Prospective Evaluation of Different Methods for Volumetric Analysis on [18F]FDG PET/CT in Pediatric Hodgkin Lymphoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
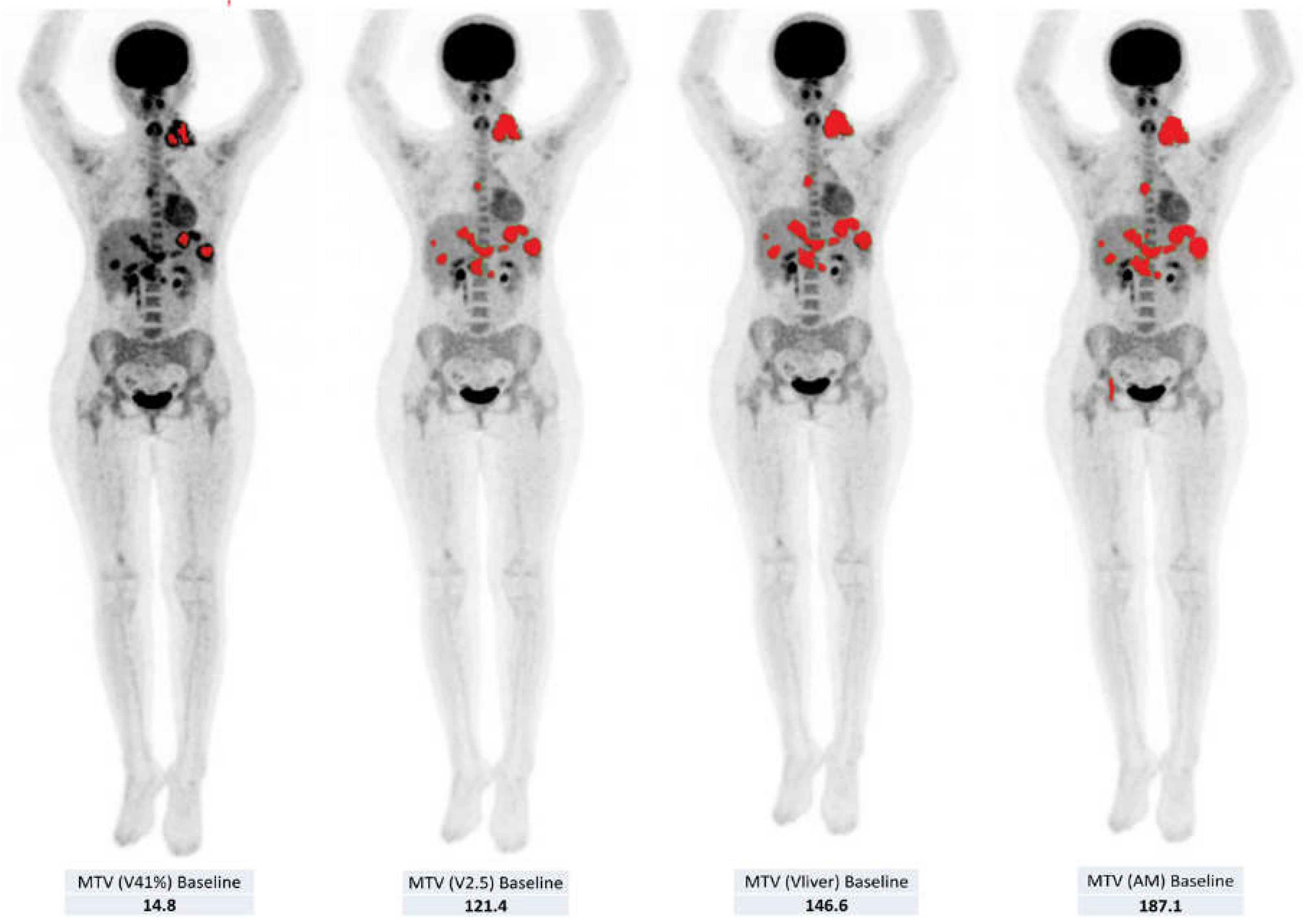
2.2. Volumetric Assessment
2.3. Response Classification
2.4. Statistical Analysis
3. Results
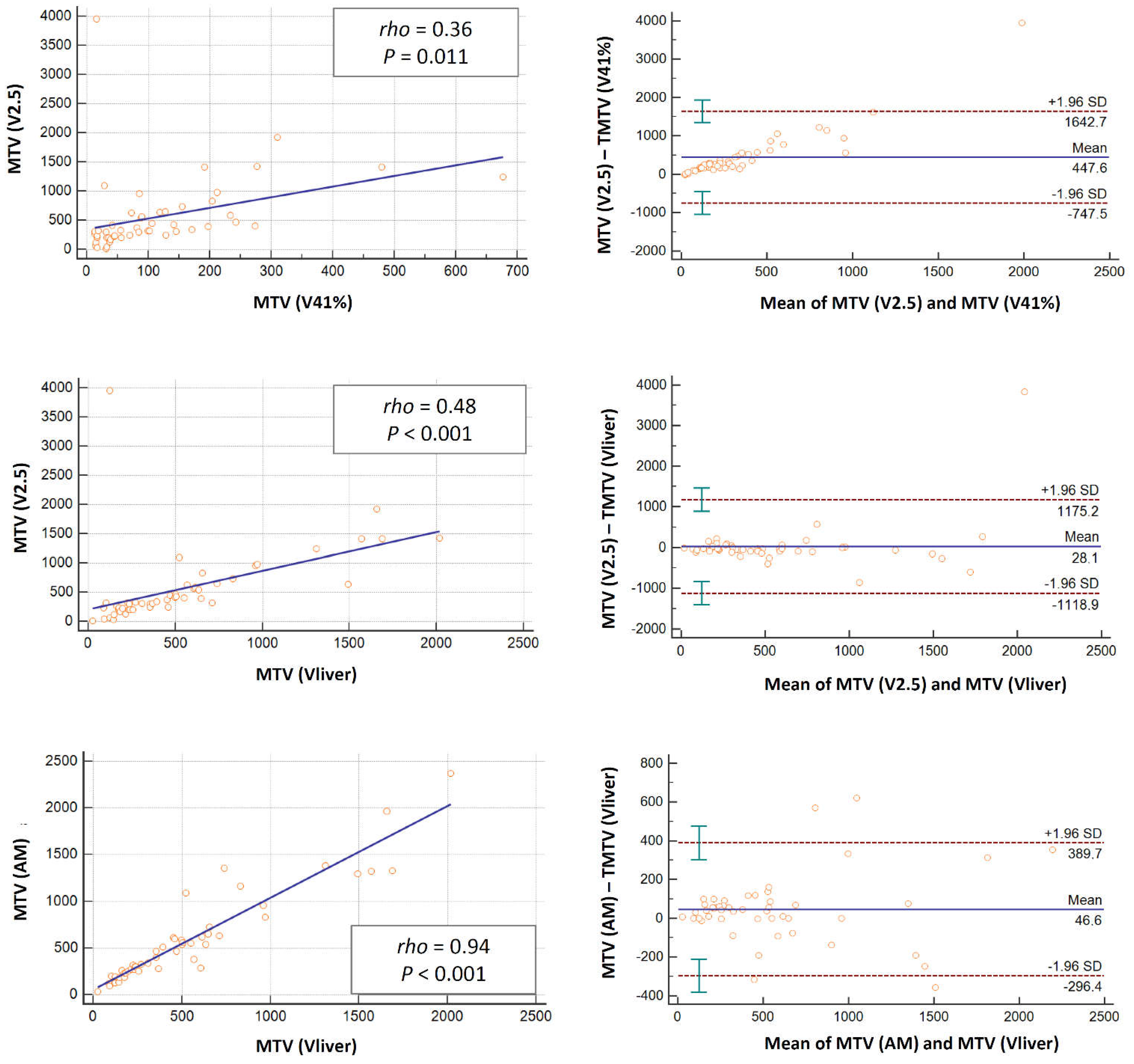
3.1. Semi-Quantitative and Volumetric Analyses
3.2. Comparison of the Parameters according to the Different Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, A.J. Epidemiology of Hodgkin’s disease and non—Hodgkin’s lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30 (Suppl. S1), 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M.; Krasin, M.J.; Kaste, S.C. PET imaging in pediatric Hodgkin’s lymphoma. Pediatr. Radiol. 2004, 34, 190–198. [Google Scholar]
- Kleis, M.; Daldrup-Link, H.; Matthay, K.; Goldsby, R.; Lu, Y.; Schuster, T.; Schreck, C.; Chu, P.W.; Hawkins, R.A.; Franc, B.L. Diagnostic value of PET/CT for the staging and restaging of pediatric tumors. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Riad, R.; Omar, W.; Kotb, M.; Hafez, M.; Sidhom, I.; Zamzam, M.; Zaky, I.; Abdel-Dayem, H. Role of PET/CT in malignant pediatric lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Franzius, C.; Juergens, K.U.; Vormoor, J. PET/CT with diagnostic CT in the evaluation of childhood sarcoma. AJR 2006, 186, 581. [Google Scholar] [CrossRef] [PubMed]
- McCarville, B. The role of positron emission tomography in pediatric musculoskeletal oncology. Skeletal. Radiol. 2006, 35, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.E.; Shulkin, B.; Gelfand, M.J.; Salisbury, S.; Kim, E.E. 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J. Nucl. Med. 2009, 50, 1237–1243. [Google Scholar] [CrossRef]
- Lopci, E.; Burnelli, R.; Ambrosini, V.; Nanni, C.; Castellucci, P.; Biassoni, L.; Rubello, M.; Fanti, S. (18)F-FDG PET in Pediatric Lymphomas: A Comparison with Conventional Imaging. Cancer Biother. Radiopharm. 2008, 23, 681–690. [Google Scholar] [CrossRef]
- Lopci, E.; Burnelli, R.; Guerra, L.; Cistaro, A.; Piccardo, A.; Zucchetta, P.; Derenzini, E.; Todesco, A.; Garaventa, A.; Schumacher, F.; et al. Postchemotherapy PET evaluation correlates with patient outcome in paediatric Hodgkin’s disease. Eur. J. Nucl. Med. Mol Imaging 2011, 38, 1620–1627. [Google Scholar] [CrossRef]
