Safety, Feasibility and Technical Considerations from a Prospective, Observational Study—CIREL: Irinotecan-TACE for CRLM in 152 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design/Setting
2.2. Patients
2.3. Study Objectives and Data Sources
- first line or consolidation treatment after response to first line systemic therapy,
- use of LP-irinotecan TACE in combination with ablation in a curative intent,
- as an intensification of treatment with/without concomitant therapy for chemo-refractory patients still eligible for further systemic treatment,
- as salvage treatment in progressive patients for chemo-refractory patients non-eligible for further systemic treatment.
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics and Patient Selection
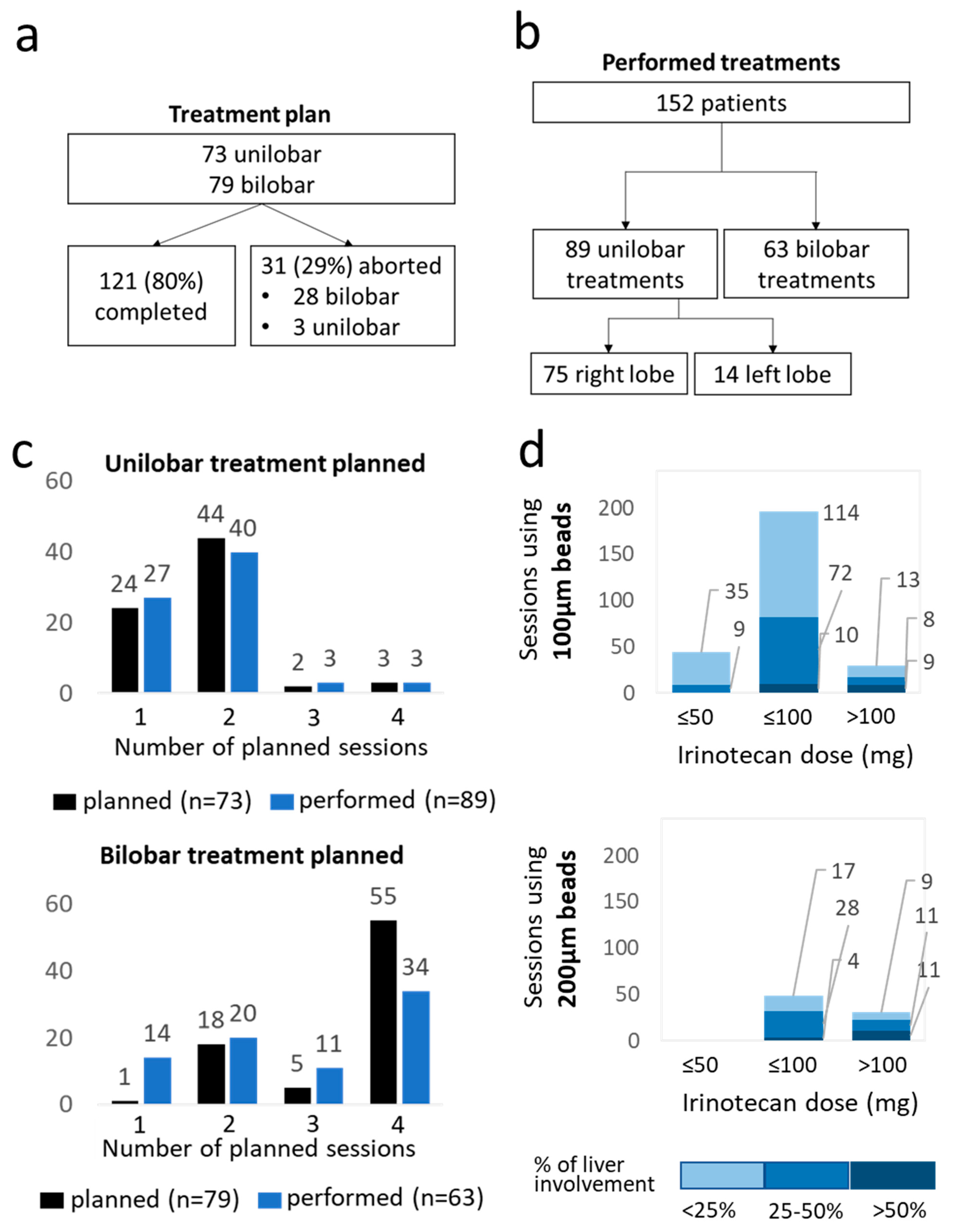
3.2. Treatment Feasibility and Technical Success
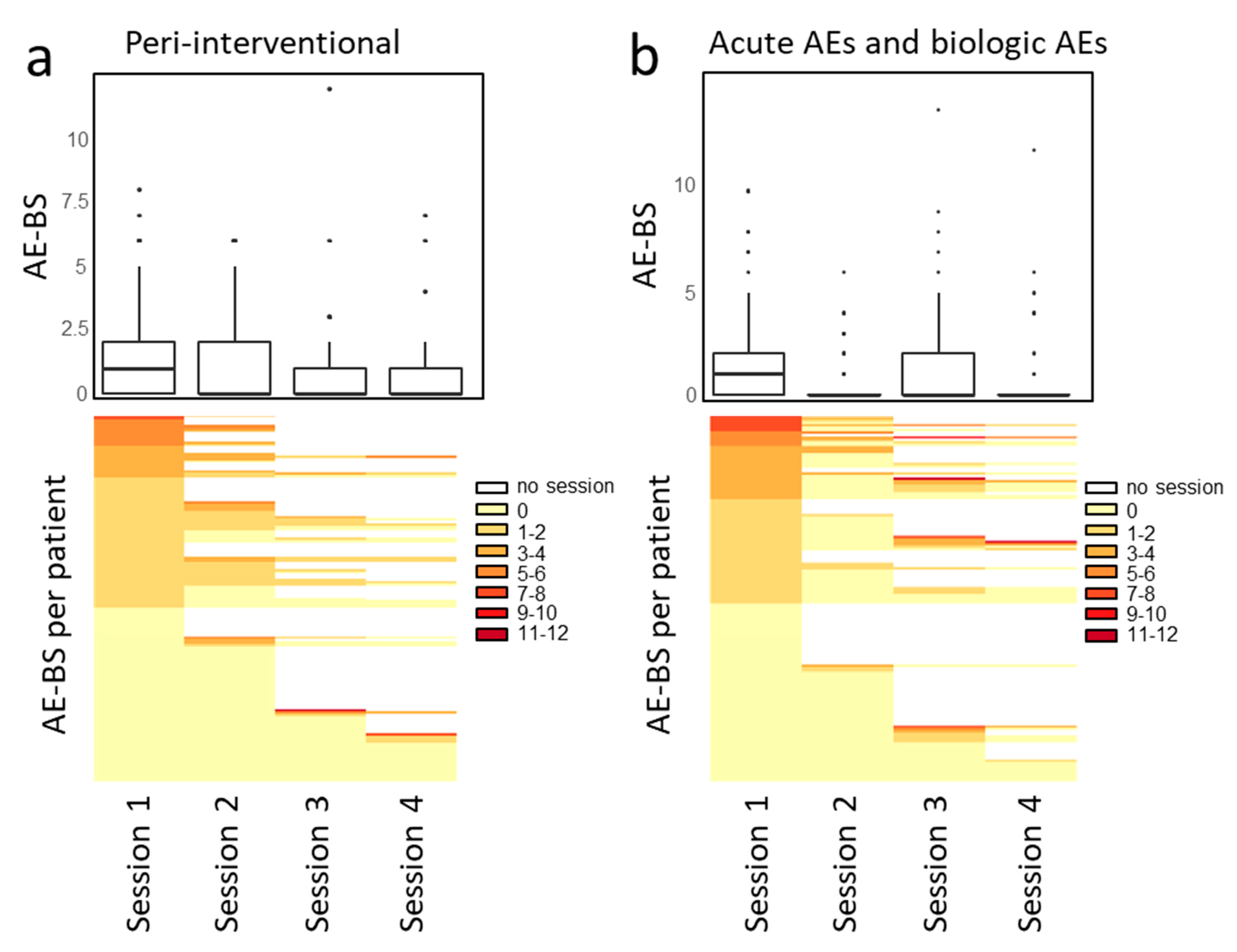
3.3. Safety and Toxicity
3.4. Relationship between Baseline or Treatment Characteristics and AE Events
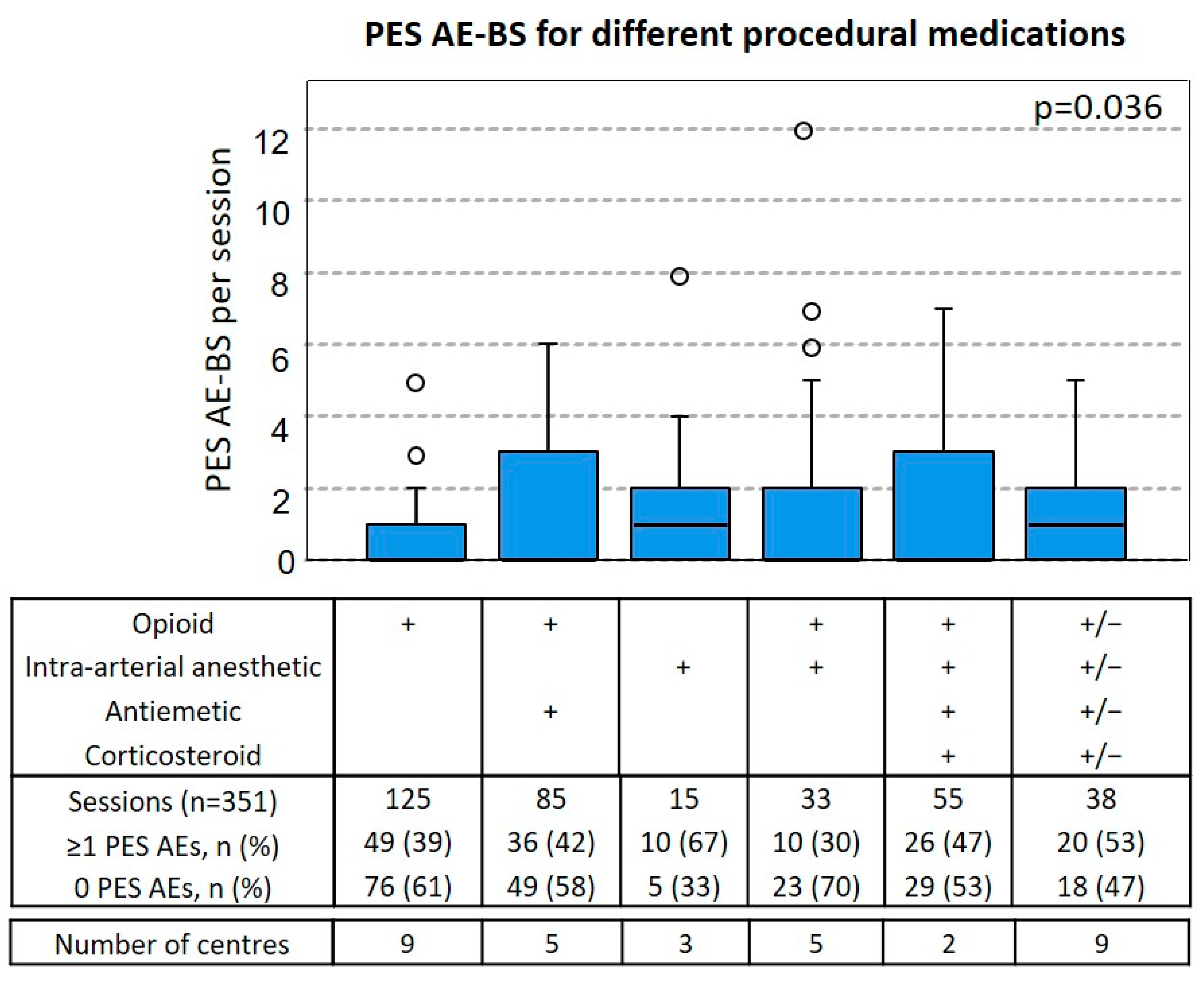
3.5. Relationship between Procedural Medication and PES AE-BS
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cance. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Malka, D.; Mendiboure, J.; Etienne, P.L.; Texereau, P.; Auby, D.; Rougier, P.; Gasmi, M.; Castaing, M.; Abbas, M.; et al. Sequential versus Combination Chemotherapy for the Treatment of Advanced Colorectal Cancer (FFCD 2000-05): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2011, 12, 1032–1044. [Google Scholar] [CrossRef]
- Adams, R.A.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Madi, A.; Fisher, D.; Kenny, S.L.; Kay, E.; Hodgkinson, E.; Pope, M.; et al. Intermittent versus Continuous Oxaliplatin and Fluoropyrimidine Combination Chemotherapy for First-Line Treatment of Advanced Colorectal Cancer: Results of the Randomised Phase 3 MRC COIN Trial. Lancet Oncol. 2011, 12, 642–653. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Martini, M.; Molinari, F.; Sartore-Bianchi, A.; Arena, S.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; Frattini, M.; Siena, S.; et al. Wild-Type BRAF Is Required for Response to Panitumumab or Cetuximab in Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 5705–5712. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive Role of BRAF Mutations in Patients with Advanced Colorectal Cancer Receiving Cetuximab and Panitumumab: A Meta-Analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Nardella, M.; Inchingolo, R.; Nestola, M.; Rebonato, A.; Fiorentini, C.; Aliberti, C.; Nani, R.; Guadagni, S. Transarterial Chemoembolization Alone or Followed by Bevacizumab for Treatment of Colorectal Liver Metastases. Hepatic Oncol. 2022, 9, HEP40. [Google Scholar] [CrossRef]
