1. Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death worldwide [
1]. Rectal cancer accounts for approximately 30% of CRC cases and has a 5-year survival rate of 67% [
2]. Locally advanced rectal cancer (LARC) is defined as a tumour invading pericolorectal tissues, adjacent organs, or regional lymph nodes. Neoadjuvant chemoradiation therapy (NCRT) followed by surgery (OP) is the standard treatment for LARC and allows for better resectability, anal sphincter preservation, local tumour control, and survival than OP alone [
3,
4]. Response to NCRT is correlated with survival in patients with LARC. However, only 18.1% of patients achieve a complete response to NCRT [
5]. Identifying prognostic factors predictive of survival for the remaining patients to guide further management remains an unmet medical need.
Microsatellite instability (MSI) is a well-known prognostic predictor of CRC. High MSI (MSI-H) is associated with better prognosis than low MSI or microsatellite stability [
6]. The prognostic benefit may result from DNA mismatch repair deficiency, which causes mutations that induce antigen and immunological cell infiltration [
7]. However, only 15% of CRCs are MSI-H. The tumour microenvironment (TME) consists of immunologic cells, surrounding blood vessels, fibroblasts, signalling molecules, and the extracellular matrix surrounding the cancer cells, and these factors may contribute to tumour occurrence, development, invasion, and metastasis [
