Abstract
COVID-19 vaccine-associated lymphadenopathy (C19-VAL) is increasingly encountered with the widespread use of the vaccine in controlling the outbreak. We aim to characterize the pathological findings of COVID-19 and non-COVID-19 vaccine-associated lymphadenopathy (NC19-VAL). A search for studies that reported pathological findings in vaccine-associated lymphadenopathy on PubMed and Google Scholar was performed on 11 December 2021. C19-VAL studies were pooled for analysis. These studies were split into clinical lymphadenopathy (CL) and subclinical lymphadenopathy detected on imaging (SLDI) for subgroup analysis. A total of 25 studies were related to COVID-19 vaccines, and 21 studies were included in the pooled analysis. The pooled analysis included 37 patients with a mean age of 47.8 ± 19.1 years old, and 62.2% were females. The mean duration from last vaccination to development of CL/SLDI was 14.5 ± 11.0 days. Most were diagnosed as reactive or negative for malignancy (28/37, 75.5%), followed by Kikuchi–Fujimoto disease (KFD) (3/37, 8.1%), florid lymphoid hyperplasia (2/37, 5.4%), and granulomatous inflammation (2/37, 5.4%). Metastases were reported in two patients with a history of malignancy (2/37, 5.4%). Cases with florid lymphoid hyperplasia and KFD were younger than those with reactive changes. A total of 14 studies were related to non-COVID-19 vaccines. Caseating granulomatous inflammation was reported in BCG vaccine-associated lymphadenopathy, while other vaccines were associated with reactive lymphoid hyperplasia, florid post-vaccinal reactions, and KFD. Although most C19-VAL cases were reported as reactive or negative for malignancy, other diagnoses included florid lymphoid hyperplasia, KFD, and granulomatous inflammation. Metastases were reported in lymphadenopathy of patients with a history of malignancy, who had been incidentally vaccinated. In conclusion, C19-VAL can yield different histopathological diagnoses when sampled, most of which require clinical and radiological correlation for optimal patient management.
Keywords:
COVID-19; vaccine; lymphadenopathy; non-COVID-19; Kikuchi–Fujimoto disease; cancer; metastases 1. Introduction
The COVID-19 global pandemic, which was caused by a novel strain of coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in December 2019 [1]. At the time of writing, at least 276 million cases, with 5.3 million deaths, have been reported worldwide [2]. In August 2021, the United States Food and Drug Administration approved the use of the Pfizer-BioNTech vaccine, a messenger ribonucleic acid (mRNA) vaccine, in individuals aged 16 years and above [3]. Since then, more than 8 billion doses of vaccine have been administered [2]. The safety and efficacy of the vaccine was evaluated in a clinical trial of 43,548 participants, where it was reported that a two-dose regimen of Pfizer-BioNTech was 95% effective in preventing COVID-19 [4], although a decline in vaccine efficacy to 91.3% after 6 months of follow-up has been reported [5]. In the landmark trial by Polack et al. [4], 0.3% of vaccine recipients reported lymphadenopathy amongst other adverse events, including reduced appetite, lethargy, malaise, and night sweats. Higher rates of axillary swelling or tenderness were also reported in the Moderna vaccine [6], where 11.6% and 16.0% experienced these symptoms after the first and second dose, respectively.
Post-vaccinal lymphadenopathy due to reactive changes in the lymph nodes is well-described [7], and it was previously reported in a number of vaccines, including bacillus Calmette–Guerin (BCG) [8], hepatitis B [9], human papillomavirus [10], and tetanus [11], amongst several others. However, SARS-CoV-2 vaccines are the first mRNA vaccines to be approved for clinical application. SARS-CoV-2 vaccines work primarily through the delivery of an mRNA into cells, where the mRNA is translated into a target protein, against which the immune system will mount an immune response, which includes antigen presentation in the regional lymph nodes, the priming of CD4+ and CD8+ T cells, germinal center reaction, and finally the formation of affinity-matured memory B cells and antibody-secreting long-lived plasma cells [12]. In a study of antigen-specific B cells in the peripheral blood and fine-needle aspirates (FNA) of draining lymph nodes from 14 participants that had received two doses of BNT162b2 (Pfizer-BioNTech) vaccine [13], a strong plasmablast response in the blood and a robust germinal center B cell and plasmablast response in the aspirates were reported, and they were persistent for at least 12 weeks after the second dose of vaccine, which is an indicator of developing a potent humoral response [14].
COVID-19 vaccine-associated lymphadenopathy (C19-VAL) is often detected either clinically as a palpable and/or painful lump or on screening and follow-up radiological studies, including breast ultrasound [15] and positron emission tomography/computed tomography (PET/CT) [16], where hypermetabolic axillary lymph nodes occurred in 37.0% of patients who had received the COVID-19 vaccine. A meta-analysis [17] of 68 cases of C19-VAL with imaging findings reported that the median duration from vaccination to development of lymphadenopathy was 12 and 5 days for the first and second dose, respectively, of which 42.6% showed diffuse or focal cortical thickening on ultrasound. Furthermore, an increased uptake on PET/CT was reported in 26.4%, with a mean maximum dimension of lymph nodes reported to be 20.9 mm [17]. Although some of these radiological findings may favor a reactive lymphadenopathy, studies have also reported suspicious ultrasound findings in lymphadenopathy after COVID-19 vaccination in patients being followed up for skin cancer [18], raising a diagnostic conundrum between a reactive and a malignant process. Similar diagnostic dilemmas occur in breast cancer screening, which involves a significant number of healthy women [15].
Histopathological and cytopathological findings, obtained through procedures including FNA and core needle biopsy (CNB), are only reported in a proportion of C19-VAL, and these findings remain to be characterized. Hence, in this review, we aim to identify the current studies that have reported pathological findings of C19-VAL, and NC19-VAL for comparison.
2. Material and Methods
2.1. Search Strategy
We performed a literature search in accordance with the PRISMA statement [19], through PubMed and Google Scholar, on 11 December 2021. The keywords included ‘vaccine’ and ‘lymphadenopathy’. We placed no restrictions on the year of publication. We searched reference lists of full-text articles for snowballing of additional studies that were not identified in the initial search.
2.2. Inclusion and Exclusion Criteria
We included studies that reported histopathological and/or cytological findings in vaccine-related lymphadenopathy, without any restrictions on the type of studies. We also included preprint articles if they met the abovementioned criteria. We excluded review, recommendation, and non-English articles.
2.3. Data Extraction and Analysis
We included studies that reported the clinical history, investigation, and pathological findings of individual patients in the pooled analysis. We extracted the pertinent details from each study, and these included: the type of publication, number of patients with pathological findings, age and sex of patients, significant history, type and dose of most recent vaccine, whether the lymphadenopathy was detected clinically or on imaging, site of vaccine, other clinical symptoms, duration from last vaccination to lymphadenopathy, laterality of lymphadenopathy compared with site of vaccination, site of lymphadenopathy, largest dimension of lymph node, ultrasound findings, additional radiological findings, indication for aspiration or biopsy, type of procedure performed, pathological diagnosis, and management and outcome. We performed pooled analysis of the variables and expressed these variables either in means and standard deviations or in percentages.
