Reduced Risk of Progression from Non-Severe to Severe COVID-19 in Hospitalized Dialysis Patients by Full COVID-19 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Full COVID-19 Vaccination
2.3. Diagnosis of COVID-19
2.4. COVID-19 Severity Classification
2.5. Management of COVID-19 during Hospitalization
2.6. Outcome
2.7. Other Clinical Parameters
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Clinical Characteristics of Dialysis Patients, and Comparisons between Those with and without Fully Vaccinated Status
3.3. Medications during Hospitalization Used for COVID-19
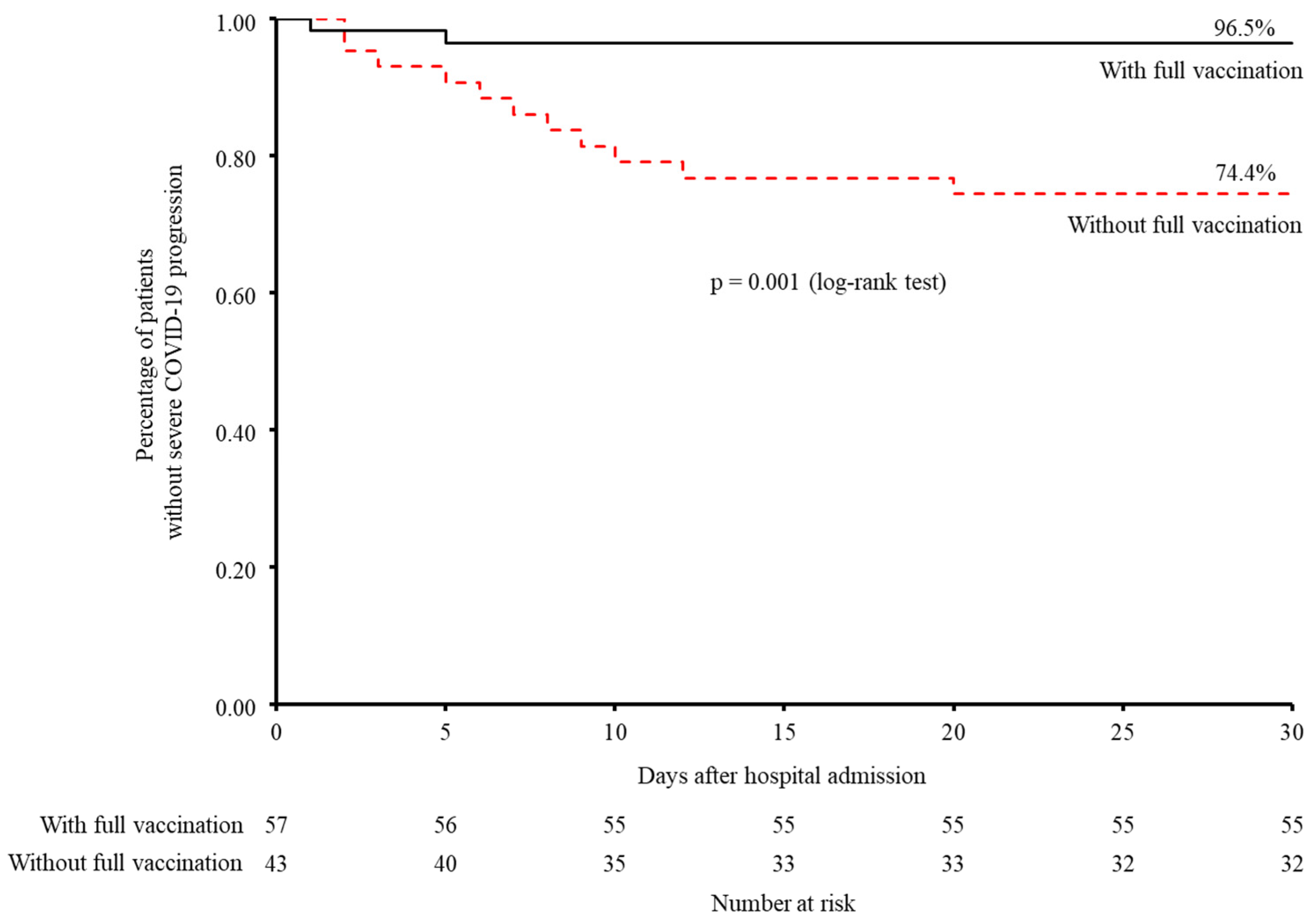
3.4. Progression from Non-Severe to Severe COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 31 August 2022).
- De Meester, J.; De Bacquer, D.; Naesens, M.; Meijers, B.; Couttenye, M.M.; De Vriese, A.S.; Group, N.K.R. Incidence, Characteristics, and Outcome of COVID-19 in Adults on Kidney Replacement Therapy: A Regionwide Registry Study. J. Am. Soc. Nephrol. 2021, 32, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kramer, A.; Chesnaye, N.C.; Couchoud, C.; Sanchez-Alvarez, J.E.; Garneata, L.; Collart, F.; Hemmelder, M.H.; Ambuhl, P.; Kerschbaum, J.; et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020, 98, 1540–1548. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernan, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Tang, L.; Hijano, D.R.; Gaur, A.H.; Geiger, T.L.; Neufeld, E.J.; Hoffman, J.M.; Hayden, R.T. Asymptomatic and Symptomatic SARS-CoV-2 Infections After BNT162b2 Vaccination in a Routinely Screened Workforce. JAMA 2021, 325, 2500–2502. [Google Scholar] [CrossRef]
- Yau, K.; Abe, K.T.; Naimark, D.; Oliver, M.J.; Perl, J.; Leis, J.A.; Bolotin, S.; Tran, V.; Mullin, S.I.; Shadowitz, E.; et al. Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis. JAMA Netw. Open 2021, 4, e2123622. [Google Scholar] [CrossRef]
- Van Praet, J.; Reynders, M.; De Bacquer, D.; Viaene, L.; Schoutteten, M.K.; Caluwe, R.; Doubel, P.; Heylen, L.; De Bel, A.V.; Van Vlem, B.; et al. Predictors and Dynamics of the Humoral and Cellular Immune Response to SARS-CoV-2 mRNA Vaccines in Hemodialysis Patients: A Multicenter Observational Study. J. Am. Soc. Nephrol. 2021, 32, 3208–3220. [Google Scholar] [CrossRef]
