Clinical Management of Pathogen-Negative Tuberculous Meningitis in Adults: A Series Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Seddon, J.A.; Tugume, L.; Solomons, R.; Prasad, K.; Bahr, N.C. The current global situation for tuberculous meningitis: Epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res. 2019, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Donovan, J.; Phu, N.H.; Nghia, H.D.T.; Thuong, N.T.T.; Thwaites, G.E. Tuberculous meningitis: Progress and remaining questions. Lancet Neurol. 2022, 21, 450–464. [Google Scholar] [CrossRef]
- Donovan, J.; Cresswell, F.V.; Thuong, N.T.T.; Boulware, D.R.; Thwaites, G.E.; Bahr, N.C. Xpert MTB/RIF Ultra for the Diagnosis of Tuberculous Meningitis: A Small Step Forward. Clin. Infect. Dis. 2020, 71, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, G.; Fisher, M.; Hemingway, C.; Scott, G.; Solomon, T.; Innes, J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J. Infect. 2009, 59, 167–187. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ryan, H.; Khaparde, S.; Sachdeva, K.S.; Singh, A.D.; Mohan, A.; Sarin, R.; Paramasivan, C.N.; Kumar, P.; Nischal, N.; et al. Index-TB guidelines: Guidelines on extrapulmonary tuberculosis for India. Indian J. Med. Res. 2017, 145, 448–463. [Google Scholar]
- Tuberculous Meningitis Professional Committee; Chinese Medical Association. Chinese guidelines for the diagnosis and treatment of central nervous system tuberculosis. Chin. J. Infect. Dis. 2020, 38, 400–408. [Google Scholar]
- Blumberg, H.; Burman, W.J.; Chaisson, R.E.; Daley, C.L.; Etkind, S.C.; Friedman, L.N.; Fujiwara, P.; Grzemska, M.; Hopewell, P.C.; Iseman, M.D.; et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 603–662. [Google Scholar]
- Marais, S.; Thwaites, G.; Schoeman, J.F.; Török, M.E.; Misra, U.K.; Prasad, K.; Donald, P.R.; Wilkinson, R.J.; Marais, B.J. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect. Dis. 2010, 10, 803–812. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Chau, T.T.; Stepniewska, K.; Phu, N.H.; Chuong, L.V.; Sinh, D.X.; White, N.J.; Parry, C.M.; Farrar, J.J. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 2002, 360, 1287–1292. [Google Scholar] [CrossRef]