As patients presenting with clinical lymphadenopathy (CL) represent a distinct population of patients compared with those with subclinical lymphadenopathy detected on imaging (SLDI), we analyzed these two groups separately. SLDI patients were detected on routine imaging follow-up for conditions including a history of malignancy, or screening for malignancy such as breast cancer. We also split patients with CL into subgroups according to their pathological diagnosis (reactive changes or negative for malignancy, florid lymphoid hyperplasia, KFD, etc.), and analyzed separately. We used the t-test to compare the means and standard deviations (www.medcalc.org, accessed on 4 January 2022). We used the chi-square to compare the percentages. We considered a p-value of less than or equal to 0.05 as statistically significant.
3. Results
3.1. Literature Search
An initial search of PubMed and Google Scholar yielded a total of 618 results, with 77 duplicates, and 541 results were screened (Figure 1). After excluding 428 articles, 108 full-text articles were obtained and assessed for eligibility. After excluding 74 articles for the reasons stated in Figure 1, and with the addition of 5 articles identified from the reference lists of articles, 39 articles were included in this review and 21 COVID-19 studies were included in the pooled analysis. A total of 25 studies [18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] were relevant to the COVID-19 vaccine and 14 studies [7,8,9,10,44,45,46,47,48,49,50,51,52,53] were related to non-COVID-19 vaccines.
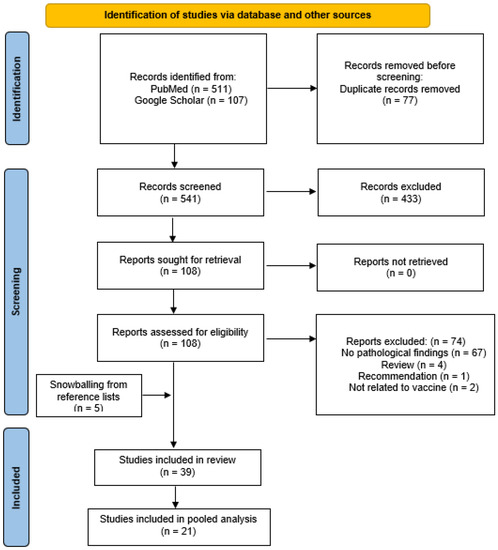
Figure 1.
PRISMA diagram.
3.2. COVID-19 Vaccine-Associated Lymphadenopathy Study Characteristics
The 25 studies were published by authors from countries including Germany [18] (n = 1), Israel [22,37] (n = 2), Japan [26] (n = 1), Portugal [20] (n = 1), Qatar [31] (n = 1), Singapore [32,33] (n = 2), South Korea [27,30,39] (n = 3), Spain [23,24,41] (n = 3), Switzerland [21] (n = 1), the United Kingdom [36] (UK) (n = 1), and the United States of America [25,28,29,34,35,38,40,43] (USA) (n = 8). Most studies are case reports (n = 12, 48.0%), followed by case series (n = 9, 36.0%), retrospective studies (n = 3, 12.0%), and not reported in one study (4.0%).
3.3. Pooled Analysis of COVID-19 Vaccine-Associated Lymphadenopathy
Findings from the pooled analysis are summarized in Table 1. Pooled analysis of 21 studies [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] included 37 patients with a mean age of 47.8 ± 19.1 years old, of which 23 were female (23/37, 62.2%). The largest proportion of patients (9/37, 24.3%) had no prior medical history, followed by 21.6% (8/37) with a history of melanoma, and 18.9% (7/37) with a history of breast cancer. The other patients had a history of lung cancer, appendix neuroendocrine tumor, Merkel cell carcinoma, and renal cell carcinoma. The largest proportion of C19-VAL occurred after the first dose (13/37, 35.1%), followed by the second dose of Pfizer-Bio-NTech vaccine (7/37, 18.9%). Other vaccines that were reported in these patients included Moderna, AstraZeneca, Vaxzevria, and CureVac.

Table 1.
Pooled analysis. CL, clinical lymphadenopathy; SLDI, subclinical lymphadenopathy detected on imaging; SD, standard deviation; N, number of; NET, neuroendocrine tumor; RCC, renal cell carcinoma; US, ultrasound; PET/CT, positron emission tomography/computed tomography; MRI, magnetic resonance imaging; KFD, Kikuchi–Fujimoto Disease; FNA, fine-needle aspiration.
The mean duration from the last vaccination to the development of CL or SLDI was 14.5 ± 11.0 days. Most cases of lymphadenopathy (23/37, 62.2%) occurred ipsilateral to the site of vaccination, while two cases (2/37, 5.4%) were contralateral, with the laterality not reported in the remaining cases. With the exclusion of Hagen et al. [21] due to the lack of individual sites of lymphadenopathy reported for each patient, most cases (18/32, 56.3%) had axillary lymphadenopathy, followed by 21.9% (7/32) with supraclavicular lymphadenopathy, and 12.5% (4/32) with cervical lymphadenopathy. Additional clinical symptoms aside from lymphadenopathy were reported in these cases. Overall, six patients (6/37, 16.2%) experienced fever [25,30,33,34], while four patients (4/37, 10.8%) also reported pain [26,27,28,29]. The other described symptoms included fatigue or malaise (2/37, 5.4%), myalgia (2/37, 5.4%), dysphagia (1/37, 2.7%), chills (1/37, 2.7%), and other symptoms such as vomiting (2/37, 5.4%).
The mean largest dimension of lymph node reported clinically or on radiologic studies was 20.8 ± 13.3 mm. Abnormal lymph node findings were reported using ultrasound in 29.7%, computed tomography/magnetic resonance imaging (CT/MRI) in 29.7%, and PET/CT in 21.6%. In terms of indication for aspiration or biopsy, 40.5% (15/37) of these procedures were performed for suspicion of malignancy, followed by other indications including suspicion of lymphadenitis and/or KFD (2/37, 5.4%), palpable mass (2/37, 5.4%), and patient’s preference (2/37, 5.4%). Other indications were family history of malignancy (1/37, 2.7%) and further oncologic management (1/37, 2.7%). Most reported cases had either a CNB (12/37, 32.4%) or excisional biopsy (12/37, 32.4%) performed for the pathological examination of lymphadenopathy, followed by FNA (9/37, 24.3%). The other procedures that were performed included surgical resection and complete lymphadenectomy [18].
Most cases of lymphadenopathy that were sent for pathological examination were diagnosed as reactive or negative for malignancy (28/37, 75.5%). Other histopathological findings were as follows: florid lymphoid hyperplasia in two patients (2/37, 5.4%); KFD in three patients [31,33] (3/37, 8.1%); granulomatous inflammation in two patients [18,42] (2/37, 5.4%); and metastases in two patients [18] (2/37, 5.4%). Further details are elaborated in the following sections.