- Lopci, E.; Mascarin, M.; Piccardo, A.; Castello, A.; Elia, C.; Guerra, L.; Borsatti, E.; Sala, A.; Todesco, A.; AIEOP Hodgkin Lymphoma Study Group, Italy; et al. FDG PET in response evaluation of bulky masses in paediatric Hodgkin’s lymphoma (HL) patients enrolled in the Italian AIEOP-LH2004 trial. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on malignant lymphomas imaging Working group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Furth, C.; Amthauer, H.; Hautzel, H.; Steffen, I.G.; Ruf, J.; Schiefer, J.; Schönberger, S.; Henze, G.; Grandt, R.; Hundsdoerfer, P.; et al. Evaluation of interim PET response criteria in paediatric Hodgkin’s lymphoma—Results for dedicated assessment criteria in a blinded dual-centre read. Ann. Oncol. 2011, 22, 1198–1203. [Google Scholar] [CrossRef]
- Kluge, R.; Chavdarova, L.; Hoffmann, M.; Kobe, C.; Malkowski, B.; Montravers, F.; Kurch, L.; Georgi, T.; Dietlein, M.; Wallace, W.H.; et al. Inter-Reader Reliability of Early FDG-PET/CT Response Assessment Using the Deauville Scale after 2 Cycles of Intensive Chemotherapy (OEPA) in Hodgkin’s Lymphoma. PLoS ONE 2016, 11, e0149072. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Kluge, R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 97–110. [Google Scholar] [CrossRef]
- Hasenclever, D.; Kurch, L.; Mauz-Körholz, C.; Georgi, T.; Wallace, H.; Landman-Parker, J.; Moryl-Bujakowska, A.; Cepelová, M.; Karlén, J.; Fernández-Teijeiro, A.Á.; et al. qPET—A quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Mauz-Körholz, C.; Hasenclever, D.; Dörffel, W.; Ruschke, K.; Pelz, T.; Voigt, A.; Stiefel, M.; Winkler, M.; Vilser, C.; Dieckmann, K.; et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: The GPOH-HD-2002 study. J. Clin. Oncol. 2010, 28, 3680–3686. [Google Scholar] [CrossRef]
- Rogasch, J.M.M.; Hundsdoerfer, P.; Hofheinz, F.; Wedel, F.; Schatka, I.; Amthauer, H.; Furth, C. Pretherapeutic FDG-PET total metabolic tumor volume predicts response to induction therapy in pediatric Hodgkin’s lymphoma. BMC Cancer 2018, 18, 521. [Google Scholar] [CrossRef]