- Sulaiman, T.; Medi, S.; Erdem, H.; Senbayrak, S.; Ozturk-Engin, D.; Inan, A.; Civljak, R.; Nechifor, M.; Akbulut, A.; Crisan, A.; et al. The diagnostic utility of the “Thwaites’ system” and “lancet consensus scoring system” in tuberculous vs. non-tuberculous subacute and chronic meningitis: Multicenter analysis of 395 adult patients. BMC Infect. Dis. 2020, 20, 788. [Google Scholar] [CrossRef]
- Mechai, F.; Bouchaud, O. Tuberculous meningitis: Challenges in diagnosis and management. Rev. Neurol. 2019, 175, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, C.; Chen, R.; Hu, Z.; Yao, H.; Zhang, Q.; Zhu, H.; Wang, Z.; Song, Z.; Zhang, C.; et al. Development and validation of a new scoring system for the early diagnosis of tuberculous meningitis in adults. Diagn. Microbiol. Infect. Dis. 2021, 101, 115393. [Google Scholar] [CrossRef] [PubMed]
- Thao, L.T.P.; Wolbers, M.; Heemskerk, A.D.; Thi Hoang Mai, N.; Thi Minh Ha, D.; Thi Hong Chau, T.; Hoan Phu, N.; Van Vinh Chau, N.; Caws, M.; Huu Lan, N.; et al. Dynamic Prediction of Death in Patients With Tuberculous Meningitis Using Time-updated Glasgow Coma Scale and Plasma Sodium Measurements. Clin. Infect. Dis. 2020, 70, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.M. Central Nervous System Tuberculosis. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Schoeman, J.F.; Donald, P.R. Tuberculous meningitis. Handb. Clin. Neurol. 2013, 112, 1135–1138. [Google Scholar]
- Luo, Y.; Xue, Y.; Lin, Q.; Mao, L.; Tang, G.; Song, H.; Liu, W.; Wu, S.; Liu, W.; Zhou, Y.; et al. Diagnostic Model for Discrimination Between Tuberculous Meningitis and Bacterial Meningitis. Front. Immunol. 2021, 12, 731876. [Google Scholar] [CrossRef]
- Jongeling, A.C.; Pisapia, D. Pearls and oy-sters: Tuberculous meningitis: Not a diagnosis of exclusion. Neurology 2013, 80. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Mokomane, M.; Leeme, T.B.; Tlhako, N.; Tsholo, K.; Chebani, T.; Stephenson, A.; Hutton, J.; Mitchell, H.K.; Patel, R.K.; et al. Mortality in adult patients with culture-positive and culture-negative meningitis in the Botswana national meningitis survey: A prevalent cohort study. Lancet Infect. Dis. 2019, 19, 740–749. [Google Scholar] [CrossRef]
- Wen, L.; Li, M.; Xu, T.; Yu, X.; Wang, L.; Li, K. Clinical features, outcomes and prognostic factors of tuberculous meningitis in adults worldwide: Systematic review and meta-analysis. J. Neurol. 2019, 266, 3009–3021. [Google Scholar] [CrossRef]