3.4. Clinical Lymphadenopathy (CL)
A total of 15 studies reported pathological findings of C19-VAL. There was a total of eight case reports [20,25,26,27,30,31,32,34], five case series [21,24,28,29,33], one retrospective study [22], and one study type was not reported. Our findings are summarized in Table 2.

Table 2.
Findings of clinical lymphadenopathy (CL) studies. NR, not reported; F, female; M, male; US, ultrasound; PET/CT, positron emission tomography/computed tomography; MRI, magnetic resonance imaging; FNA, fine-needle aspiration.
Three studies [22,23,24] were not included in the pooled analysis as individual patient data was not reported. Each one of these three studies reported five to eleven patients, of which those in Faermann et al. [22] had a history of breast cancer or were BRCA carriers, and included patients with both CL and SLDI. The vaccines that were used in these studies included Pfizer-Bio-NTech and Moderna. The lymphadenopathy was ipsilateral to the site of vaccination and occurred in the axillary and supraclavicular regions. Further investigation of the lymphadenopathy was performed due to a suspicion of malignancy [22,23]. In terms of pathological findings obtained by ultrasound-guided core biopsy or FNA, these three studies reported reactive findings, with Felices-Farias et al. [23] describing reactive paracortical and interfollicular hyperplasia and Fernandez-Prada et al. [24] reporting reactive lymphocytic infiltrate and active germinal centers.
The pooled analysis of 18 cases with CL showed a mean age of 37.8 ± 15.6 years old, with 50.0% (9/18) females (Table 1). Half of these patients had no prior medical history, while three patients (3/18, 16.7%) had prior non-neoplastic medical history, including asthma, eczema, and hypothyroidism in Tintle et al. [34], steroid-dependent minimal-change renal disease in Soub et al. [31], and diabetes mellitus and hypertension in Tan et al. [33]. The other cases had a family history of breast cancer [20,29], lung cancer [21], and appendix neuroendocrine tumor (NET) [21].
Most cases of lymphadenopathy occurred ipsilaterally to the site of vaccination, with contra-laterality reported in two cases (2/18, 11.1%) [21,30]. The most common site of lymphadenopathy was the supraclavicular region (6/13, 46.2%), followed by the axillary (4/13, 30.8%) and cervical regions (3/13, 23.1%). The most reported associated symptoms included fever (6/13, 46.2%), pain (4/13, 30.8%), as well as other symptoms mentioned in Table 1 and Table 2. The mean largest dimension of lymph node reported was 21.1 ± 14.7 mm.
In terms of radiological findings, eight patients (8/18, 44.4%) had abnormal CT findings, which included ‘irregular thickening and inflammation in the sternocleidomastoid area’ [25] and conglomerated lymph nodes with perinodal infiltration [30], while other CT findings mainly reported that the lymph nodes were enlarged. Seven patients (7/18, 38.9%) had abnormal US findings, which included the loss of a defined hilum or a partially detectable hilum [20,21,26,27,32], and ill-defined borders [27]. Two patients (2/18, 11.1%) had abnormal PET/CT findings with increased FDG uptake [21].
FNA and CNB were most frequently performed for further pathological investigation, with the most commonly reported indication being suspicion of malignancy (8/18, 44.4%). Most cases of CL were reported as either reactive or negative for malignancy (13/18, 72.2%), two cases (2/18, 11.1%) were reported to have florid lymphoid hyperplasia, and KFD was reported in three patients [31,33] (3/18, 16.7%). Notably, Cardoso et al. [20] reported atypical lymphoid findings using FNA; however, the subsequent biopsy disclosed reactive follicular hyperplasia. Similarly, Larkin et al. [28] reported that there was a focal increase in Epstein–Barr Virus (EBV)-positive cells, with other findings suggestive of a prior infection. In the two cases of florid lymphoid hyperplasia [28,34], Larkin et al. [28] also reported that there was progressive transformation of germinal centers, while Tintle et al. [34] reported Langerhans cell hyperplasia. The management and outcomes of CL are outlined in Table 2.
Cases diagnosed with reactive changes or negative for malignancy were compared with those diagnosed with florid lymphoid hyperplasia and KFD. Cases of florid lymphoid hyperplasia and KFD had a mean age of 18.0 ± 5.0 and 23.3 ± 7.5 years old, respectively, which are significantly younger than those diagnosed with reactive changes or as negative for malignancy (44.2 ± 13.1 years old, p = 0.02). The duration from the last vaccination to CL in the KFD cases was 20.7 ± 10.5 days, which is significantly longer than those diagnosed with reactive changes or as negative for malignancy (10.9 ± 6.3 days, p = 0.048). There was no statistically significant difference in the duration from last vaccination to CL between those diagnosed with reactive changes or as negative for malignancy (10.9 ± 6.3 days), and those with florid lymphoid hyperplasia (10.5 ± 3.5 days). The largest dimension of lymph node did not differ significantly amongst these three diagnoses (reactive changes or negative for malignancy: 22.1 ± 18.2 mm, florid lymphoid hyperplasia: 15.5 ± 5.5 mm, KFD: 22.3 ± 7.1 mm).
3.5. Subclinical Lymphadenopathy Detected on Imaging (SLDI)
The findings are summarized in Table 3. A total of eleven studies reported the pathological findings of SLDI, and these included five case series [18,29,36,38,39], four case reports [35,40,41,42], and two retrospective studies [37,43]. Robinson et al. [43] reported a breast cancer patient with SLDI and a biopsy that was negative for malignancy; however, this report was excluded from the pooled analysis as individual patient data was not reported. There were 19 patients identified from these studies with a mean age of 57.2 ± 17.3 years old, which is significantly older than the cases with CL (37.8 ± 15.6 years, p = 0.001). Most of these patients were females (14/19, 73.7%). All patients had a prior history of malignancy, with a history of melanoma [18,40,42] in 42.1% (8/19), breast cancer [29,36,37,38,39] in 36.8% (7/19), and Merkel cell carcinoma [18] in 10.5% (2/19), with other malignancies including cecum-appendix NET [41] and renal cell carcinoma [35].

Table 3.
Findings of subclinical lymphadenopathy detected on imaging (SLDI) studies. NR, not reported; F, female; M, male; US, ultrasound; PET/CT, positron emission tomography/computed tomography; MRI, magnetic resonance imaging; FNA, fine-needle aspiration.