- Cao, F.; Zheng, J.; Luo, J.; Zhang, Z.; Shao, G. Treatment Efficacy and Safety of Regorafenib plus Drug-Eluting Beads-Transarterial Chemoembolization versus Regorafenib Monotherapy in Colorectal Cancer Liver Metastasis Patients Who Fail Standard Treatment Regimens. J. Cancer Res. Clin. Oncol. 2021, 147, 2993–3002. [Google Scholar] [CrossRef]
- Martin, R.C.G.; Scoggins, C.R.; Tomalty, D.; Schreeder, M.; Metzger, T.; Tatum, C.; Sharma, V. Irinotecan Drug-Eluting Beads in the Treatment of Chemo-Naive Unresectable Colorectal Liver Metastasis with Concomitant Systemic Fluorouracil and Oxaliplatin: Results of Pharmacokinetics and Phase I Trial. J. Gastrointest. Surg. 2012, 16, 1531–1538. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Nani, R.; Aliberti, C.; Fiorentini, C.; Guadagni, S. Updates of Colorectal Cancer Liver Metastases Therapy: Review on DEBIRI. Hepatic Oncol. 2020, 7, HEP16. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Asabella, A.N.; Altini, C.; Fazio, V.; Caporusso, L.; Marech, I.; Vinciarelli, G.; Macina, F.; de Ceglia, D.; Fanelli, M.; et al. Apilot Study Employing Hepatic Intra-Arterial Irinotecan Injection of Drug-Eluting Beads as Salvage Therapy in Liver Metastatic Colorectal Cancer Patients without Extrahepatic Involvement: The First Southern Italy Experience. OncoTargets Ther. 2016, 9, 7527–7535. [Google Scholar] [CrossRef] [PubMed]
- Stutz, M.; Mamo, A.; Valenti, D.; Hausvater, A.; Cabrera, T.; Metrakos, P.; Chaudhury, P.; Steacy, G.; Garoufalis, E.; Kavan, P. Real-Life Report on Chemoembolization Using Debiri for Liver Metastases from Colorectal Cancer. Gastroenterol. Res. Pract. 2015, 2015, 715102. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Marsico, V.A.; Guerra, A.; Cerchiaro, E.; Cassano, A.; Basso, M.; Devicienti, E.; Rodolfino, E.; Barone, C.; Bonomo, L. Trans-Arterial Chemoembolization with Irinotecan-Loaded Drug-Eluting Beads (DEBIRI) and Capecitabine in Refractory Liver Prevalent Colorectal Metastases: A Phase II Single-Center Study. Gastroenterol. Res. Pract. 2015, 38, 1523–1531. [Google Scholar] [CrossRef]
- Bhutiani, N.; Akinwande, O.; Martin, R.C.G. Efficacy and Toxicity of Hepatic Intra-Arterial Drug-Eluting (Irinotecan) Bead (DEBIRI) Therapy in Irinotecan-Refractory Unresectable Colorectal Liver Metastases. World J. Surg. 2016, 40, 1178–1190. [Google Scholar] [CrossRef]
- Akinwande, O.; Scoggins, C.; Martin, R.C.G. Early Experience with 70-150 m Irinotecan Drug-Eluting Beads (M1-DEBIRI) for the Treatment of Unresectable Hepatic Colorectal Metastases. Anticancer Res. 2016, 36, 3413–3418. [Google Scholar]