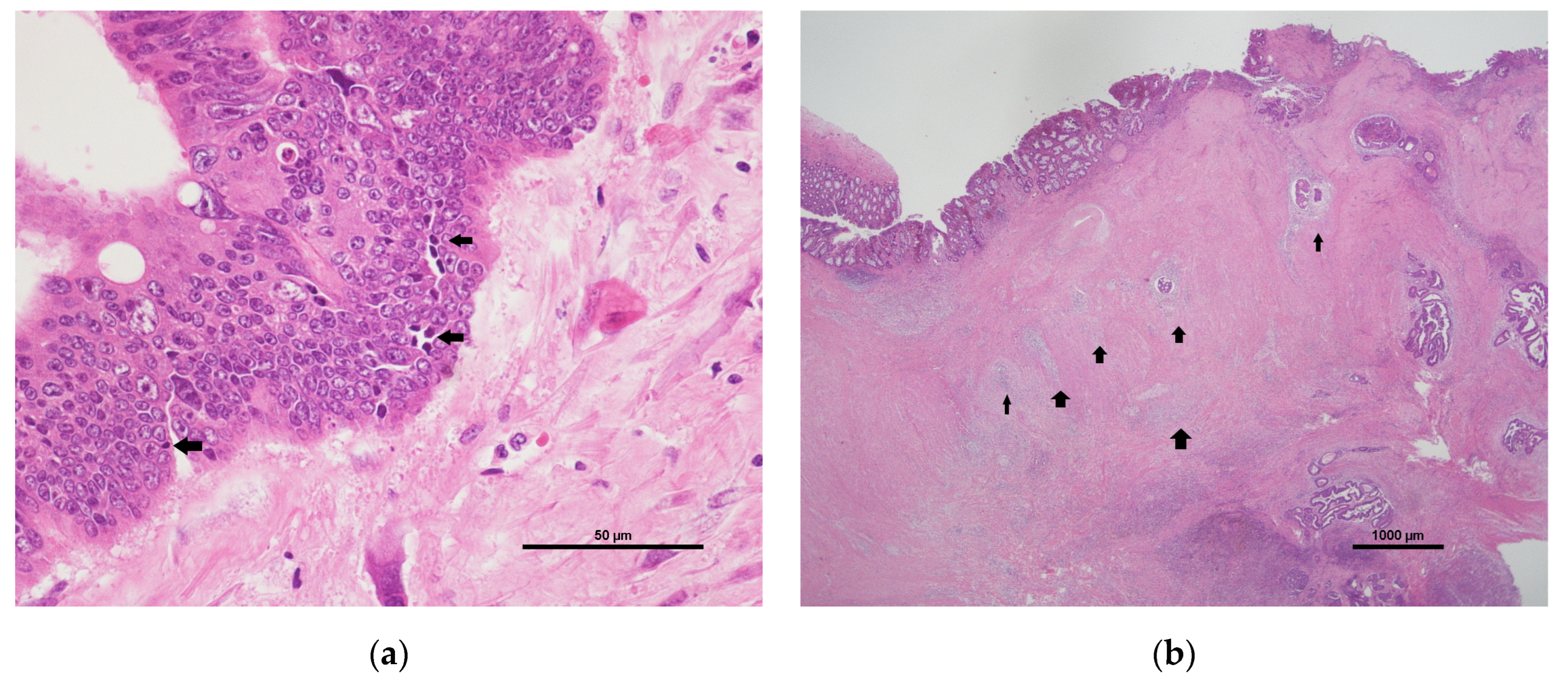
8]. The intratumoural lymphocytic response (ILR) and peritumoural lymphocytic reaction (PLR) are important components of the TME and may be related to tumour progression and treatment outcome [
9]. The ILR is a measurement of tumour-infiltrating lymphocytes (TILs), which can trigger the lysis of cancer cells. The PLR presents as peritumoural Crohn’s-like lymphoid aggregates. The ILR and PLR are also prognostic predictors of CRC [