Most cases were associated with the Pfizer-Bio-Ntech vaccine, with equal proportions associated with the first and second dose (31.6%). The other vaccines implicated in these patients with SLDI are presented in Table 1 and Table 3. The mean duration from last vaccination to SLDI was 16.5 ± 12.9 days; however, this was not significantly different from the mean duration from last vaccination to CL (12.5 ± 7.9 days). Most cases of SLDI occurred ipsilateral to the site of vaccination (13/19, 68.4%), while the site of the remaining cases was not reported. The axillary region was the most commonly reported site of SLDI (14/19, 73.7%), and this is significantly higher than that reported in CL (4/13, 30.8%, p = 0.018). A total of 5.3% (1/19) of SLDI occurred in the supraclavicular region, which is significantly lower than that reported in CL (6/13, 46.2%, p = 0.0069). Of note, Trikannad et al. reported SLDI in the mediastinum [42].
The largest dimension of the lymph node was 19.7 ± 2.9 mm, which is not significantly different from that reported in CL. In terms of radiological findings, 31.6% (6/19) had abnormal PET/CT findings, while 21.1% (4/19) had abnormal US findings and 15.8% had abnormal CT/MRI findings. Abnormal PET/CT findings included FDG uptake or hypermetabolic lymph nodes [29,37,40,41,42], while abnormal US findings included thickened cortex [36,39]. Abnormal CT/MRI findings included a length/width ratio of less than 1.5 [39], cortical thickening [39], and asymmetricity [38].
The indications for aspiration or biopsy are summarized in Table 1 and Table 3. Excision biopsy was performed in 36.8% (7/19), CNB in 26.3% (5/19), and FNA in 10.5% (2/19). The other procedures (4/19, 21.1%) included complete lymphadenectomy and surgical resection.
The majority of SLDI was reactive and/or negative for malignancy (15/19, 78.9%). However, granulomatous inflammation was reported in two cases [18,42] (2/19, 10.5%) and metastases were reported in two cases (2/19, 10.5%) [18]. Trikannad et al. [42] reported non-caseating granulomas in the mediastinal FNA of a 57-year-old female with a history of melanoma. Placke et al. [18] reported a sarcoid-like reaction in a patient with a history of melanoma who underwent complete lymphadenectomy. Unfortunately, this patient experienced post-operative lymphorrhea requiring multiple sclerotherapies [18]. Placke et al. [18] described two patients (without further elaboration of whether these two patients had melanoma or Merkel cell carcinoma) in whom ultrasound of the SLDI could not exclude malignancy. Histopathological examination confirmed metastatic disease in each case.
3.6. Non-COVID-19 Vaccine-Associated Lymphadenopathy
Findings are summarized in Table 4. A total of 14 studies that reported pathological findings in NC19-VAL were identified, and these included nine case reports [9,10,11,44,45,47,48,50,51], three retrospective studies [7,8,53], one prospective study [46], and one case series [49]. Seven studies [8,9,44,45,46,47,53] reported on the BCG vaccine, while the others included hepatitis B [9], H1N1 [48], HPV [10,49], Japanese encephalitis virus (JEV) [10], measles [50], rubella [51], and tetanus vaccines [11]. Hartsock et al. [7] reported a range of vaccines including smallpox, cholera, typhus, tetanus, pertussis, Salk (polio), and influenza.

Table 4.
Findings from non-COVID-19 vaccine-associated lymphadenopathy. BCG, bacillus Calmette–Guérin; HPV, human papillomavirus; JEV, Japanese encephalitis virus.
The BCG studies predominantly described caseating granulomatous inflammation either on biopsy [8,9,45,47] or aspiration [8,46,53] of the involved lymph nodes. Of note, Dotlic et al. [9] reported a 2-week-old male with inguinal lymphadenopathy who received both the BCG and hepatitis B virus vaccines, with an initial FNA showing atypical lymphoid cells that were suspicious of lymphoma. Subsequently, the excision biopsy showed an effacement of nodal architecture, with an atypical T cell proliferation that showed an active cytotoxic phenotype, as well as a high proliferative index of 90% [9]. This case was initially diagnosed as a T cell lymphoma, possibly with a lymphoblastic subtype; however, further immunohistochemistry was negative for immature T cell markers, including TdT, CD34, and CD117 [9]. This case was subsequently diagnosed as BCG lymphadenitis with a reactive hyperimmune post-vaccinal reaction [9].
Lymphadenopathy associated with other vaccines showed mainly reactive lymphoid hyperplasia [7,49,50]; however, florid reactions to these vaccines may raise a concern for lymphomas. In the case of H1N1 vaccine-associated lymphadenopathy reported by Toy et al. [48], there were CD30-positive immunoblasts as well as large cells that showed a resemblance to Hodgkin cells, raising the differential diagnosis of Hodgkin lymphoma. This case was subsequently diagnosed as post-vaccinal lymphadenitis. White et al. [11] reported a case of tetanus-associated lymphadenopathy in a 50-year-old female, with excisional biopsy showing sheets of small-to-medium-sized lymphocytes and a flow cytometry study interpreted as atypical T cell population; however, the diagnosis was subsequently reviewed to ‘pseudolymphomatous florid proliferation of CD4 + T cells in response to tetanus toxoid immune stimulation’. KFD was reported by Watanabe et al. [10] in a 14-year-old female who received HPV and JEV vaccines.
4. Discussion
In this systematic review, we identified 25 studies that reported the pathological findings of C19-VAL, with 21 studies subsequently included in the pooled analysis, and 14 studies that reported pathological findings of NC19-VAL for comparison. The pooled analysis of the 21 C19-VAL studies included 37 patients with a mean age of 47.8 ± 19.1 years old, of which 62.2% were females. The mean duration from the last vaccination to the development of CL or SLDI was 14.5 ± 11.0 days. Most cases were diagnosed as reactive or negative for malignancy (75.5%), followed by KFD (8.1%), florid lymphoid hyperplasia (5.4%), and granulomatous inflammation (5.4%). Metastases were reported in two patients (5.4%) who had a history of malignancy.
Furthermore, cases with florid lymphoid hyperplasia and KFD were significantly younger than those with reactive changes or negative for malignancy. The duration from last vaccination to CL in KFD cases was significantly longer than those with reactive changes or negative for malignancy. The axillary region was the most commonly reported site of biopsy or FNA for SLDI while the supraclavicular region was the most commonly reported site of biopsy or FNA for CL. For NC19-VAL, caseating granulomatous inflammation was reported in BCG vaccine-associated lymphadenopathy, while other vaccines were associated with reactive lymphoid hyperplasia, florid post-vaccinal reactions, and KFD.