- Fiorentini, G.; Carandina, R.; Sarti, D.; Nardella, M.; Zoras, O.; Guadagni, S.; Inchingolo, R.; Nestola, M.; Felicioli, A.; Navarro, D.B.; et al. Polyethylene Glycol Microspheres Loaded with Irinotecan for Arterially Directed Embolic Therapy of Metastatic Liver Cancer. World J. Gastrointest. Oncol. 2017, 9, 379–384. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Giordani, P.; Graziano, F.; Catalano, V.; Aliberti, C.; Coschiera, P.; Mulazzani, L.; Tilli, M.; Gonzalez, A.M.; et al. Locoregional Therapy and Systemic Cetuximab to Treat Colorectal Liver Metastases. World J. Gastrointest. Oncol. 2015, 7, 47–54. [Google Scholar] [CrossRef]
- Fiorentini, G.; Aliberti, C.; Tilli, M.; Mulazzani, L.; Graziano, F.; Giordani, P.; Mambrini, A.; Montagnani, F.; Alessandroni, P.; Catalano, V.; et al. Intra-Arterial Infusion of Irinotecan-Loaded Drug-Eluting Beads (DEBIRI) versus Intravenous Therapy (FOLFIRI) for Hepatic Metastases from Colorectal Cancer: Final Results of a Phase III Study. Anticancer Res. 2012, 32, 1387–1395. [Google Scholar]
- Aliberti, C.; Fiorentini, G.; Muzzio, P.C.; Pomerri, F.; Tilli, M.; Dallara, S.; Benea, G. Trans-Arterial Chemoembolization of Metastatic Colorectal Carcinoma to the Liver Adopting DC Bead®, Drug-Eluting Bead Loaded with Irinotecan: Results of a Phase II Clinical Study. Anticancer Res. 2011, 31, 4581–4587. [Google Scholar]
- Eichler, K.; Zangos, S.; Mack, M.G.; Hammerstingl, R.; Gruber-Rouh, T.; Gallus, C.; Vogl, T.J. First Human Study in Treatment of Unresectable Liver Metastases from Colorectal Cancer with Irinotecan-Loaded Beads (DEBIRI). Int. J. Oncol. 2012, 41, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Scevola, G.; Loreni, G.; Rastelli, M.; Sposato, S.; Ramponi, S.; Miele, V. Third-Line Treatment of Colorectal Liver Metastases Using DEBIRI Chemoembolization. Med. Oncol. 2017, 34, 37. [Google Scholar] [CrossRef]
- Mauri, G.; Rossi, D.; Frassoni, S.; Bonomo, G.; Camisassi, N.; Della Vigna, P.; Bagnardi, V.; Maiettini, D.; Varano, G.M.; Zampino, M.G.; et al. Small-Size (40 Μm) Beads Loaded with Irinotecan in the Treatment of Patients with Colorectal Liver Metastases. CardioVascular Interv. Radiol. 2022, 45, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Szemitko, M.; Golubinska-Szemitko, E.; Wilk-Milczarek, E.; Falkowski, A. Side Effect/Complication Risk Related to Injection Branch Level of Chemoembolization in Treatment of Metastatic Liver Lesions from Colorectal Cancer. J. Clin. Med. 2021, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Kovacs, A.; Prenen, H.; Chevallier, P.; Pereira, P.L. Transarterial Chemoembolisation of Colorectal Liver Metastases with Irinotecan-Loaded Beads: What Every Interventional Radiologist Should Know. Eur. J. Radiol. Open 2020, 7, 100236. [Google Scholar] [CrossRef]