- Segura, R.M.; Pascual, C.; Ocaña, I.; Martínez-Vázquez, J.M.; Ribera, E.; Ruiz, I.; Pelegrí, M.D. Adenosine deaminase in body fluids: A useful diagnostic tool in tuberculosis. Clin. Biochem. 1989, 22, 141–148. [Google Scholar] [CrossRef]
- Shaw, J.A.; Diacon, A.H.; Koegelenberg, C.F.N. Tuberculous pleural effusion. Respirology 2019, 24, 962–971. [Google Scholar] [CrossRef]
- Tuon, F.F.; Higashino, H.R.; Lopes, M.I.B.F.; Litvoc, M.N.; Atomiya, A.N.; Antonangelo, L.; Leite, O.M. Adenosine deaminase and tuberculous meningitis—A systematic review with meta-analysis. Scand. J. Infect. Dis. 2010, 42, 198–207. [Google Scholar] [CrossRef]
- Corral, I.; Quereda, C.; Navas, E.; Martín-Dávila, P.; Pérez-Elías, M.J.; Casado, J.L.; Pintado, V.; Cobo, J.; Pallarés, E.; Rubí, J.; et al. Adenosine deaminase activity in cerebrospinal fluid of HIV-infected patients: Limited value for diagnosis of tuberculous meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 471–476. [Google Scholar] [CrossRef]
- Kalita, J.; Prasad, S.; Misra, U.K. Predictors of paradoxical tuberculoma in tuberculous meningitis. Int. J. Tuberc. Lung Dis. 2014, 18, 486–491. [Google Scholar] [CrossRef]
- Azeemuddin, M.; Alvi, A.; Sayani, R.; Khan, M.K.; Farooq, S.; Beg, M.A.; Awan, S.; Wasay, M. Neuroimaging Findings in Tuberculosis: A Single-Center Experience in 559 Cases. J. Neuroimaging 2019, 29, 657–668. [Google Scholar] [CrossRef]
- Siahaan, A.M.P.; Tandean, S.; Indharty, R.S.; Nainggolan, B.W.M.; Susanto, M. Paroxysmal sympathetic hyperactivity syndrome in tuberculous meningitis with paradoxical reaction. Int. J. Surg. Case Rep. 2022, 99, 107619. [Google Scholar] [CrossRef]
- Kaur, H.; Mittal, G.K.; Singhdev, J. Intradural extramedullary tuberculoma of the spinal cord in patient of tubercular meningitis—An uncommon scenario. Indian J. Tuberc. 2020, 67, 426–429. [Google Scholar] [CrossRef]
- Esposito, S.B.; Levi, J.; Matuzsan, Z.M.; Amaducci, A.M.; Richardson, D.M. A Case Report of Widely Disseminated Tuberculosis in Immunocompetent Adult Male. Clin. Pract. Cases Emerg. Med. 2020, 4, 375–379. [Google Scholar] [CrossRef]
- Vasconcelos, G.; Santos, L.; Couto, C.; Cruz, M.; Castro, A. Miliary Brain Tuberculomas and Meningitis: Tuberculosis Beyond the Lungs. Eur. J. Case Rep. Intern. Med. 2020, 7, 001931. [Google Scholar] [CrossRef]