- Mauz-Körholz, C.; Landman-Parker, J.; Balwierz, W.; Ammann, R.A.; Anderson, R.A.; Attarbaschi, A.; Bartelt, J.M.; Beishuizen, A.; Boudjemaa, S.; Cepelová, M.; et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): A titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol. 2022, 23, 125–137. [Google Scholar] [PubMed]
- Akhtari, M.; Milgrom, S.A.; Pinnix, C.C.; Reddy, J.P.; Dong, W.; Smith, G.L.; Mawlawi, O.; Yehia, Z.A.; Gunther, J.; Osborne, E.M.; et al. Re-classifying patients with early-stage Hodgkin lymphoma based on functional radiographic markers at presentation. Blood 2018, 131, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-K.; Chung, J.-S.; Lee, J.-J.; Jeong, S.Y.; Lee, S.-M.; Hong, J.-S.; Chong, A.; Moon, J.-H.; Kim, J.-H.; Lee, S.-M.; et al. Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci. 2013, 104, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, J.; Kim, S.G.; Hwang, K.H.; Kim, M.; Ahn, H.K.; Sym, S.J.; Park, J.; Cho, E.K.; Shin, D.B.; et al. Prognostic value of metabolic tumor volume estimated by (18) F-FDG positron emission tomography/computed tomography in patients with diffuse large B-cell lymphoma of stage II or III disease. Nucl. Med. Mol. Imaging. 2014, 48, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Paeng, J.C.; Chun, I.K.; Keam, B.; Jeon, Y.K.; Lee, S.-H.; Kim, D.-W.; Lee, D.S.; Kim, C.W.; Chung, J.-K.; et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer 2013, 119, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Cottereau, A.S.; Versari, A.; Luminari, S.; Dupuis, J.; Chartier, L.; Casasnovas, R.-O.; Berriolo-Riedinger, A.; Menga, M.; Haioun, C.; Tilly, H.; et al. Prognostic model for high-tumor-burden follicular lymphoma integrating baseline and end-induction PET: A LYSA/FIL study. Blood 2018, 131, 2449–2453. [Google Scholar] [CrossRef]
- Cottereau, A.S.; Versari, A.; Loft, A.; Casasnovas, O.; Bellei, M.; Ricci, R.; Bardet, S.; Castagnoli, A.; Brice, P.; Raemaekers, J.; et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood 2018, 131, 1456–1463. [Google Scholar] [CrossRef]