- Lucatelli, P.; Burrel, M.; Guiu, B.; de Rubeis, G.; van Delden, O.; Helmberger, T. CIRSE Standards of Practice on Hepatic Transarterial Chemoembolisation. CardioVascular Interv. Radiol. 2021, 44, 1851–1867. [Google Scholar] [CrossRef]
- Pereira, P.L.; Arnold, D.; de Baère, T.; Gomez, F.; Helmberger, T.; Iezzi, R.; Maleux, G.; Prenen, H.; Sangro, B.; Nordlund, A.; et al. A Multicentre, International, Observational Study on Transarterial Chemoembolisation in Colorectal Cancer Liver Metastases: Design and Rationale of CIREL. Dig. Liver Dis. 2020, 52, 857–861. [Google Scholar] [CrossRef]
- Le-Rademacher, J.G.; Hillman, S.; Storrick, E.; Mahoney, M.R.; Thall, P.F.; Jatoi, A.; Mandrekar, S.J. Adverse Event Burden Score—A Versatile Summary Measure for Cancer Clinical Trials. Cancers 2020, 12, 3251. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Nardella, M.; Inchingolo, R.; Nestola, M.; Rebonato, A.; Guadagni, S. Chemoembolization Alone or Associated with Bevacizumab for Therapy of Colorectal Cancer Metastases: Preliminary Results of a Randomized Study. In Vivo 2020, 34, 683–686. [Google Scholar] [CrossRef]
- Pernot, S.; Pellerin, O.; Artru, P.; Montérymard, C.; Smith, D.; Raoul, J.L.; De La Fouchardière, C.; Dahan, L.; Guimbaud, R.; Sefrioui, D.; et al. Intra-Arterial Hepatic Beads Loaded with Irinotecan (DEBIRI) with MFOLFOX6 in Unresectable Liver Metastases from Colorectal Cancer: A Phase 2 Study. Br. J. Cancer 2020, 123, 518–524. [Google Scholar] [CrossRef]
- Pereira, P.L.; Iezzi, R.; Manfredi, R.; Carchesio, F.; Bánsághi, Z.; Brountzos, E.; Spiliopoulos, S.; Echevarria-Uraga, J.J.; Gonçalves, B.; Inchingolo, R.; et al. The CIREL Cohort: A Prospective Controlled Registry Studying the Real-Life Use of Irinotecan-Loaded Chemoembolisation in Colorectal Cancer Liver Metastases: Interim Analysis. CardioVascular Interv. Radiol. 2021, 44, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Gaba, R.C.; Lokken, R.P.; Hickey, R.M.; Lipnik, A.J.; Lewandowski, R.J.; Salem, R.; Brown, D.B.; Walker, T.G.; Silberzweig, J.E.; Baerlocher, M.O.; et al. Quality Improvement Guidelines for Transarterial Chemoembolization and Embolization of Hepatic Malignancy. J. Vasc. Interv. Radiol. 2017, 28, 1210–1223.e3. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Aliberti, C.; De Baere, T.; Garcia-Monaco, R.; Narayanan, G.; O’Grady, E.; Rilling, W.S.; Walker, D.; Martin, R.C.G. Transarterial Treatment of Colorectal Cancer Liver Metastases with Irinotecan-Loaded Drug-Eluting Beads: Technical Recommendations. J. Vasc. Interv. Radiol. 2014, 25, 365–369. [Google Scholar] [CrossRef]