To the best of our knowledge, this is the first systematic review to characterize the pathological findings in C19-VAL. Two key patient populations are implicated: patients with CL and patients with SLDI, who have a history of malignancy and are on active follow-up. Most of the patients with CL had other symptoms and abnormal imaging findings, which may raise a suspicion of conditions other than post-vaccinal lymphadenitis. Regarding histopathological findings, patients with florid lymphoid hyperplasia and KFD were significantly younger than those with reactive changes or negative for malignancy, and patients with KFD developed lymphadenopathy significantly later than those with reactive changes or negative for malignancy. Although patients with KFD have been reported to be younger [54], these findings were based on a limited sample size and need to be interpreted in the appropriate context. Among patients with SLDI, although most cases were histologically diagnosed as reactive or negative for malignancy, granulomatous inflammation and metastases were also reported. Of note, metastases were reported in 2 out of 19 patients (10.5%) in this population. Given a clinical history of a previous malignancy, this differential diagnosis needs to be considered in any patient with C19-VAL. Despite the association of the COVID-19 vaccine with lymphadenopathies diagnosed histologically as KFD, and granulomatous inflammation, it remains unclear whether these conditions are related to the vaccine. The etiology and pathogenesis of KFD remains unclear, with viruses being postulated to be a key inciting agent [55,56]. On the other hand, the cases that were found to have metastatic lymphadenopathy were previously known to have a primary malignancy.
Several systematic reviews investigating the imaging findings in C19-VAL have been performed. Garreffa et al. [57] reported the incidence of clinical and subclinical lymphadenopathy to range from 14.5% to 53% of 2057 patients, and the lymphadenopathy persisted beyond 6 weeks in more than a quarter of these patients. Treglia et al. [16] performed a meta-analysis of 2354 patients who underwent PET/CT after COVID-19 vaccination and reported a prevalence of hypermetabolic axillary lymph nodes in 37% of these patients. Keshavarz et al. [17] performed a pooled analysis of 68 cases of C19-VAL and reported that cortical thickening was seen on the US in 42.6%, and other findings included preserved nodal hilar fat and necrotic patterns. The mean maximum dimension of the lymph nodes reported in the imaging modalities was 20.9 ± 5.8 mm, which is similar to what we have found in this systematic review. A systematic review reported that the cases of C19-VAL detected in patients undergoing follow-up PET/CT were ipsilateral to the vaccine injection site [58]. Brown et al. [59] also provided a narrative review of the imaging findings and reported that loss of normal fatty hilum can be expected on ultrasound. Numerous guidelines and recommendations [15,57,59,60] have been proposed for the management of C19-VAL. A case-by-case patient-centered approach has been suggested in determining whether further investigations or management are warranted in patients with a history of malignancy [15,57]. Diagnostic algorithms have also been suggested for better assessment of axillary lymphadenopathy [61,62], and follow-up with PET/CT [63].
In light of these current findings, it appears that the pathological findings of C19-VAL and NC19-VAL are similar. Traditionally, vaccines are classified as live vaccines, non-live vaccines, as well as viral vectors, RNA, DNA, and virus-like particles vaccines [64]. With the exception of the BCG vaccine that induces T cell responses (cellular immunity), all other routine vaccines confer immune protection through the production of antibodies mediated by B cells (humoral immunity) [64]. Once the vaccine antigen is introduced to the immune system, it is transported to the draining lymph nodes where antigen presentation activates T cells, which subsequently activates B cells, leading to a cascade of events that ultimately result in production of short-lived plasma cells that secrete antibodies in the first 2 weeks after vaccination, and memory B cells and long-lived plasma cells that produce antibodies for decades [64].
In this study, most of the cases were associated with the use of the Pfizer-Bio-Ntech vaccine, with more than one-third associated with the first dose. Numerous COVID-19 vaccines have been developed, and these include DNA and RNA vaccines (Pfizer-Bio-NTech, Moderna), adenoviral-vectored vaccines (AstraZeneca) and whole-cell-inactivated vaccines (Sinovac, Sinopharm) [65]. These vaccines are designed to induce an immune response that is mediated by neutralizing antibodies against the SARS-CoV-2 spike protein [65]. The immune responses induced by COVID-19 vaccines are similar to non-COVID-19 vaccines [12,65,66]. In addition to neutralizing bodies, T cell responses are also implicated in conferring protection, although the exact mechanisms remain undetermined and may influence whether booster doses are necessary or not in the future [65]. The BCG vaccine has also been proposed to be involved in the induction of COVID-19 vaccine immune responses through ‘trained immunity’, where monocytes and natural killer cells undergo epigenetic changes to mount an enhanced response against pathogens [67,68].
COVID-19 vaccines have also been associated with lymphoproliferative disorders [69,70,71] and hyperinflammatory syndromes [72].
Goldman et al. [71] described a case of a 66-year-old man who presented with moderate asthenia and mild inflammatory syndrome without abnormal blood cell counts 6 months after the second dose of an mRNA COVID-19 vaccine. Using PET/CT, he was found to have hypermetabolic adenopathies above and below the diaphragm. Biopsy demonstrated angioimmunoblastic T cell lymphoma (AITL) and next-generation sequencing (NGS) of the biopsy showed mutations, such as DNMT3A and TET2, which correlate with clonal hematopoiesis [73]. Interestingly, he was later administered a booster dose of the same vaccine in the right deltoid area in order to prepare him to receive chemotherapy. A few days later, he developed swelling of the right cervical lymph nodes and a second PET/CT demonstrated increased avidity in all lymphadenopathies and the appearance of new lesions. He was treated immediately with combination chemotherapy and anti-CD30 monoclonal antibody, with a reduction in disease within 2 weeks. Since the presence or absence of initial disease before the vaccination could not be ascertained in this case, the authors highlighted the rapid progression of the recently diagnosed AITL after the booster vaccine rather than implicating the vaccine in the development of the disease itself. They invoked a possibility of interaction of already malignant T cells with the vaccine mechanisms of stimulation of T helper cell immunity.
Sekizawa et al. [69] reported the case of an 80-year-old Japanese female who developed a right temporal mass after the administration of the first COVID-19 vaccine, which was also associated with multiple lymphadenopathies in other sites, including the cervical and supraclavicular regions. This was subsequently diagnosed as a marginal zone lymphoma [69], reinforcing the importance of considering a neoplastic etiology in the differential diagnosis of lymphadenopathies associated with COVID-19 vaccines. Tang et al. [70] reported a case of a 51-year-old male heart transplant recipient who presented with a mediastinal mass one week after receiving the first dose of a COVID-19 vaccine, which was diagnosed as an Epstein–Barr virus (EBV)-positive diffuse large B-cell lymphoma. The authors hypothesized that the COVID-19 vaccine may reactivate latent EBV infection, thus contributing to the development of a neoplastic process [70]. mRNA COVID-19 vaccines have also been associated with immune dysregulation resulting in hemophagocytic lymphohistiocytosis, mainly in patients with pre-existent autoimmune disorders [72]. It is important to recognize these clinical associations with mRNA COVID-19 vaccines in order to be able to perform a risk/benefit analysis in each specific circumstance. Similarly, further research is needed to establish the mechanisms of interaction of the immune response to mRNA COVID19 vaccination and pre-existent immune alterations as well as possible neoplastic predispositions, such as mutations that may correlate with clonal hematopoiesis.71 Further research is needed to elucidate the possible mechanisms of pathogenesis and tumorigenesis in these contexts.