- Albano, D.; Bosio, G.; Bianchetti, N.; Pagani, C.; Alessandro, R.; Tucci, A.; Giubbini, R.; Bertagna, F. Prognostic role of baseline 18FFDG PET/CTmetabolic parameters in mantle cell lymphoma. Ann. Nucl. Med. 2019, 33, 449–458. [Google Scholar] [CrossRef]
- Albano, D.; Bertoli, M.; Battistotti, M.; Rodella, C.; Statuto, M.; Giubbini, R.; Bertagna, F. Prognostic role of pretreatment 18F-FDG PET/CT in primary brain lymphoma. Ann. Nucl. Med. 2018, 32, 532–541. [Google Scholar] [CrossRef]
- Albano, D.; Mazzoletti, A.; Spallino, M.; Muzi, C.; Zilioli, V.R.; Pagani, C.; Tucci, A.; Rossetti, C.; Giubbini, R.; Bertagna, F. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in elderly HL: A two-center experience in 123 patients. Ann. Hematol. 2020, 99, 1321–1330. [Google Scholar] [CrossRef]
- Albano, D.; Bosio, G.; Pagani, C.; Re, A.; Tucci, A.; Giubbini, R.; Bertagna, F. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in Burkitt lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 87–96. [Google Scholar] [CrossRef]
- Sasanelli, M.; Meignan, M.; Haioun, C.; Berriolo-Riedinger, A.; Casasnovas, R.-O.; Biggi, A.; Gallamini, A.; Siegel, B.A.; Cashen, A.F.; Véra, P.; et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2017–2022. [Google Scholar] [CrossRef]
- Esfahani, S.A.; Heidari, P.; Halpern, E.F.; Hochberg, E.P.; Palmer, E.L.; Mahmood, U. Baseline total lesion glycolysis measured with (18)F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: A pilot study. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 272–281. [Google Scholar] [PubMed]
- Mikhaeel, N.G.; Smith, D.; Dunn, J.T.; Phillips, M.; Møller, H.; Fields, P.A.; Wrench, D.; Barrington, S.F. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Meignan, M.; Cottereau, A.S.; Versari, A.; Chartier, L.; Dupuis, J.; Boussetta, S.; Grassi, I.; Casasnovas, R.-O.; Haioun, C.; Tilly, H.; et al. Baseline Metabolic Tumor Volume Predicts Outcome in High-Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J. Clin. Oncol. 2016, 34, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Second International Inter-Group Study for Classical Hodgkin Lymphoma in Children and Adolescents. Available online: https://clinicaltrials.gov/ct2/show/NCT02684708 (accessed on 29 August 2022).