- Levy, J.; Zuckerman, J.; Garfinkle, R.; Acuna, S.A.; Touchette, J.; Vanounou, T.; Pelletier, J.S. Intra-Arterial Therapies for Unresectable and Chemorefractory Colorectal Cancer Liver Metastases: A Systematic Review and Meta-Analysis. Hpb 2018, 20, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Voizard, N.; Ni, T.; Kiss, A.; Pugash, R.; Raphael, M.J.; Coburn, N.; David, E. Small Particle DEBIRI TACE as Salvage Therapy in Patients with Liver Dominant Colorectal Cancer Metastasis: Retrospective Analysis of Safety and Outcomes. Curr. Oncol. 2022, 29, 209–220. [Google Scholar] [CrossRef]
- Seager, M.J.; Jakobs, T.F.; Sharma, R.A.; Bandula, S. Combination of Ablation and Embolization for Intermediate-Sized Liver Metastases from Colorectal Cancer: What Can We Learn from Treating Primary Liver Cancer? Diagn. Interv. Radiol. 2021, 27, 677–683. [Google Scholar] [CrossRef]
- Lewis, A.L.; Hall, B. Toward a Better Understanding of the Mechanism of Action for Intra-Arterial Delivery of Irinotecan from DC Bead(TM) (DEBIRI). Future Oncol. 2019, 15, 2053–2068. [Google Scholar] [CrossRef]
- Sobhani, F.; Xu, C.; Murano, E.; Pan, L.; Rastegar, N.; Kamel, I.R. Hypo-Vascular Liver Metastases Treated with Transarterial Chemoembolization: Assessment of Early Response by Volumetric Contrast-Enhanced and Diffusion-Weighted Magnetic Resonance Imaging. Transl. Oncol. 2016, 9, 287–294. [Google Scholar] [CrossRef]
- Akinwande, O.K.; Philips, P.; Duras, P.; Pluntke, S.; Scoggins, C.; Martin, R.C.G. Small Versus Large-Sized Drug-Eluting Beads (DEBIRI) for the Treatment of Hepatic Colorectal Metastases: A Propensity Score Matching Analysis. CardioVascular Interv. Radiol. 2015, 38, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.G.; Howard, J.; Tomalty, D.; Robbins, K.; Padr, R.; Bosnjakovic, P.M.; Tatum, C. Toxicity of Irinotecan-Eluting Beads in the Treatment of Hepatic Malignancies: Results of a Multi-Institutional Registry. CardioVascular Interv. Radiol. 2010, 33, 960–966. [Google Scholar] [CrossRef]
- Tanaka, T.; Sato, T.; Nishiofuku, H.; Masada, T.; Tatsumoto, S.; Marugami, N.; Otsuji, T.; Kanno, M.; Koyama, F.; Sho, M.; et al. Selective TACE with Irinotecan-Loaded 40 Μm Microspheres and FOLFIRI for Colorectal Liver Metastases: Phase i Dose Escalation Pharmacokinetic Study. BMC Cancer 2019, 19, 758. [Google Scholar] [CrossRef]
- Kekez, D.; Badzek, S.; Prejac, J.; Gorsic, I.; Golem, H.; Librenjak, N.; Perkov, D.; Smiljanic, R.; Plestina, S. Fluorouracil, Leucovorin and Irinotecan Combined with Intra-Arterial Hepatic Infusion of Drugeluting Beads Preloaded with Irinotecan in Unresectable Colorectal Liver Metastases: Side Effects and Results of a Concomitant Treatment Schedule. Clinical Invest. Tumori J. 2014, 100, 499–503. [Google Scholar] [CrossRef]
