2) | |
| CKD G3 | ||||
| Gryp, T.; et al. [33] | 44 | 7 | 27 | 10 |
| Ramos, C.I.; et al. [40] | 6 | 1 | 5 | 0 |
| Ruszkowski, J.; et al. [9,46] | 62 | 19 | 37 | 6 |
| Prevalence (95% CI) b | 23.0% (10.9–37.3) | 63.8% (47.5–77.6) | 13.2% (4.1–25.5) | |
| CKD G4–5 | ||||
| Gryp, T.; et al. [33] | 33 | 9 | 18 | 6 |
| Meade, A.; et al. [47] | 134 | 18 | 98 | 18 |
| Ramos, C.I.; et al. [40] | 36 | 13 | 18 | 5 |
| Ruszkowski, J.; et al. [9,46] | 22 | 6 | 12 | 4 |
| Lee, A.; et al. [55] | 21 | 4 | 17 | |
| Prevalence (95% CI) c | 24.5% (13.2–37.8) | 59.8% (45.3–73.2) | 15.7% (6.5–27.6) | |
| CKD: eGFR unknown | ||||
| Trimingham, C.; et al. [12] | 95 | 9 | 77 | 9 |
| Prevalence (95% CI) d | 9.5% (3.2–17.6) | 81.1% (74.7–89.1) | 9.5% (3.2–17.6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruszkowski, J.; Majkutewicz, K.; Heleniak, Z.; Witkowski, J.M.; Dębska-Ślizień, A. Prevalence and Severity of Lower Gastrointestinal Symptoms amongst Non-Dialysis Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6363. https://doi.org/10.3390/jcm11216363
Ruszkowski J, Majkutewicz K, Heleniak Z, Witkowski JM, Dębska-Ślizień A. Prevalence and Severity of Lower Gastrointestinal Symptoms amongst Non-Dialysis Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(21):6363. https://doi.org/10.3390/jcm11216363
Chicago/Turabian StyleRuszkowski, Jakub, Katarzyna Majkutewicz, Zbigniew Heleniak, Jacek M. Witkowski, and Alicja Dębska-Ślizień. 2022. "Prevalence and Severity of Lower Gastrointestinal Symptoms amongst Non-Dialysis Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 21: 6363. https://doi.org/10.3390/jcm11216363
APA StyleRuszkowski, J., Majkutewicz, K., Heleniak, Z., Witkowski, J. M., & Dębska-Ślizień, A. (2022). Prevalence and Severity of Lower Gastrointestinal Symptoms amongst Non-Dialysis Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(21), 6363. https://doi.org/10.3390/jcm11216363