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging—Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Lopci, E.; Burnelli, R.; Elia, C.; Piccardo, A.; Castello, A.; Borsatti, E.; Zucchetta, P.; Cistaro, A.; Mascarin, M. Additional value of volumetric and texture analysis on FDG PET assessment in paediatric Hodgkin lymphoma: An Italian multicentric study protocol. BMJ Open 2021, 11, e041252. [Google Scholar] [CrossRef]
- Meignan, M.; Gallamini, A.; Meignan, M.; Haioun, C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk. Lymphoma. 2009, 50, 1257–1260. [Google Scholar] [CrossRef]
- Barrington, S.F.; Meignan, M. Time to prepare for risk adaptation in lymphoma by standardizing measurement of metabolic tumor burden. J. Nucl. Med. 2019, 60, 1096–1102. [Google Scholar] [CrossRef]
- Kanoun, S.; Tal, I.; Berriolo-Riedinger, A.; Rossi, C.; Riedinger, J.-M.; Vrigneaud, J.-M.; Legrand, L.; Humbert, O.; Casasnovas, O.; Brunotte, F.; et al. Influence of software tool and methodological aspects of total metabolic tumor volume calculation on baseline [18F] FDG PET to predict survival in Hodgkin lymphoma. Clin. Trial 2015, 10, e0140830. [Google Scholar] [CrossRef]
- Martín-Saladich, Q.; Reynés-Llompart, G.; Sabaté-Llobera, A.; Palomar-Muñoz, A.; Domenech, E.D.; Cortés-Romera, M. Comparison of different automatic methods for the delineation of the total metabolic tumor volume in I-II stage Hodgkin Lymphoma. Sci. Rep. 2020, 10, 12590. [Google Scholar] [CrossRef]
- Parvez, A.; Tau, N.; Hussey, D.; Maganti, M.; Metser, U. 18F-FDG PET/CT metabolic tumor parameters and radiomics features in aggressive non-Hodgkin’s lymphoma as predictors of treatment outcome and survival. Ann. Nucl. Med. 2018, 32, 410–416. [Google Scholar] [CrossRef]
- Eude, F.; Toledano, M.N.; Vera, P.; Tilly, H.; Mihailescu, S.-D.; Becker, S. Reproducibility of Baseline Tumour Metabolic Volume Measurements in Diffuse Large B-Cell Lymphoma: Is There a Superior Method? Metabolites 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Driessen, J.; Zwezerijnen, G.J.; Schöder, H.; Drees, E.E.; Kersten, M.J.; Moskowitz, A.J.; Moskowitz, C.H.; Eertink, J.J.; de Vet, H.C.; Hoekstra, O.S.; et al. The impact of semi-automatic segmentation methods on metabolic tumor volume, intensity and dissemination radiomics in 18F-FDG PET scans of patients with classical Hodgkin lymphoma. J. Nucl. Med. 2022, 63, 1424–1433. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Number (%) |