| Performance Status (ECOG) n (%) | |||||
|---|---|---|---|---|---|
| 0 | 89 (59) | ||||
| 1 | 52 (34) | ||||
| 2 | 10 (7) | ||||
| 3 | 1 (1) | ||||
| Primary tumour location, n (%) | |||||
| Rectum (from the anal verge to 15 cm above) | 45 (30) | ||||
| Right colon (before splenic flexure) | 35 (23) | ||||
| Left colon (after splenic flexure) | 72 (47) | ||||
| TNM status of the primary tumour, n (%) | |||||
| Tis | N0 | 22 (14) | M0 | 40 (26) | |
| T1 | 9 (6) | N1a | 22 (14) | M1 | 87 (57) |
| T2 | 17 (12) | N1b | 29 (19) | Mx | 25 (16) |
| T3 | 98 (64) | N1c | 13 (9) | ||
| T4 | 28 (18) | N2a | 26 (17) | ||
| N2b | 21 (14) | ||||
| Nx | 19 (13) | ||||
| Time since primary cancer diagnosis, n (%) | |||||
| Synchronous (<6 months) | 103 (68) | ||||
| Metachronous (>6 months) | 49 (32) | ||||
| Previous lines of systemic chemotherapy n (%) | |||||
| no lines | 24 (16) | ||||
| 1–2 lines | 112 (73) | ||||
| 3 or more lines | 16 (11) | ||||
| Previous treatment with systemic irinotecan n (%) | 89 (58) | ||||
| Previous ablation on liver metastases n (%) | 17 (11) | ||||
| Previous intra-arterial treatment on liver metastases n (%) | 17 (11) | ||||
| Location, n (%) | |||||
| Whole liver | 87 (57) | ||||
| Only left liver lobe | 10 (7) | ||||
| Only right liver lobe | 55 (36) | ||||
| % of liver involvement, n (%) | |||||
| <25% | 82 (54) | ||||
| 25–50% | 59 (39) | ||||
| >50% | 11 (7) | ||||
| Number of lesions, n (%) | |||||
| 1 | 25 (16) | ||||
| 2–3 | 46 (30) | ||||
| 4–10 | 52 (34) | ||||
| >10 | 29 (19) | ||||
| Extrahepatic metastases, n (%) | |||||
| Yes | 66 (43) | ||||
| No | 86 (57) | ||||
| CEA increased, n (%) | 80 (53) | ||||
| CA 19.9 increased, n (%) | 64 (42) | ||||
| Molecular characterisation | |||||
| RAS, n (%) | BRAF, n (%) | ||||
| Yes | 49 (32) | Yes | 9 (6) | ||
| No | 56 (37) | No | 60 (39) | ||
| N/A | 47 (31) | N/A | 83 (55) | ||
| Treatment intention n (%) | |||||
| First line treatment or consolidation therapy after response to first line | 41 (27) | ||||
| Combination treatment with ablation with a curative intent | 19 (13) | ||||
| Intensification of treatment with/without concomitant therapy | 41 (27) | ||||
| Salvage treatment in progressive patients | 46 (30) | ||||
| Other | 5 (3) | ||||
| Number of treatments per patients | |||||
| 1 | 41 (27) | ||||
| 2 | 60 (39) | ||||
| 3 | 14 (9) | ||||
| 4 | 36 (24) | ||||
| 5 | 1 (<1) | ||||
| Bead size per treatment session (µm) | |||||