In November 2021, the SARS-CoV-2 variant B.1.1.529, also named Omicron, was designated by the World Health Organization as a variant of concern, with an increased risk of reinfection with Omicron in those who had prior COVID-19 [74]. The BNT162b2 (Pfizer-Bio-NTech) vaccine was evaluated in the setting of hospitalized patients with the Omicron variant of COVID-19 [75]. The vaccine effectiveness was reported to be 70% and the authors [75] suggested that a third booster dose of vaccine may be warranted to improve effectiveness, as the vaccine effectiveness was reported to be 93% for hospital admission, 92% for severe disease, and 81% for COVID-19-related mortality [76].
This study is limited by its small sample size. Larger sample sizes may allow the characterization of patients who developed a florid lymphoid hyperplasia or KFD. Furthermore, none of the patients in this review had a history of, or a clinical suspicion for, a lymphoproliferative disorder. As such, the interpretation of these results in a patient that recently received a COVID-19 vaccine and that is suspicious for a lymphoproliferative disorder needs to be performed cautiously, although there have been reported associations between COVID-19 vaccines and lymphoproliferative disorders as mentioned above. Additionally, the majority of the included studies did not detail the pathological findings aside from a diagnostic line, and this further limits a deeper analysis of these results. It would also be of interest to determine whether each vaccine is associated with a different rate of diagnoses with larger sample sizes. There is also an inherent selection bias, especially for SLDI cases, as these cases of incidental lymphadenopathy would likely be more frequently detected by surveillance imaging. However, considering that only a proportion of C19-VAL is further investigated with FNA or biopsy, this review has the largest number of C19-VAL with pathological findings. As a result of the methodology of pooling individual patient data from published studies, there is heterogeneity in the patient population, demographics, and clinical characteristics. Additionally, most of the studies identified did not have a detailed report of the pathological findings, including for the further ancillary immunohistochemical studies that were performed.
This study does not include specifically pediatric patients, since the vaccine was only later approved to be used in children aged 5 and above and recently for 6 months and above.
5. Conclusions
C19-VAL is gaining recognition with the widespread use of these vaccines in controlling the outbreak. Although most cases of C19-VAL were diagnosed histologically as reactive or negative for malignancy, other diagnoses including florid lymphoid hyperplasia, KFD, and granulomatous inflammation have been reported. Metastases can occur in patients with a history of malignancy who have been recently vaccinated, and the lymphadenopathy in these cases is likely related to the underlying malignancy. Awareness of these pathological findings, along with their associated clinical and radiological findings, may help to guide the management of this population of patients.
Author Contributions
T.H.C. and A.T. conceptualized the manuscript. T.H.C. collected and analyzed the data. T.H.C. and A.T. contributed to the interpretation of data, writing of manuscript and approved the final version of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data presented are available within the manuscript.
Acknowledgments
We acknowledge the SingHealth Duke-NUS Pathology Academic Clinical Program for their support in the provision of open access publication fee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Johns Hopkins Coronavirus Resource Center. COVID-19 Map. 2021. Available online: https://coronavirus.jhu.edu/map.html (accessed on 4 January 2022).
- Administration FaD. FDA Approves First COVID-19 Vaccine. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 4 January 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Prevention CfDCa. Vaccines and Immunizations: Reactions and Adverse Reactions. 2021. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html (accessed on 4 January 2022).
- Hartsock, R.J. Postvaccinial lymphadenitis. Hyperplasia of lymphoid tissue that simulates malignant lymphomas. Cancer 1968, 21, 632–649. [Google Scholar] [CrossRef]
- Aelami, M.H.; Alborzi, A.; Pouladfar, G.; Geramizadeh, B.; Pourabbas, B.; Mardaneh, J. Post-Vaccination Disseminated Bacillus Calmette Guerin Infection Among Children in Southern Iran. Jundishapur J. Microbiol. 2015, 8, e25663. [Google Scholar] [CrossRef]
- Dotlic, S.; Vranic, S.; Jakovljevic, G.; Ilic, I.; Kardum-Paro, M.M.; Dojcinov, S.D. Neonatal hyperimmune T-cell reaction mimicking T-cell non-Hodgkin’s lymphoma following BCG and hepatitis B co-vaccination. Virchows Arch. 2012, 461, 601–605. [Google Scholar] [CrossRef]
- Watanabe, T.; Hashidate, H.; Hirayama, Y.; Iinuma, Y. Kikuchi-Fujimoto disease following vaccination against human papilloma virus infection and Japanese encephalitis. Eur. J. Pediatr. 2012, 171, 1409–1411. [Google Scholar] [CrossRef]
- White, C.K.; Al-Saleem, T.; Skarbnik, A.P.; Smith, M.R. Tetanus toxoid reactive lymphadenopathy masquerading as T-cell lymphoma. Future Oncol. 2012, 8, 631–634. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Atria, D.G.; Oguin, T.H., III; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Lehman, C.D.; D’Alessandro, H.A.; Mendoza, D.P.; Succi, M.D.; Kambadakone, A.; Lamb, L.R. Unilateral Lymphadenopathy After COVID-19 Vaccination: A Practical Management Plan for Radiologists Across Specialties. J. Am. Coll. Radiol. 2021, 18, 843–852. [Google Scholar] [CrossRef]
- Treglia, G.; Cuzzocrea, M.; Giovanella, L.; Elzi, L.; Muoio, B. Prevalence and Significance of Hypermetabolic Lymph Nodes Detected by 2-[(18)F]FDG PET/CT after COVID-19 Vaccination: A Systematic Review and a Meta-Analysis. Pharmaceuticals 2021, 14, 762. [Google Scholar] [CrossRef]
- Keshavarz, P.; Yazdanpanah, F.; Rafiee, F.; Mizandari, M. Lymphadenopathy Following COVID-19 Vaccination: Imaging Findings Review. Acad. Radiol. 2021, 28, 1058–1071. [Google Scholar] [CrossRef]