| Sex | |
| Male | 31 (62%) |
| Female | 19 (38%) |
| Age (years) | 14.4 |
| Range | 7–25 |
| Stage | |
| II | 20 (40%) |
| III | 8 (16%) |
| IV | 22 (44%) |
| Bulky masses | 30 (60%) |
| B symptoms | 25 (50%) |
| Treatment levels (TLs) * | |
| TL-1 | 0 (0%) |
| TL-2 | 21 (42%) |
| TL-3 | 29 (58%) |
| PET1 | PET2 | PET3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thresholds | SUVmean | MTV | TLG | SUVmean | MTV | TLG | SUVmean | MTV | TLG | |||||||||
| rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | |
| 2.5 vs. 41% | 0.83 | <0.001 | 0.36 | 0.011 | 0.88 | <0.001 | 0.97 | <0.001 | 0.31 | 0.037 | 0.42 | 0.009 | 0.88 | <0.001 | 0.90 | <0.001 | 0.96 | <0.001 |
| 2.5 vs. AM | 0.79 | <0.001 | 0.47 | 0.001 | 0.98 | <0.001 | 0.93 | <0.001 | 0.91 | <0.001 | 0.96 | <0.001 | 0.99 | <0.001 | 0.98 | <0.001 | 1 | <0.001 |
| 2.5 vs. liver | 0.81 | <0.001 | 0.48 | <0.001 | 0.98 | <0.001 | 0.87 | <0.001 | 0.91 | <0.001 | 0.95 | <0.001 | 0.98 | <0.001 | 0.99 | <0.001 | 1 | <0.001 |
| AM vs. liver | 0.90 | <0.001 | 0.94 | <0.001 | 0.98 | <0.001 | 0.92 | <0.001 | 0.95 | <0.001 | 0.97 | <0.001 | 0.99 | <0.001 | 0.99 | <0.001 | 1 | <0.001 |
| 41% vs. AM | 0.80 | <0.001 | 0.65 | <0.001 | 0.86 | <0.001 | 0.93 | <0.001 | 0.45 | 0.001 | 0.5 | 0.001 | 0.88 | <0.001 | 0.95 | <0.001 | 0.97 | <0.001 |
| 41% vs. liver | 0.77 | <0.001 | 0.70 | <0.001 | 0.89 | <0.001 | 0.87 | <0.001 | 0.39 | 0.006 | 0.47 | 0.002 | 0.91 | <0.001 | 0.92 | <0.001 | 0.96 | <0.001 |
| Δ PET2 | Δ PET3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thresholds | ΔSUVmean | ΔMTV | ΔTLG | ΔSUVmean | ΔMTV | ΔTLG | ||||||
| rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | |
| 2.5 vs. 41% | 0.83 | <0.001 | 0.19 | 0.198 | 0.37 | 0.008 | 0.90 | <0.001 | 0.92 | <0.001 | 0.91 | <0.001 |
| 2.5 vs. AM | 0.88 | <0.001 | 0.93 | <0.001 | 0.97 | <0.001 | 0.96 | <0.001 | 0.94 | <0.001 | 0.98 | <0.001 |
| 2.5 vs. liver | 0.88 | <0.001 | 0.94 | <0.001 | 0.97 | <0.001 | 0.93 | <0.001 | 0.98 | <0.001 | 0.98 | <0.001 |