| 100 | 270 (77) | ||||
| 200 | 80 (23) | ||||
| 400 | 1 (<1) | ||||
| Dose per treatment session (mg) | |||||
| ≤50 | 44 (13) | ||||
| ≤100 | 246 (70) | ||||
| >100 | 61 (17) | ||||
| Treatment plan | |||||
| Completed | 121 (80) | ||||
| Aborted | 31 (20) | ||||
| Technical success | |||||
| Yes | 346 (99) | ||||
| No | 5 (1) | ||||
| Technical success due to | |||||
| Complete stasis | 59 (17) | ||||
| Complete delivery of the dose | 212 (60) | ||||
| Both | 75 (21) | ||||
| No technical success due to | |||||
| No complete delivery of the dose due to | 2 (<1) | ||||
| Immediate spasm of the artery | 3 (<1) | ||||
| Adverse Events at the Same Day as a Treatment Session | ||||
| Total AEs | 269 | |||
| Patients with at least 1 AE, n (%) | 91 (60) | |||
| Total grade 3 or 4 | 19 | |||
| Patients with at least 1 grade 3 or 4 AE, n (%) | 12 (8) | |||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Infusion related reaction | 0 | 0 | 1 | 0 |
| Post-embolization syndrome | 0 | 0 | 0 | 0 |
| Pain | 87 | 34 | 4 | 0 |
| Nausea/vomiting | 66 | 18 | 8 | 0 |
| Fever | 9 | 1 | 0 | 0 |
| Hypertension | 0 | 1 | 1 | 0 |
| Diarrhea | 0 | 1 | 0 | 0 |
| Hemorrhage (liver subcapsular hematoma) | 1 | 0 | 0 | 0 |
| Hypertonia | 0 | 0 | 3 | 0 |
| Platelet count decreased | 2 | 0 | 0 | 0 |
| Other | 3 1 | 6 2 | 2 3 | 0 |
| Adverse events within 30 days after any treatment session | ||||
| Total AEs | 115 | |||
| Patients with at least 1 AE, n (%) | 49 (32) | |||
| Total grade 3 or 4 | 33 | |||
| Patients with at least 1 grade 3 or 4 AE, n (%) | 21 (14) | |||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Pain | 15 | 18 | 4 | 0 |
| Fever | 8 | 2 | 0 | 1 |
| Nausea/vomiting | 6 | 10 | 5 | 0 |
| Diarrhoea | 2 | 2 | 0 | 0 |
| Alopecia | 0 | 0 | 1 | 0 |
| Hepatic failure | 0 | 0 | 1 | 0 |
| Cholecystitis | 0 | 1 | 1 | 0 |
| Vertigo | 2 | 0 | 0 | 0 |
| Asthenia/Fatigue | 0 | 2 | 0 | 0 |
| Neutropenia | 1 | 0 | 0 | 2 |
| Increase in serum creatinine | 1 | 0 | 0 | 0 |
| Increase in alkaline phosphatase | 28 | 4 | 0 | 0 |
| Increase in ALT | 21 | 3 | 2 | 0 |
| Increase in AST | 19 | 3 | 0 | 0 |
| Blood bilirubin increase | 6 | 0 | 2 | 1 |
| Thrombocytopenia | 2 | 0 | 0 | 0 |
| Lymphocyte count decreased | 23 | 4 | 0 | 0 |
| Hypoalbuminemia | 22 | 6 | 1 | 0 |
| LDH | 8 | 0 | 0 | 0 |
| Other | 4 4 | 5 5 | 6 6 | 4 7 |
| Variable | Reference/Units of Increase | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Treated liver lobes | unilobar | 1 | |
| bilobar | 3.1 (1.5–6.4) | 0.002 | |
| Percentage of liver involvement | <25% | 1 | |
| >25% | 2.9 (1.8–4.9) | <0.001 | |
| Bead size | 100 µm | 1 | |
| >100 µm | 3.9 (2.0–7.5) | <0.001 | |