- Toda, M.; Yoshifuji, A.; Kikuchi, K.; Koinuma, M.; Komatsu, M.; Fujii, K.; Kato, A.; Kikuchi, T.; Nakazawa, A.; Ryuzaki, M. Factors associated with SARS-CoV-2 antibody titers and prognosis of breakthrough infection in hemodialysis patients. Clin. Exp. Nephrol. 2022, 26, 571–580. [Google Scholar] [CrossRef]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chen, J.; et al. Outcome and effect of vaccination in SARS-CoV-2 Omicron infection in hemodialysis patients: A cohort study. Nephrol. Dial. Transplant. 2022, 37, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chen, J.; et al. Severity of COVID-19 after Vaccination among Hemodialysis Patients: An Observational Cohort Study. Clin. J. Am. Soc. Nephrol. 2022, 17, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Nangaku, M.; Ryuzaki, M.; Yamakawa, T.; Yoshihiro, O.; Hanafusa, N.; Sakai, K.; Kanno, Y.; Ando, R.; Shinoda, T.; et al. Effectiveness of SARS-CoV-2 vaccines on hemodialysis patients in Japan: A nationwide cohort study. Ther. Apher. Dial. 2022. [Google Scholar] [CrossRef]
- Oliver, M.J.; Thomas, D.; Balamchi, S.; Ip, J.; Naylor, K.; Dixon, S.N.; McArthur, E.; Kwong, J.; Perl, J.; Atiquzzaman, M.; et al. Vaccine Effectiveness Against SARS-CoV-2 Infection and Severe Outcomes in the Maintenance Dialysis Population in Ontario, Canada. J. Am. Soc. Nephrol. 2022, 33, 839–849. [Google Scholar] [CrossRef]
- Bell, S.; Campbell, J.; Lambourg, E.; Watters, C.; O’Neil, M.; Almond, A.; Buck, K.; Carr, E.J.; Clark, L.; Cousland, Z.; et al. The Impact of Vaccination on Incidence and Outcomes of SARS-CoV-2 Infection in Patients with Kidney Failure in Scotland. J. Am. Soc. Nephrol. 2022, 33, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, G.; Fantini, M.; Righini, M.; Flachi, M.; Semprini, S.; Hu, L.; Chiappo, F.; Veterani, B.; Ambri, K.; Ferrini, F.; et al. Efficacy of SARS-CoV-2 Vaccination in Dialysis Patients: Epidemiological Analysis and Evaluation of the Clinical Progress. J. Clin. Med. 2022, 11, 4723. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Abhilash, K.P.P.; Mathiyalagan, P.; Krishnaraj, V.R.K.; Selvan, S.; Kanagarajan, R.; Reddy, N.P.; Rajendiran, N.; Hazra, D.; Gunasekaran, K.; Moorthy, M.; et al. Impact of prior vaccination with Covishield(TM) and Covaxin(R) on mortality among symptomatic COVID-19 patients during the second wave of the pandemic in South India during April and May 2021: A cohort study. Vaccine 2022, 40, 2107–2113. [Google Scholar] [CrossRef]