| AM vs. liver | 0.94 | <0.001 | 0.95 | <0.001 | 0.97 | <0.001 | 0.94 | <0.001 | 0.93 | <0.001 | 0.97 | <0.001 |
| 41% vs. AM | 0.89 | <0.001 | 0.29 | 0.040 | 0.45 | 0.001 | 0.90 | <0.001 | 0.82 | <0.001 | 0.91 | <0.001 |
| 41% vs. liver | 0.88 | <0.001 | 0.14 | 0.345 | 0.37 | 0.008 | 0.89 | <0.001 | 0.96 | <0.001 | 0.97 | <0.001 |
| DS 2 | DS 3 | DS 4 | DS 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thresholds | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | |
| 2.5 | SUVmean | 0.37 | 1.3 | 0.7–2.3 | 0.01 | 1.4 | 0.9–2 | 0.01 | 1.6 | 1–2.6 | 0.77 | 1 | 0.7–1.7 |
| MTV | 0.01 | 1 | 0.9–1 | 0.07 | 1 | 0.9–1 | 0.02 | 1 | 0.9–1 | 0.9 | 1 | 0.9–1 | |
| TLG | 0.03 | 1 | 0.9–1 | 0.2 | 1 | 0.9–1 | 0.08 | 1 | 0.9–1 | 0.7 | 1 | 0.9–1 | |
| 41% | SUVmean | 0.03 | 1.1 | 0.9–1.5 | 0.1 | 1.1 | 0.9–1.4 | 0.005 | 1.3 | 1–1.6 | 0.3 | 1.1 | 0.9–1.4 |
| MTV | 0.14 | 1 | 0.9–1 | 0.8 | 1 | 0.9–1 | 0.7 | 1 | 0.9–1 | 0.6 | 0.9 | 0.9–1 | |
| TLG | 0.13 | 1 | 0.9–1 | 0.4 | 1 | 0.9–1 | 0.25 | 1 | 0.9–1 | 0.7 | 0.9 | 0.9–1 | |
| Liver | SUVmean | 0.3 | 1.3 | 0.7–2.3 | 0.07 | 1.4 | 0.9–2.1 | 0.02 | 1.6 | 1–2.4 | 0.3 | 1.2 | 0.8–1.9 |
| MTV | 0.04 | 1 | 0.9–1 | 0.4 | 1 | 0.9–1 | 0.2 | 1 | 0.9–1 | 0.5 | 1 | 0.9–1 | |
| TLG | 0.04 | 1 | 0.9–1 | 0.3 | 1 | 0.9–1 | 0.1 | 1 | 0.9–1 | 0.7 | 1 | 0.9–1 | |
| AM | SUVmean | 0.2 | 1.4 | 0.8–2.4 | 0.06 | 1.4 | 0.9–2 | 0.01 | 1.6 | 1–2.3 | 0.1 | 1.3 | 0.8–2 |
| MTV | 0.08 | 1 | 0.9–1 | 0.7 | 1 | 0.9–1 | 0.4 | 1 | 0.9–1 | 0.8 | 1 | 0.9–1 | |
| TLG | 0.05 | 1 | 0.9–1 | 0.3 | 1 | 0.9–1 | 0.15 | 1 | 0.9–1 | 0.9 | 1 | 0.9–1 | |
| DS 2 | DS 3 | DS 4 | DS 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thresholds | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | |
| 2.5 | ΔSUVmean | <0.0001 | 0.4 | - | 0.0004 | 0.9 | 0.94–0.98 | 0.0005 | 0.9 | 0.95–0.99 | 0.009 | 0.9 | 0.92–0.99 |
| ΔMTV | - | - | - | 0.2 | 0.9 | 0.9–1 | 0.09 | 0.9 | 0.9–1 | 0.8 | 0.9 | 0.9–1 | |
| ΔTLG | - | - | - | 0.1 | 0.9 | 0.9–1 | 0.07 | 0.9 | 0.8–1 | 0.75 | 0.9 | 0.9–1 | |
| 41% | ΔSUVmean | - | - | - | 0.0009 | 0.9 | 0.92–0.98 | 0.007 | 0.9 | 0.94–0.99 | 0.008 | 0.9 | 0.94–0.99 |
| ΔMTV | - | - | - | 0.5 | 0.9 | 0.9–1 | 0.3 | 0.9 | 0.98–1 | 0.8 | 1 | 0.98–1 | |