- Placke, J.-M.; Reis, H.; Hadaschik, E.; Roesch, A.; Schadendorf, D.; Stoffels, I.; Klode, J. Coronavirus disease 2019 vaccine mimics lymph node metastases in patients undergoing skin cancer follow-up: A monocentre study. Eur. J. Cancer 2021, 154, 167–174. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Cardoso, F.; Reis, A.; Osório, C.; Scigliano, H.; Nora, M. A Case of Cervical Lymphadenopathy After Vaccination Against COVID-19. Cureus 2021, 13, e15050. [Google Scholar] [CrossRef]
- Hagen, C.; Nowack, M.; Messerli, M.; Saro, F.; Mangold, F.; Bode, P.K. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med. Wkly. 2021, 151, w20557. [Google Scholar] [CrossRef]
- Faermann, R.; Nissan, N.; Halshtok-Neiman, O.; Shalmon, A.; Gotlieb, M.; Yagil, Y.; Samoocha, D.; Friedman, E.; Sklair-Levy, M. COVID-19 Vaccination Induced Lymphadenopathy in a Specialized Breast Imaging Clinic in Israel: Analysis of 163 cases. Acad. Radiol. 2021, 28, 1191–1197. [Google Scholar] [CrossRef]
- Felices-Farias, J.M.; Martínez-Martínez, J.F.; Guzmán-Aroca, F. Unusual lymphadenopathies secondary to the BNT162b2 mRNA COVID-19 vaccine. Med. Clin. 2021, 158, 343. [Google Scholar] [CrossRef]
- Fernández-Prada, M.; Rivero-Calle, I.; Calvache-González, A.; Martinón-Torres, F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Eurosurveillance 2021, 26, 2100193. [Google Scholar] [CrossRef] [PubMed]
- Ganga, K.; Solyar, A.Y.; Ganga, R. Massive Cervical Lymphadenopathy Post-COVID-19 Vaccination. Ear Nose Throat J. 2021. [Google Scholar] [CrossRef]
- Kado, S.; Kamiya, K.; Iwabuchi, S.; Kajii, E.; Ohtsuki, M. Unilateral lymphadenopathy associated with COVID-19 vaccination. J. Cutan. Immunol. Allergy 2021, 5, 100–101. [Google Scholar] [CrossRef]
- Kim, B.; Park, Y.; Kim, E.K.; Lee, S.H. Supraclavicular lymphadenopathy after COVID-19 vaccination in Korea: Serial follow-up using ultrasonography. Clin. Imaging 2021, 79, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Larkin, K.; Sharma, A.; Salaru, G.; Drachtman, R. Supraclavicular Lymphadenopathy after COVID-19 vaccination. Authorea Prepr. 2021. Available online: https://www.authorea.com/users/444098/articles/543906-supraclavicular-lymphadenopathy-after-covid-19-vaccination (accessed on 4 January 2022). [CrossRef]
- Özütemiz, C.; Krystosek, L.A.; Church, A.L.; Chauhan, A.; Ellermann, J.M.; Domingo-Musibay, E.; Steinberger, D. Lymphadenopathy in COVID-19 vaccine recipients: Diagnostic dilemma in oncologic Patients. Radiology 2021, 300, E296–E300. [Google Scholar] [CrossRef]
- Park, J.Y.; Yi, S.Y. Rare case of contralateral supraclavicular lymphadenopathy after COVID-19 vaccination: Computed tomography and ultrasonography findings. Radiol. Case Rep. 2021, 16, 3879–3881. [Google Scholar] [CrossRef]
- Al Soub, H.; Ibrahim, W.; Al Maslamani, M.; Ali, G.A.; Ummer, W. Kikuchi-Fujimoto disease following SARS CoV2 vaccination: Case report. IDCases 2021, 25, e01253. [Google Scholar] [CrossRef]
- Tan, N.J.H.; Tay, K.X.J.; Wong, S.B.J.; Nga, M.E. COVID-19 post-vaccination lymphadenopathy: Report of cytological findings from fine needle aspiration biopsy. Diagn. Cytopathol. 2021, 49, E467–E470. [Google Scholar] [CrossRef]
- Tan, H.M.; Hue, S.S.; Wee, A.; See, K.C. Kikuchi-Fujimoto Disease Post COVID-19 Vaccination: Case Report and Review of Literature. Vaccines 2021, 9, 1251. [Google Scholar] [CrossRef]
- Tintle, S.; Chen, M. Lymphadenopathy with florid lymphoid and Langerhans cell hyperplasia and hemophagocytosis mimicking lymphoma after COVID-19 mRNA vaccination. eJHaem 2021, 2, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Aalberg, J.J.; Collins, T.P.; Dobrow, E.M. Axillary lymphadenopathy in a renal cell carcinoma patient after COVID-19 Vaccination. Radiol. Case Rep. 2021, 16, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Ashoor, A.; Shephard, J.; Lissidini, G.; Nicosia, L. Axillary Adenopathy in Patients with Recent COVID-19 Vaccination: A New Diagnostic Dilemma. Korean J. Radiol. 2021, 22, 2124–2126. [Google Scholar] [CrossRef]
- Eifer, M.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Shams, J.; Keret, N.; Gorfine, M.; Eshet, Y. COVID-19 mRNA Vaccination: Age and Immune Status and its Association with Axillary Lymph Node PET/CT Uptake. J. Nucl. Med. 2021, 63, 134–139. [Google Scholar] [CrossRef]
- Lane, D.L.; Neelapu, S.S.; Xu, G.; Weaver, O. COVID-19 Vaccine-Related Axillary and Cervical Lymphadenopathy in Patients with Current or Prior Breast Cancer and Other Malignancies: Cross-Sectional Imaging Findings on MRI, CT, and PET-CT. Korean J. Radiol. 2021, 22, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, S.A.; Khil, E.K.; Byeon, S.J.; Kang, H.J.; Choi, J.A. COVID-19 vaccine-related axillary lymphadenopathy in breast cancer patients: Case series with a review of literature. Semin. Oncol. 2021, 48, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.A.; Mannava, K.; Sahasrabudhe, D.M. COVID-19 mRNA vaccine-related adenopathy mimicking metastatic melanoma. Lancet Oncol. 2021, 22, e281. [Google Scholar] [CrossRef]
- Pudis, M.; Vercher Conejero, J.L.; Martín Marcuartu, J.J.; Cortés Romera, M. 68Ga-DOTATOC-avid lymphadenopathies induced from COVID-19 mRNA vaccination. Jpn. J. Clin. Oncol. 2021, 51, 1765. [Google Scholar] [CrossRef]
- Trikannad, A.; Vellanki, S.; Kunapareddy, G. Mediastinal Lymphadenopathy after COVID-19 Vaccine: Staging Dilemma in Oncology Patients. Chest 2021, 160, A1460. [Google Scholar] [CrossRef]
- Robinson, K.A.; Maimone, S.; Gococo-Benore, D.A.; Li, Z.; Advani, P.P.; Chumsri, S. Incidence of Axillary Adenopathy in Breast Imaging After COVID-19 Vaccination. JAMA Oncol. 2021, 7, 1395–1397. [Google Scholar] [CrossRef]