- Japanese Ministry of Health, Labour and Welfare. Clinical Management of Patients with COVID-19: A Guide for Front-Line Healthcare Workers; Version 4; Japanese Ministry of Health, Labour and Welfare: Tokyo, Japan, 2020. [Google Scholar]
- Japanese Ministry of Health, Labour and Welfare. Clinical Management of Patients with COVID-19: A Guide for Front-Line Healthcare Workers; Version 6; Japanese Ministry of Health, Labour and Welfare: Tokyo, Japan, 2021. [Google Scholar]
- Japanese Ministry of Health, Labour and Welfare. Clinical Management of Patients with COVID-19: A Guide for Front-Line Healthcare Workers; Version 7; Japanese Ministry of Health, Labour and Welfare: Tokyo, Japan, 2022. [Google Scholar]
- Numaguchi, R.; Kurajoh, M.; Hiura, Y.; Imai, T.; Morioka, T.; Saito, M.; Shiraishi, S.; Emoto, M.; Nishiguchi, Y. Glycated hemoglobin level on admission associated with progression to severe disease in hospitalized patients with non-severe coronavirus disease 2019. J. Diabetes Investig. 2022, 13, 1779–1787. [Google Scholar] [CrossRef]
- Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus; Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Daida, H.; Dohi, S.; Egusa, G.; Hiro, T.; Hirobe, K.; Iida, M.; et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J. Atheroscler. Thromb. 2013, 20, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [Green Version]
- Udomkarnjananun, S.; Takkavatakarn, K.; Praditpornsilpa, K.; Nader, C.; Eiam-Ong, S.; Jaber, B.L.; Susantitaphong, P. Hepatitis B virus vaccine immune response and mortality in dialysis patients: A meta-analysis. J. Nephrol. 2020, 33, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Collado, S.; Mir, M.; Cao, H.; Barbosa, F.; Serra, C.; Hidalgo, C.; Faura, A.; Montero, M.; Garcia de Lomas, J.; et al. Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2208–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, C.; Patel, P.; Pilishvili, T.; Moore, M.; Murphy, T.R.S. Guidelines for Vaccinating Kidney Dialysis Patients and Patients with Chronic Kidney Disease, Atlanta, GA, Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/dialysis/pdfs/vaccinating_dialysis_patients_and_patients_dec2012 (accessed on 31 August 2022).
- Hecking, M.; Karaboyas, A.; Rayner, H.; Saran, R.; Sen, A.; Inaba, M.; Bommer, J.; Horl, W.H.; Pisoni, R.L.; Robinson, B.M.; et al. Dialysate sodium prescription and blood pressure in hemodialysis patients. Am. J. Hypertens. 2014, 27, 1160–1169. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 31 August 2022).