| ΔTLG | - | - | - | 0.9 | 1 | 0.9–1 | 0.9 | 0.9 | 0.97–1 | 0.9 | 0.9 | 0.97–1 | |
| Liver | ΔSUVmean | - | - | - | 0.004 | 0.9 | 0.95–0.99 | 0.01 | 0.97 | 0.95–0.99 | 0.05 | 0.97 | 0.95–1 |
| ΔMTV | - | - | - | 0.09 | 0.9 | 0.8–1 | 0.04 | 0.9 | 0.8–1 | 0.3 | 0.97 | 0.92–1 | |
| ΔTLG | - | - | - | 0.1 | 0.9 | 0.8–1 | 0.06 | 0.9 | 0.8 | 0.4 | 0.97 | 0.92–1 | |
| AM | ΔSUVmean | - | - | - | 0.01 | 0.9 | 0.95–0.99 | 0.03 | 0.9 | 0.95–0.99 | 0.07 | 0.97 | 0.95–1 |
| ΔMTV | - | - | - | 0.1 | 0.9 | 0.9–1 | 0.05 | 0.9 | 0.9–1 | 0.6 | 0.98 | 0.94–1 | |
| ΔTLG | - | - | - | 0.1 | 0.9 | 0.9–1 | 0.07 | 0.9 | 0.8–1 | 0.7 | 0.98 | 0.94–1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopci, E.; Elia, C.; Catalfamo, B.; Burnelli, R.; De Re, V.; Mussolin, L.; Piccardo, A.; Cistaro, A.; Borsatti, E.; Zucchetta, P.; et al. Prospective Evaluation of Different Methods for Volumetric Analysis on [18F]FDG PET/CT in Pediatric Hodgkin Lymphoma. J. Clin. Med. 2022, 11, 6223. https://doi.org/10.3390/jcm11206223
Lopci E, Elia C, Catalfamo B, Burnelli R, De Re V, Mussolin L, Piccardo A, Cistaro A, Borsatti E, Zucchetta P, et al. Prospective Evaluation of Different Methods for Volumetric Analysis on [18F]FDG PET/CT in Pediatric Hodgkin Lymphoma. Journal of Clinical Medicine. 2022; 11(20):6223. https://doi.org/10.3390/jcm11206223
Chicago/Turabian StyleLopci, Egesta, Caterina Elia, Barbara Catalfamo, Roberta Burnelli, Valli De Re, Lara Mussolin, Arnoldo Piccardo, Angelina Cistaro, Eugenio Borsatti, Pietro Zucchetta, and et al. 2022. "Prospective Evaluation of Different Methods for Volumetric Analysis on [18F]FDG PET/CT in Pediatric Hodgkin Lymphoma" Journal of Clinical Medicine 11, no. 20: 6223. https://doi.org/10.3390/jcm11206223
APA StyleLopci, E., Elia, C., Catalfamo, B., Burnelli, R., De Re, V., Mussolin, L., Piccardo, A., Cistaro, A., Borsatti, E., Zucchetta, P., Bianchi, M., Buffardi, S., Farruggia, P., Garaventa, A., Sala, A., Vinti, L., Mauz-Koerholz, C., & Mascarin, M. (2022). Prospective Evaluation of Different Methods for Volumetric Analysis on [18F]FDG PET/CT in Pediatric Hodgkin Lymphoma. Journal of Clinical Medicine, 11(20), 6223. https://doi.org/10.3390/jcm11206223