- Bongomin, F.; Khan, S.A.; Oravec, T. A Complete Triad: Horner’s Syndrome in Tuberculous Meningitis. Am. J. Med. Sci. 2020, 360, 204–205. [Google Scholar] [CrossRef]
- Flynn, W.P.; Ntuli, Y.; Zhang, H.; Tiberi, S. A case of Clival Tuberculosis and associated meningitis. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 25, 100273. [Google Scholar] [CrossRef]
- Elavarasi, A.; Goyal, V. Brainstem tuberculoma: A delayed IRIS. Indian J. Tuberc. 2020, 67, 343–345. [Google Scholar] [CrossRef]
- Tala-Ighil, T.; Greffe, S.; Trad, S.; Delaroche, M.; Coutte, L.; Rouveix, E.; Kahn, J.E.; Hanslik, T. Cerebral infarction and tuberculosis: Case report and literature review. Rev. Med. Interne 2020, 41, 704–707. [Google Scholar] [CrossRef]
- Zafar, Z.; Hafeez, M.A.-O.; Butt, M. Elusive tuberculous meningitis with rare neurological complication of longitudinally extensive transverse myelitis: A case report. Spinal Cord Ser. Cases 2021, 14, 82. [Google Scholar] [CrossRef]
- Shao, K.; Dong, F.; Guo, S.; Wang, J.; Sun, Z. Eight-and-a-half syndrome caused by tuberculous meningitis: A case report. Acta Neurol. Belg. 2021, 121, 591–593. [Google Scholar] [CrossRef]
- Oka, Y.; Tabu, H.; Matsumoto, S. Tuberculous meningitis presenting with nonconvulsive status epilepticus and transient diffusion restriction: A rare case. Neurol. India 2020, 68, 512. [Google Scholar] [CrossRef]
- Kitazaki, Y.; Ikawa, M.; Enomoto, S.; Shirafuji, N.; Hayashi, K.; Yamamura, O.; Yamada, S.; Arishima, H.; Noriki, S.; Nakamoto, Y.; et al. An autopsy case of tuberculous meningitis undiagnosed by nested-PCR of CSF samples and brain biopsy. J. Neurol. Sci. 2020, 415, 116968. [Google Scholar] [CrossRef]
- Gaba, S.; Gupta, M.; Lamba, A.S.; Bhardwaj, A.; Gupta, H. Bilateral Complete Oculomotor Palsy in Tubercular Meningitis. Cureus 2020, 12, 11001. [Google Scholar] [CrossRef]
- Desai, N.; Krishnan, R.; Rukmangadachar, L. Central Nervous System Tuberculosis Presenting With Multiple Ring-Enhancing Lesions: A Diagnostic Challenge. Cureus 2022, 14, 21819. [Google Scholar] [CrossRef]
- Chesdachai, S.; Katz, B.; Sapkota, S. Diagnostic Challenges and Dilemmas in Tuberculous Meningitis. Am. J. Med. Sci. 2020, 359, 372–377. [Google Scholar] [CrossRef]