| Dose | 1 mg | 0.99 (0.98–1.0) | <0.001 |
| Session number | 1 session | 0.7 (0.5–0.9) | 0.006 |
| Total number of sessions performed | 1 session | 0.6 (0.4–0.8) | 0.003 |
| Variable | Reference/Units of Increase | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Treated liver lobes | unilobar | 1 | |
| bilobar | 3.2 (1.4–7.5) | 0.06 | |
| ECOG | 0 | 1 | |
| >0 | 3.3 (1.8–6.1) | <0.001 | |
| Dose | 1 mg | 0.99 (0.98–1.00) | 0.04 |
| Total number of sessions performed | 1 session | 0.5 (0.3–0.7) | <0.001 |
| PES AE per Session | Grade | No Corticosteroids, n (%) n = 277 | Corticosteroids + Antiemetic, n (%) n = 74 | p Value |
|---|---|---|---|---|
| Fever | ||||
| G1 | 9 (3) | 0 (0) | ns | |
| G2 | 1 (<1) | 0 (0) | ns | |
| Nausea | ||||
| G1 | 32 (12) | 5 (7) | ns | |
| G2 | 9 (3) | 5 (7) | ns | |
| Vomiting | ||||
| G1 | 36 (13) | 2 (3) | 0.01 | |
| G2 | 6 (2) | 2 (3) | ns | |
| G3 | 8 (3) | 0 (0) | ns | |
| Pain | ||||
| G1 | 76 (27) | 10 (14) | 0.01 | |
| G2 | 22 (8) | 14 (19) | 0.009 | |
| G3 | 3 (1) | 0 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmberger, T.; Lucatelli, P.; Pereira, P.L.; Gjoreski, A.; Jovanoska, I.; Bansaghi, Z.; Spiliopoulos, S.; Carchesio, F.; Arnold, D.; Baierl, A.; et al. Safety, Feasibility and Technical Considerations from a Prospective, Observational Study—CIREL: Irinotecan-TACE for CRLM in 152 Patients. J. Clin. Med. 2022, 11, 6178. https://doi.org/10.3390/jcm11206178
Helmberger T, Lucatelli P, Pereira PL, Gjoreski A, Jovanoska I, Bansaghi Z, Spiliopoulos S, Carchesio F, Arnold D, Baierl A, et al. Safety, Feasibility and Technical Considerations from a Prospective, Observational Study—CIREL: Irinotecan-TACE for CRLM in 152 Patients. Journal of Clinical Medicine. 2022; 11(20):6178. https://doi.org/10.3390/jcm11206178
Chicago/Turabian StyleHelmberger, Thomas, Pierleone Lucatelli, Philippe L. Pereira, Aleksandar Gjoreski, Ivona Jovanoska, Zoltan Bansaghi, Stavros Spiliopoulos, Francesca Carchesio, Dirk Arnold, Andreas Baierl, and et al. 2022. "Safety, Feasibility and Technical Considerations from a Prospective, Observational Study—CIREL: Irinotecan-TACE for CRLM in 152 Patients" Journal of Clinical Medicine 11, no. 20: 6178. https://doi.org/10.3390/jcm11206178
APA StyleHelmberger, T., Lucatelli, P., Pereira, P. L., Gjoreski, A., Jovanoska, I., Bansaghi, Z., Spiliopoulos, S., Carchesio, F., Arnold, D., Baierl, A., Zeka, B., Kaufmann, N. C., Taieb, J., & Iezzi, R. (2022). Safety, Feasibility and Technical Considerations from a Prospective, Observational Study—CIREL: Irinotecan-TACE for CRLM in 152 Patients. Journal of Clinical Medicine, 11(20), 6178. https://doi.org/10.3390/jcm11206178