- Xu, Y.; Chen, Y.; Tang, X. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Glob. Health Med. 2020, 2, 66–72. [Google Scholar] [CrossRef]

| Total (n = 100) | With Full Vaccination (n = 57) | Without Full Vaccination (n = 43) | ASD | p Value | |
|---|---|---|---|---|---|
| Age, years | 71.0 (62.0–76.0) | 72.0 (62.0–78.0) | 70.0 (63.0–74.0) | 0.045 | 0.482 |
| Male | 64 (64) | 37 (64.9) | 27 (62.8) | 0.044 | 0.827 |
| BMI, kg/m2 | 22.0 (19.5–24.4) | 21.1 (19.0–24.2) | 22.6 (20.6–24.4) | 0.136 | 0.193 |
| Dialysis duration, years | 6.0 (2.8–15.3) | 6.0 (2.0–16.0) | 7.0 (3.0–15.0) | 0.020 | 0.727 |
| Diabetes | 46 (46) | 24 (42.1) | 22 (51.2) | 0.182 | 0.368 |
| Hypertension | 81 (81) | 45 (78.9) | 36 (83.7) | 0.123 | 0.547 |
| Dyslipidemia | 50 (50) | 28 (49.1) | 22 (51.2) | 0.041 | 0.84 |
| Cardiovascular disease | 70 (70) | 42 (73.7) | 28 (65.1) | 0.187 | 0.355 |
| Chronic respiratory disease | 13 (13) | 8 (14.0) | 5 (11.6) | 0.072 | 0.723 |
| Use of ACE inhibitors/ARBs | 47 (47) | 29 (50.9) | 18 (41.9) | 0.182 | 0.371 |
| Use of immunosuppressants | 5 (5) | 3 (5.3) | 2 (4.7) | 0.028 | 0.889 |
| Days from disease onset to hospitalization | 3.0 (1.0–4.0) | 3.0 (1.0–3.0) | 3.0 (1.0–5.0) | 0.092 | 0.774 |
| COVID-19 severity on admission | 0.135 | 0.802 | |||
| Mild | 41 (41) | 22 (38.6) | 19 (44.2) | ||
| Moderate I | 48 (48) | 29 (50.9) | 19 (44.2) | ||
| Moderate II | 11 (11) | 6 (10.5) | 5 (11.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichii, M.; Kurajoh, M.; Okute, Y.; Ihara, Y.; Imai, T.; Morioka, T.; Mori, K.; Shoji, T.; Tsujimoto, Y.; Ubai, T.; et al. Reduced Risk of Progression from Non-Severe to Severe COVID-19 in Hospitalized Dialysis Patients by Full COVID-19 Vaccination. J. Clin. Med. 2022, 11, 6348. https://doi.org/10.3390/jcm11216348
Ichii M, Kurajoh M, Okute Y, Ihara Y, Imai T, Morioka T, Mori K, Shoji T, Tsujimoto Y, Ubai T, et al. Reduced Risk of Progression from Non-Severe to Severe COVID-19 in Hospitalized Dialysis Patients by Full COVID-19 Vaccination. Journal of Clinical Medicine. 2022; 11(21):6348. https://doi.org/10.3390/jcm11216348
Chicago/Turabian StyleIchii, Mitsuru, Masafumi Kurajoh, Yujiro Okute, Yasutaka Ihara, Takumi Imai, Tomoaki Morioka, Katsuhito Mori, Tetsuo Shoji, Yoshihiro Tsujimoto, Takanobu Ubai, and et al. 2022. "Reduced Risk of Progression from Non-Severe to Severe COVID-19 in Hospitalized Dialysis Patients by Full COVID-19 Vaccination" Journal of Clinical Medicine 11, no. 21: 6348. https://doi.org/10.3390/jcm11216348
APA StyleIchii, M., Kurajoh, M., Okute, Y., Ihara, Y., Imai, T., Morioka, T., Mori, K., Shoji, T., Tsujimoto, Y., Ubai, T., & Emoto, M. (2022). Reduced Risk of Progression from Non-Severe to Severe COVID-19 in Hospitalized Dialysis Patients by Full COVID-19 Vaccination. Journal of Clinical Medicine, 11(21), 6348. https://doi.org/10.3390/jcm11216348