- Arif, S.; Arif, S.; Slehria, A.U.; Yousaf, G.; Nawaz Sr, K.H. Central Nervous System Tuberculosis With Shower Like Pattern of Intracranial Tuberculomas in an Immunocompetent Patient. Cureus 2020, 12, 9922. [Google Scholar] [CrossRef]
- Marais, B.J.; Heemskerk, A.D.; Marais, S.S.; van Crevel, R.; Rohlwink, U.; Caws, M.; Meintjes, G.; Misra, U.K.; Mai, N.T.H.; Ruslami, R.; et al. Standardized Methods for Enhanced Quality and Comparability of Tuberculous Meningitis Studies. Clin. Infect. Dis. 2017, 64, 501–509. [Google Scholar] [CrossRef]
- Qu, J.; Zhou, T.; Zhong, C.; Deng, R.; Lü, X. Comparison of clinical features and prognostic factors in HIV-negative adults with cryptococcal meningitis and tuberculous meningitis: A retrospective study. BMC Infect. Dis. 2017, 17, 51. [Google Scholar] [CrossRef]
- Soares, C.N.; Angelim, A.I.M.; Brandão, C.O.; Santos, R.Q.; Mehta, R.; Silva, M. Neurobrucellosis: The great mimicker. Rev. Soc. Bras. Med. Trop. 2022, 55, e05672021. [Google Scholar] [CrossRef]
- Elavarasi, A.; Dash, D.; Warrier, A.R.; Jain, D. Leptomeningeal leukaemia misdiagnosed as tubercular meningitis. BMJ Case Rep. 2019, 12, e228328. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, S.-Q.; Chen, X.; Tang, Y.; Chen, M.; Shang, K.; Deng, G.; Qin, C.; Tian, D.-S. Comparison of clinical and radiological characteristics in autoimmune GFAP astrocytopathy, MOGAD and AQP4-IgG+ NMOSD mimicking intracranial infection as the initial manifestation. Mult. Scler. Relat. Disord. 2022, 66, 104057. [Google Scholar] [CrossRef]
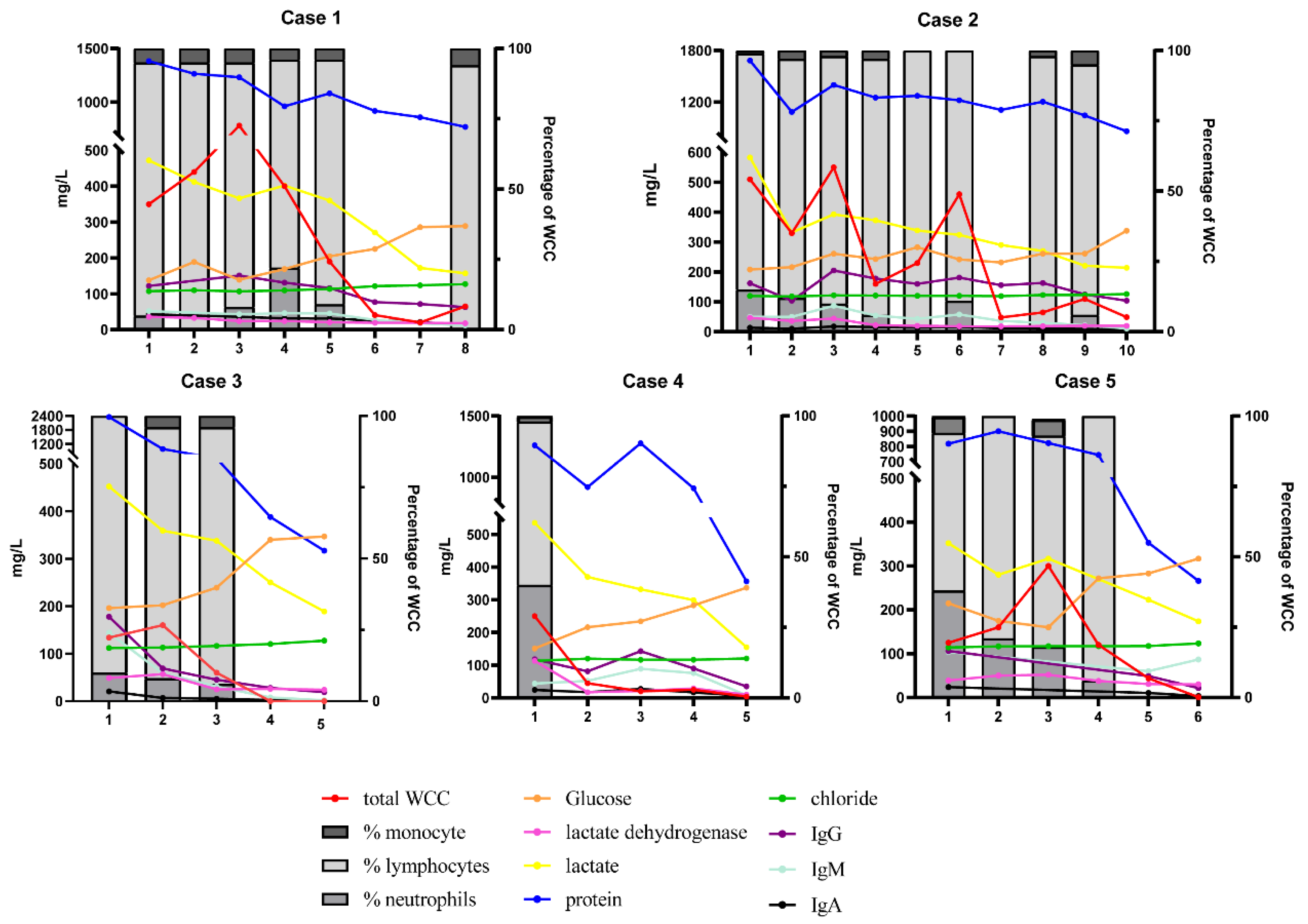

| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | ||
|---|---|---|---|---|---|---|---|
| Age | 27 | 56 | 48 | 40 | 62 | ||
| Sex | Male | Male | Male | Male | Female | ||
| Potentially relevant personal history | Eats seafood | Neighbors have pigeons | Breakfast shop owner | Construction worker | Retiree | ||
| Exposure history of tuberculosis | Yes | No | No | No | No | ||
| Illness history | No | No | No | No | No | ||
| History of Smoke/Drink/Drug | No | No | No | No | No | ||
| Symptom duration (days) | 29 | 14 | 11 | 19 | 12 | ||
| Prodrome | Cold and cough | Cold and anorexia | Toothache and muscle soreness and anorexia | Diarrhea and fatigue | Cold | ||
| Clinical syndrome | Headache | Yes | Yes | Yes | Yes | Yes | |
| Fever | Yes | Yes | Yes | Yes | Yes | ||
| Night sweat | Yes | No | Yes | No | No | ||
| Hyponatremia | Yes | No | Yes | No | Yes | ||