- Barouni, A.S.; Augusto, C.; Queiroz, M.V.; Lopes, M.T.; Zanini, M.S.; Salas, C.E. BCG lymphadenopathy detected in a BCG-vaccinated infant. Braz. J. Med. Biol. Res. 2004, 37, 697–700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biers, S.M.; Di Marco, A.; Mostafid, A.H. Case report: Differential diagnosis of isolated iliac lymphadenopathy following Bacillus Calmette-Guérin treatment for high-risk superficial bladder cancer. Int. Urol. Nephrol. 2007, 39, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chakarabarti, S.; Phukan, J.P.; Biswas, S.; Sinha, A.; Sinha, R. Role of needle aspiration in diagnosis and management of suppurative bacille calmette-guerin adenitis: An institutional study of 30 cases. J. Lab. Physicians 2015, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Wu, H.J.; Yong, S.B. Bacillus Calmette-Guérin vaccination-associated axillary lymphadenopathy in a 2-year-old girl: Case report. J. Formos. Med. Assoc. 2019, 118, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Toy, H.; Karasoy, D.; Keser, M. Lymphadenitis caused by H1N1 vaccination: Case report. Vaccine 2010, 28, 2158–2160. [Google Scholar] [CrossRef]
- Pereira, M.P.; Flores, P.; Neto, A.S. Neck and supraclavicular lymphadenopathy secondary to 9-valent human papillomavirus vaccination. BMJ Case Rep. 2019, 12, e231582. [Google Scholar] [CrossRef]
- Dorfman, R.F.; Herweg, J.C. Live, attenuated measles virus vaccine. Inguinal lymphadenopathy complicating administration. JAMA 1966, 198, 320–321. [Google Scholar] [CrossRef]
- Sumaya, C.V.; Cherry, J.D.; Gohd, R. Exaggerated antibody response following rubella vaccination in a child with sinus histiocytosis with massive lymphadenopathy. J. Pediatr. 1976, 89, 81–83. [Google Scholar] [CrossRef]
- White, C. Tetanus vaccine Supraclavicular lymphadenopathy: Case report. Reactions 2012, 1415, 18. [Google Scholar]
- Gupta, K.; Singh, N.; Bhatia, A.; Arora, V.K.; Singh, U.R.; Singh, B. Cytomorphologic patterns in Calmette Guerin bacillus lymphadenitis. Acta Cytol. 1997, 41, 348–350. [Google Scholar] [CrossRef]
- Bosch, X.; Guilabert, A.; Miquel, R.; Campo, E. Enigmatic Kikuchi-Fujimoto disease: A comprehensive review. Am. J. Clin. Pathol. 2004, 122, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, C.B.; Wang, E. Kikuchi-Fujimoto disease. Arch. Pathol. Lab. Med. 2010, 134, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X.; Guilabert, A. Kikuchi-Fujimoto disease. Orphanet J. Rare Dis. 2006, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Garreffa, E.; Hamad, A.; O’Sullivan, C.C.; Hazim, A.Z.; York, J.; Puri, S.; Turnbull, A.; Robertson, J.F.; Goetz, M.P. Regional lymphadenopathy following COVID-19 vaccination: Literature review and considerations for patient management in breast cancer care. Eur. J. Cancer 2021, 159, 38–51. [Google Scholar] [CrossRef]
- Bshesh, K.; Khan, W.; Vattoth, A.L.; Janjua, E.; Nauman, A.; Almasri, M.; Ali, A.M.; Ramadorai, V.; Mushannen, B.; Al Subaie, M.; et al. Lymphadenopathy post-COVID-19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: A systematic review. J. Med. Virol. 2022, 94, 1833–1845. [Google Scholar] [CrossRef]
- Brown, A.; Shah, S.; Dluzewski, S.; Musaddaq, B.; Wagner, T.; Szyszko, T.; Wan, S.; Groves, A.; Mokbel, K.; Malhotra, A. Unilateral axillary adenopathy following COVID-19 vaccination: A multimodality pictorial illustration and review of current guidelines. Clin. Radiol. 2021, 76, 553–558. [Google Scholar] [CrossRef]
- Schiaffino, S.; Pinker, K.; Magni, V.; Cozzi, A.; Athanasiou, A.; Baltzer, P.A.; Camps Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; et al. Axillary lymphadenopathy at the time of COVID-19 vaccination: Ten recommendations from the European Society of Breast Imaging (EUSOBI). Insights Imaging 2021, 12, 119. [Google Scholar] [CrossRef]
- Samkowski, J.; Sklinda, K.; Walecki, J.M. Lymphadenopathy in the era of COVID-19 vaccination—An oncological dilemma in diagnostic imaging. Pol. J. Radiol. 2022, 87, e304–e310. [Google Scholar] [CrossRef]
- Van Nijnatten, T.J.A.; Jochelson, M.S.; Lobbes, M.B.I. Axillary lymph node characteristics in breast cancer patients versus post-COVID-19 vaccination: Overview of current evidence per imaging modality. Eur. J. Radiol. 2022, 152, 110334. [Google Scholar] [CrossRef]
- McIntosh, L.J.; Bankier, A.A.; Vijayaraghavan, G.R.; Licho, R.; Rosen, M.P. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management. AJR Am. J. Roentgenol. 2021, 217, 975–983. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Mascellino, M.T.; Di Timoteo, F.; de Angelis, M.; Oliva, A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Netea, M.G. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.; Sparrow, A.; Ghebreyesus, T.A.; Netea, M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef]
- Sekizawa, A.; Hashimoto, K.; Kobayashi, S.; Kozono, S.; Kobayashi, T.; Kawamura, Y.; Kimata, M.; Fujita, N.; Ono, Y.; Obuchi, Y.; et al. Rapid progression of marginal zone B-cell lymphoma after COVID-19 vaccination (BNT162b2): A case report. Front. Med. 2022, 9, 963393. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-R.; Hsu, C.-W.; Lee, C.-C.; Huang, W.-L.; Lin, C.-Y.; Hsu, Y.-T.; Chang, C.; Tsai, M.-T.; Hu, Y.-N.; Hsu, C.-H.; et al. A Case Report of Posttransplant Lymphoproliferative Disorder After AstraZeneca Coronavirus Disease 2019 Vaccine in a Heart Transplant Recipient. Transplant. Proc. 2022, 54, 1575–1578. [Google Scholar] [CrossRef]
- Goldman, S.; Bron, D.; Tousseyn, T.; Vierasu, I.; Dewispelaere, L.; Heimann, P.; Cogan, E.; Goldman, M. Rapid Progression of Angioimmunoblastic T Cell Lymphoma Following BNT162b2 mRNA Vaccine Booster Shot: A Case Report. Front. Med. 2021, 8, 798095. [Google Scholar] [CrossRef]
- Rocco, J.M.; Mallarino-Haeger, C.; Randolph, A.H.; Ray, S.M.; Schechter, M.C.; Zerbe, C.S.; Holland, S.M.; Sereti, I. Hyperinflammatory Syndromes After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Messenger RNA vaccination in Individuals With Underlying Immune Dysregulation. Clin. Infect. Dis. 2022, 75, e912–e915. [Google Scholar] [CrossRef]
- Buscarlet, M.; Provost, S.; Zada, Y.F.; Barhdadi, A.; Bourgoin, V.; Lépine, G.; Mollica, L.; Szuber, N.; Dubé, M.-P.; Busque, L. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 2017, 130, 753–762. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Update on Omicron. 2021. Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron (accessed on 4 January 2022).
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2021, 386, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).