| Cranial nerve palsy symptoms | intractable hiccup | No | No | No | No | ||
| Altered consciousness | Lethargy | Psychiatric symptoms and cognitive impairment | Lethargy | No | Epileptic seizures | ||
| Stiff-Neck | Yes | Yes | Yes | Yes | Yes | ||
| Laboratory results | CSF Specimen | M. tb culture | − | − | − | − | - |
| ZN smear | − | − | − | − | - | ||
| mNGS | − | − | − | Human M.tb | - | ||
| Xpert | − | − | − | − | - | ||
| Bacterial culture | − | − | − | − | - | ||
| Bacterial staining | − | − | − | − | - | ||
| India ink staining | − | − | − | − | - | ||
| Virus antibody * | − | / | − | − | - | ||
| Ab related to AE * | − | / | / | / | - | ||
| WCC * (106/L) | 350 | 510 | 134 | 250 | 125 | ||
| Protein * (mg/L) | 1383 | 1683 | 2356 | 1260 | 819 | ||
| Glucose * (mmol/L) | 1.38 | 2.08 | 1.96 | 1.51 | 2.15 | ||
| Blood specimen | Fungal antigen * | + | − | − | − | - | |
| Virus antibody * | − | / | − | − | - | ||
| mNGS | − | − | − | − | - | ||
| T-SPOT | + | + | + | + | - | ||
| ANA | − | / | / | / | / | ||
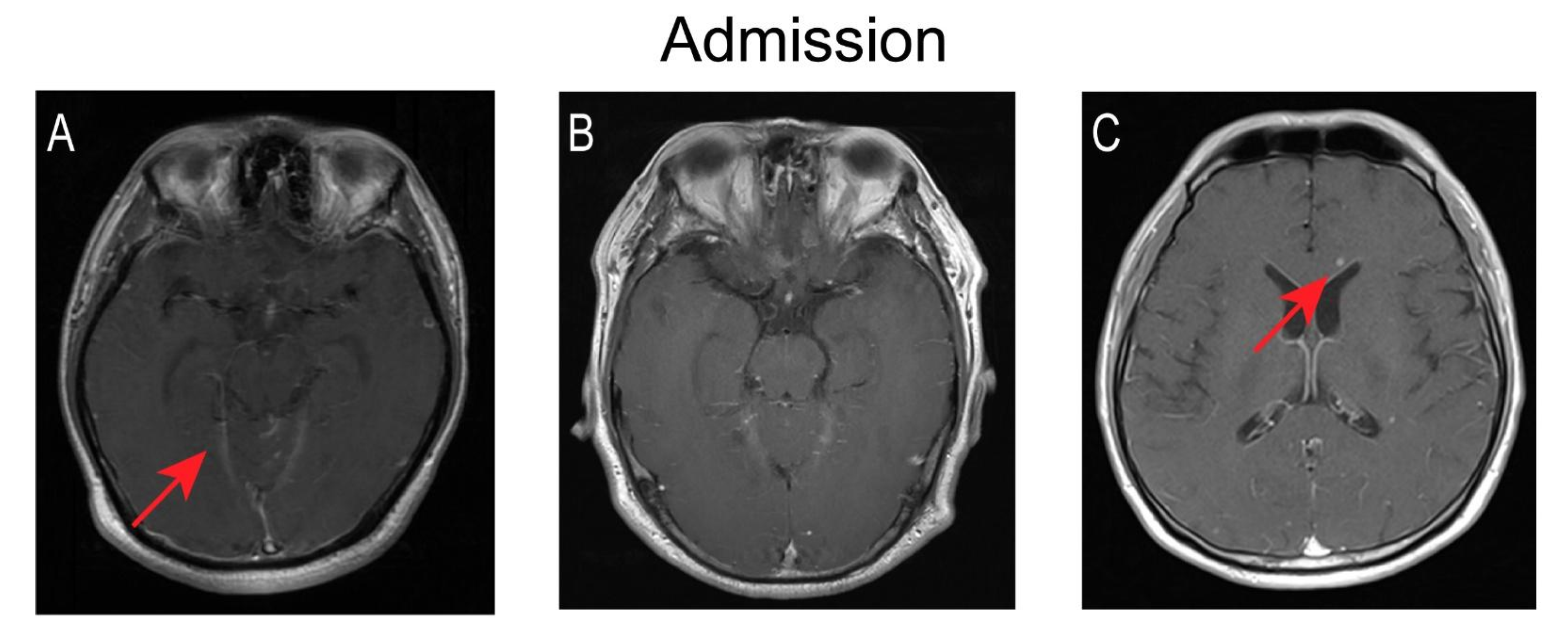
| Evidence of extracranial tuberculosis | Chest-CT | - | − | − | − | − | |
| Cervical Spinal cord MRI | - | − | − | / | − | ||
| ATT time(days) | 30 | 15 | 12 | 20 | 31 | ||
| Follow up time(months) | 8 | 3 | 6 | 6 | 3 | ||
| Neurological sequelae | No | No | No | No | No | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Huang, Y.; Wu, D.; Wu, Y.; Wang, M. Clinical Management of Pathogen-Negative Tuberculous Meningitis in Adults: A Series Case Study. J. Clin. Med. 2022, 11, 6250. https://doi.org/10.3390/jcm11216250
He Y, Huang Y, Wu D, Wu Y, Wang M. Clinical Management of Pathogen-Negative Tuberculous Meningitis in Adults: A Series Case Study. Journal of Clinical Medicine. 2022; 11(21):6250. https://doi.org/10.3390/jcm11216250
Chicago/Turabian StyleHe, Yuqin, Yanzhu Huang, Di Wu, Yingying Wu, and Minghuan Wang. 2022. "Clinical Management of Pathogen-Negative Tuberculous Meningitis in Adults: A Series Case Study" Journal of Clinical Medicine 11, no. 21: 6250. https://doi.org/10.3390/jcm11216250
APA StyleHe, Y., Huang, Y., Wu, D., Wu, Y., & Wang, M. (2022). Clinical Management of Pathogen-Negative Tuberculous Meningitis in Adults: A Series Case Study. Journal of Clinical Medicine, 11(21), 6250. https://doi.org/10.3390/jcm11216250