10,
11]. Further, the ILR and PLR have been used for phenotypic characterisation of MSI. Therefore, these histopathological features could be used to interpret the immune phenotype of the TME. Finally, there are long-term follow-up data on patients in whom the ILR and PLR were evaluated, which allows assessment of the correlation between the TME and NCRT effects.
The interaction between the local immune response and systemic inflammation is the seventh hallmark of cancer. This interaction is associated with the initiation, development, and progression of several types of malignancies [
12]. White blood cell, neutrophil, lymphocyte, platelet, and C-reactive protein levels are closely related to cancer-related inflammation [
13]. Combinations of these parameters, including the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PtLR), play a crucial role in predicting tumour progression, recurrence, and metastasis [
14,
15,
16]. Further, several recent studies have identified NLR and PtLR as prognostic predictors in patients with CRC [
17,
18].
A previous study demonstrated that NCRT-induced DNA damage resulting in immunogenic cell death can potentially prime T-cell responses in LARC [
19]. However, the importance of the local immune response and systemic inflammation in patients with LARC receiving NCRT is not fully understood. This study aimed to investigate the significance of NCRT-induced local and systemic immune responses by evaluating prognostic predictors, such as the ILR, PLR, NLR, and PtLR, in LARC patients without a complete response to NCRT.
4. Discussion
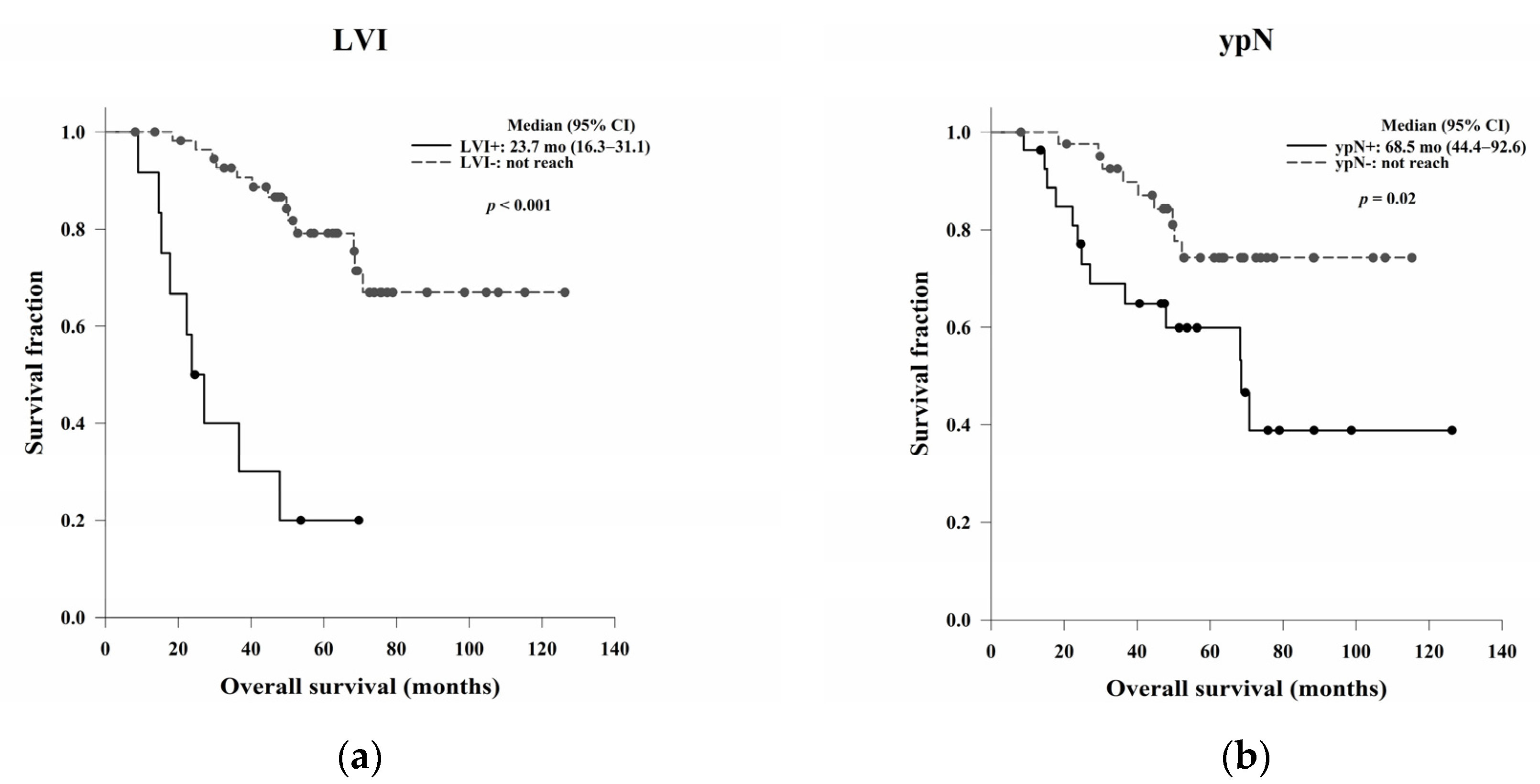
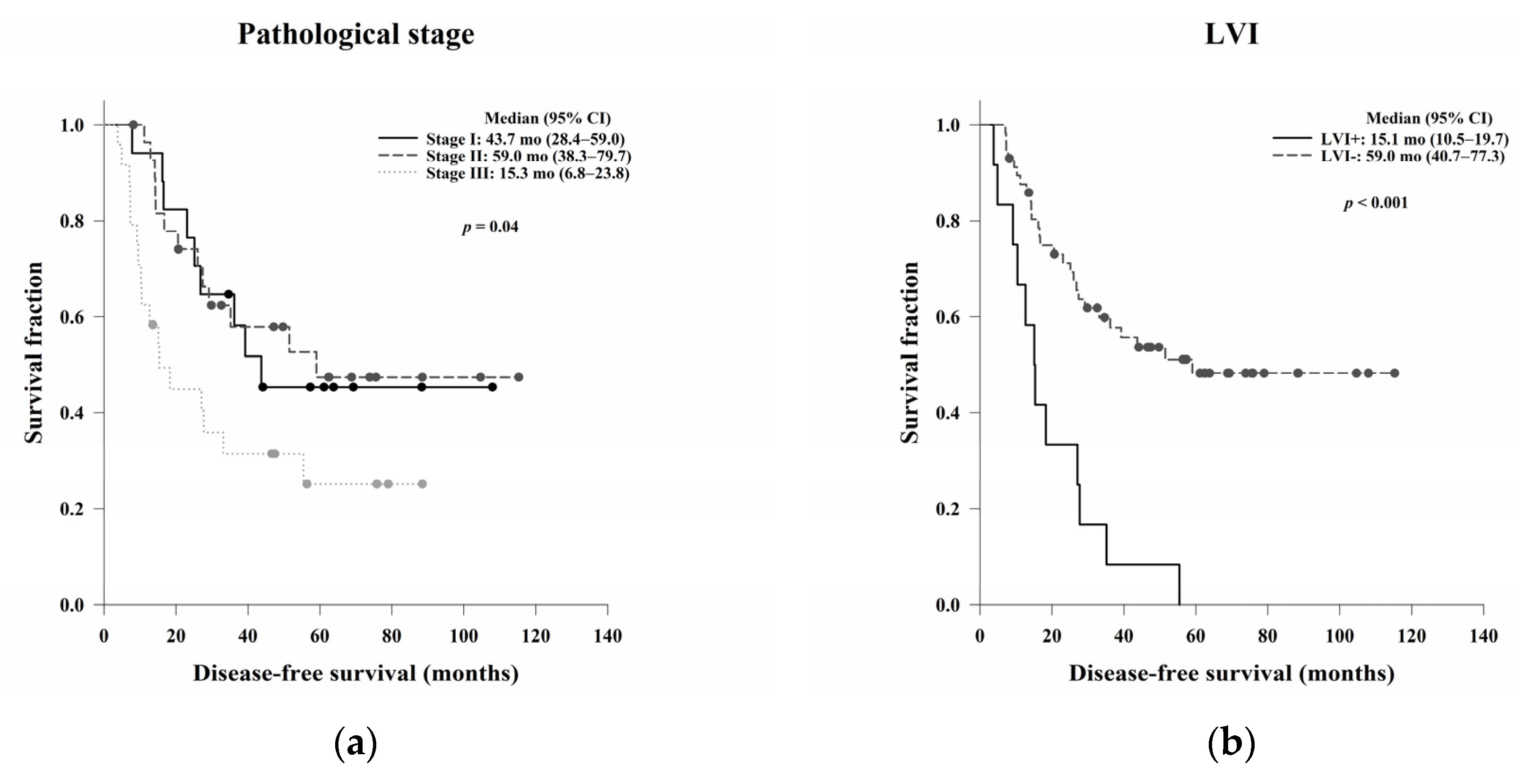
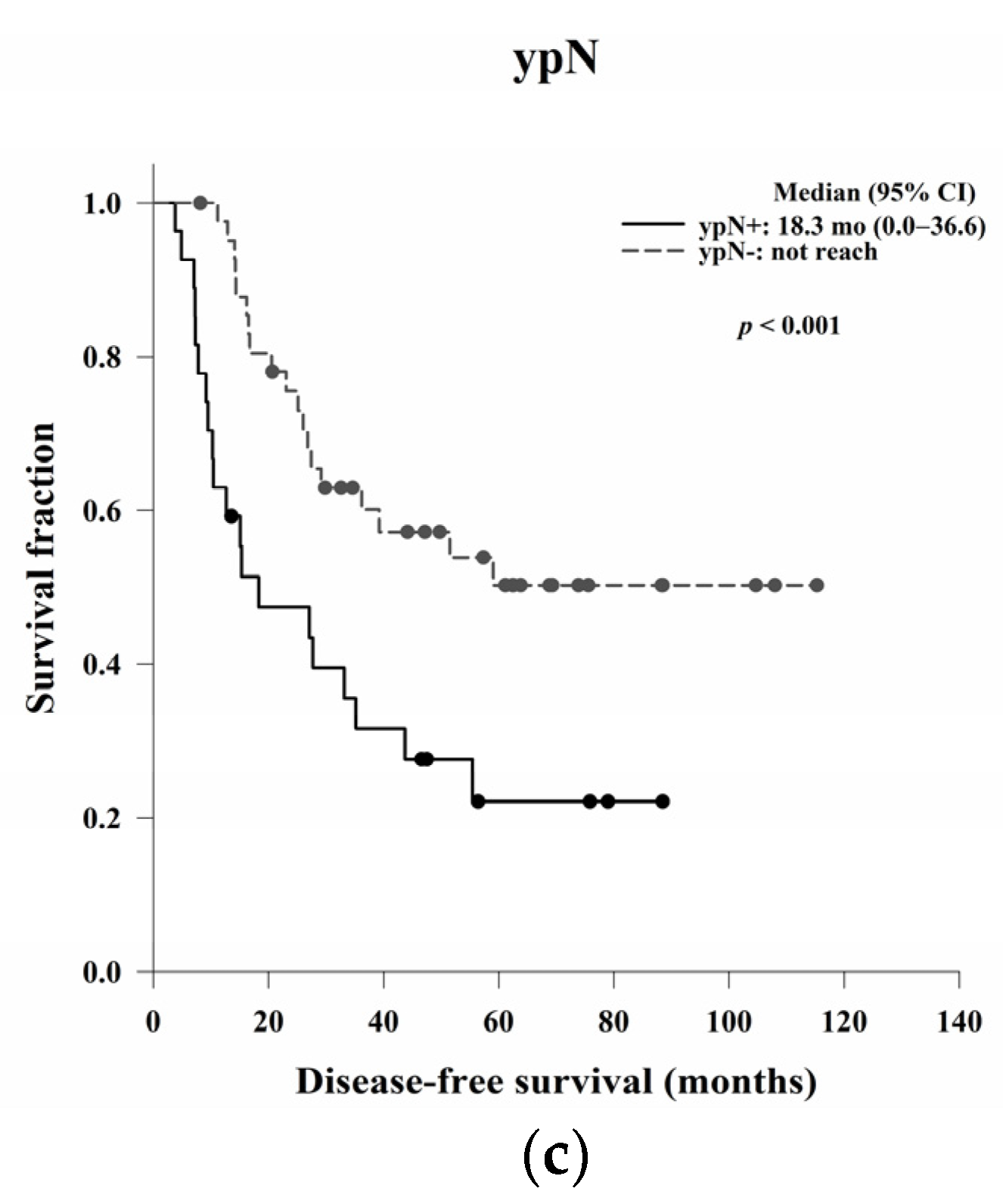
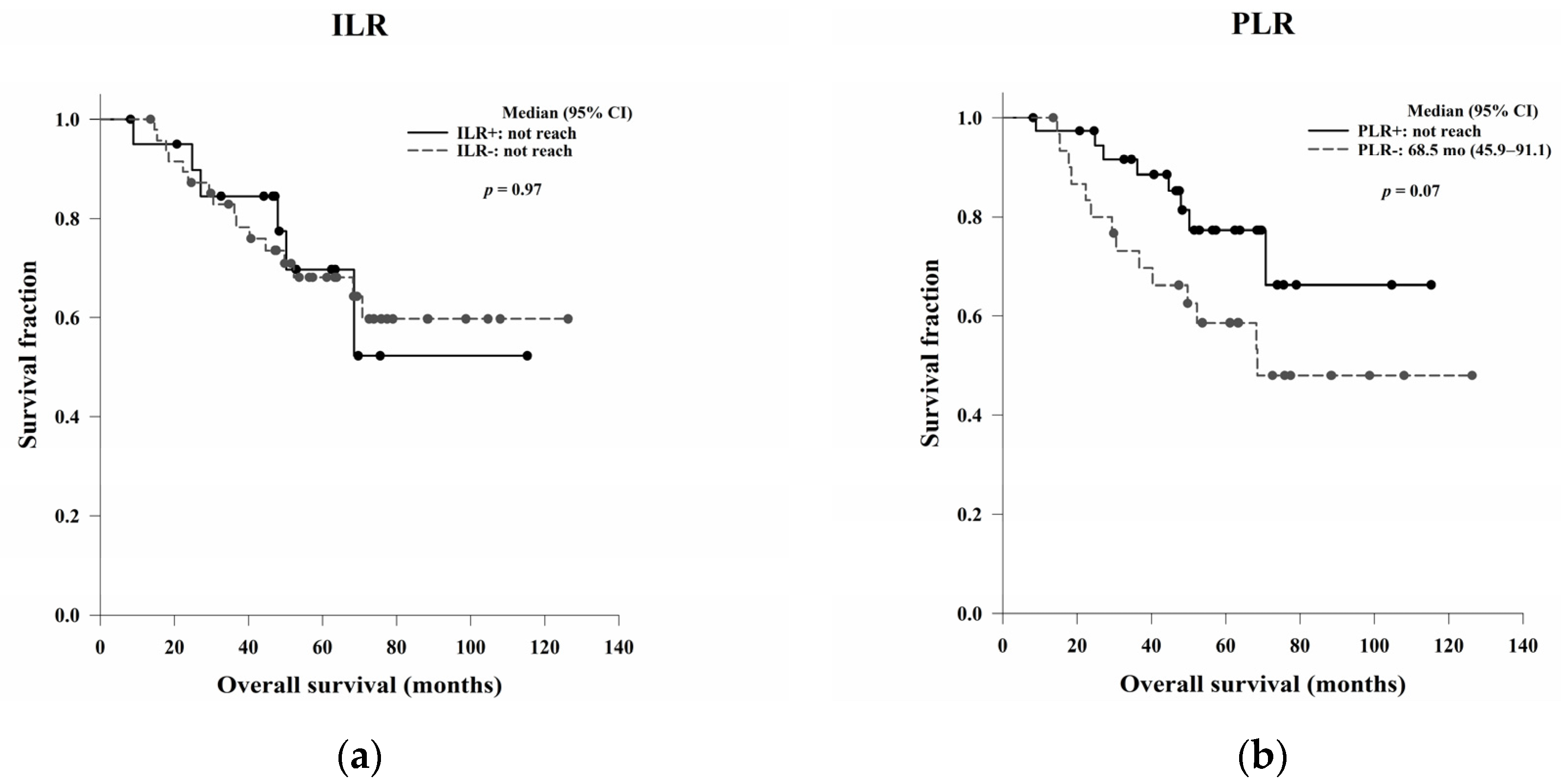
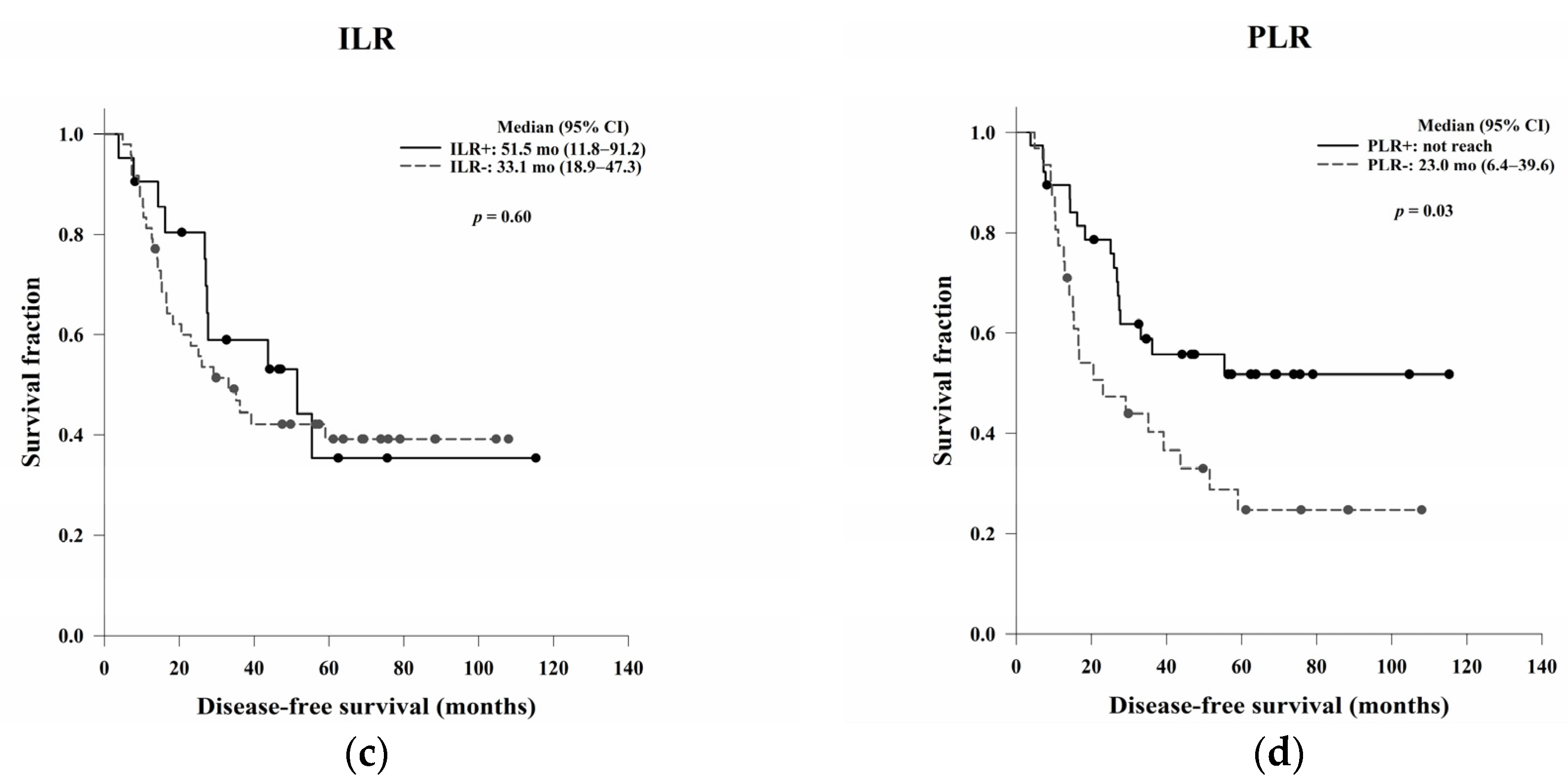
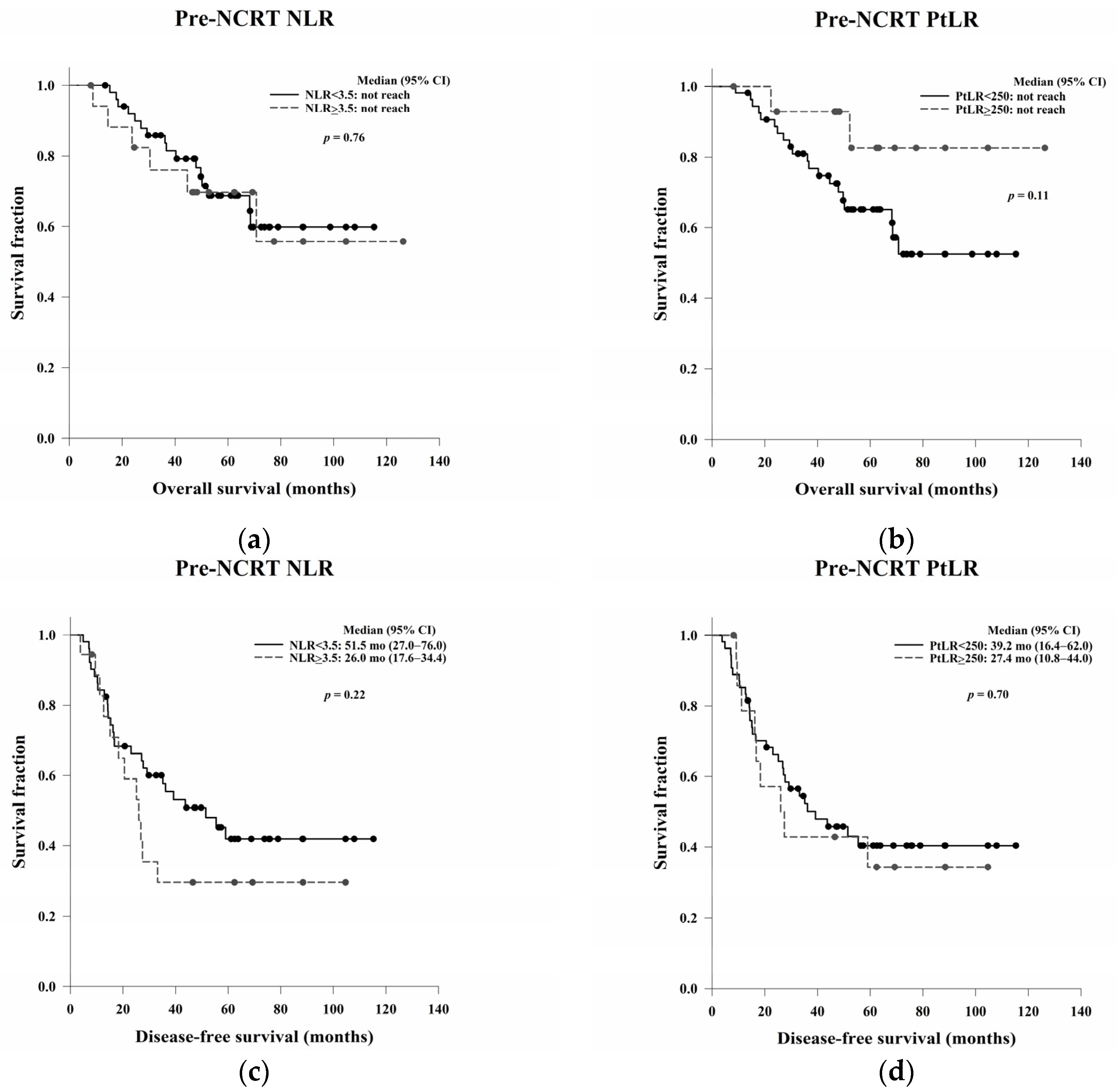
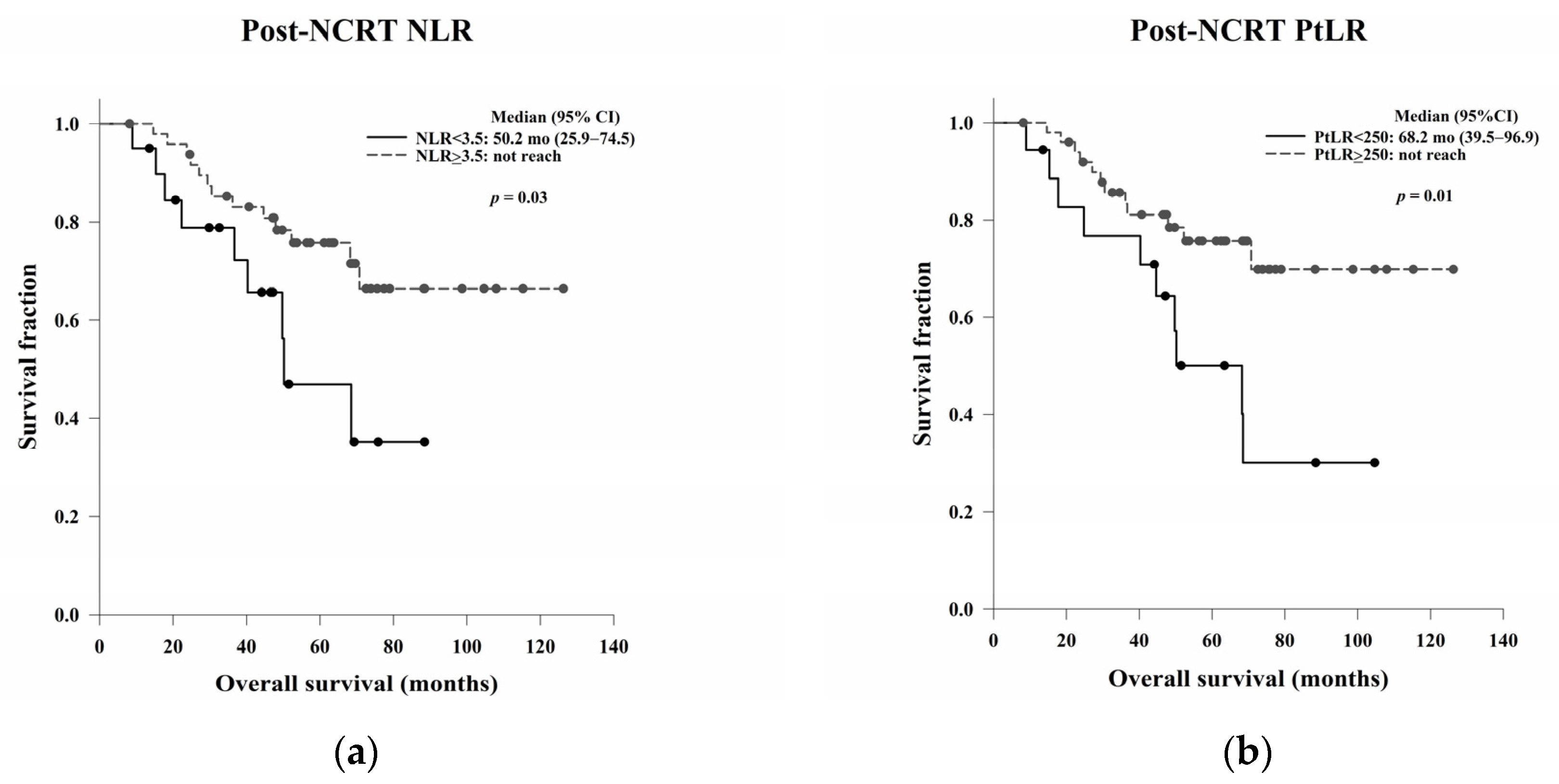
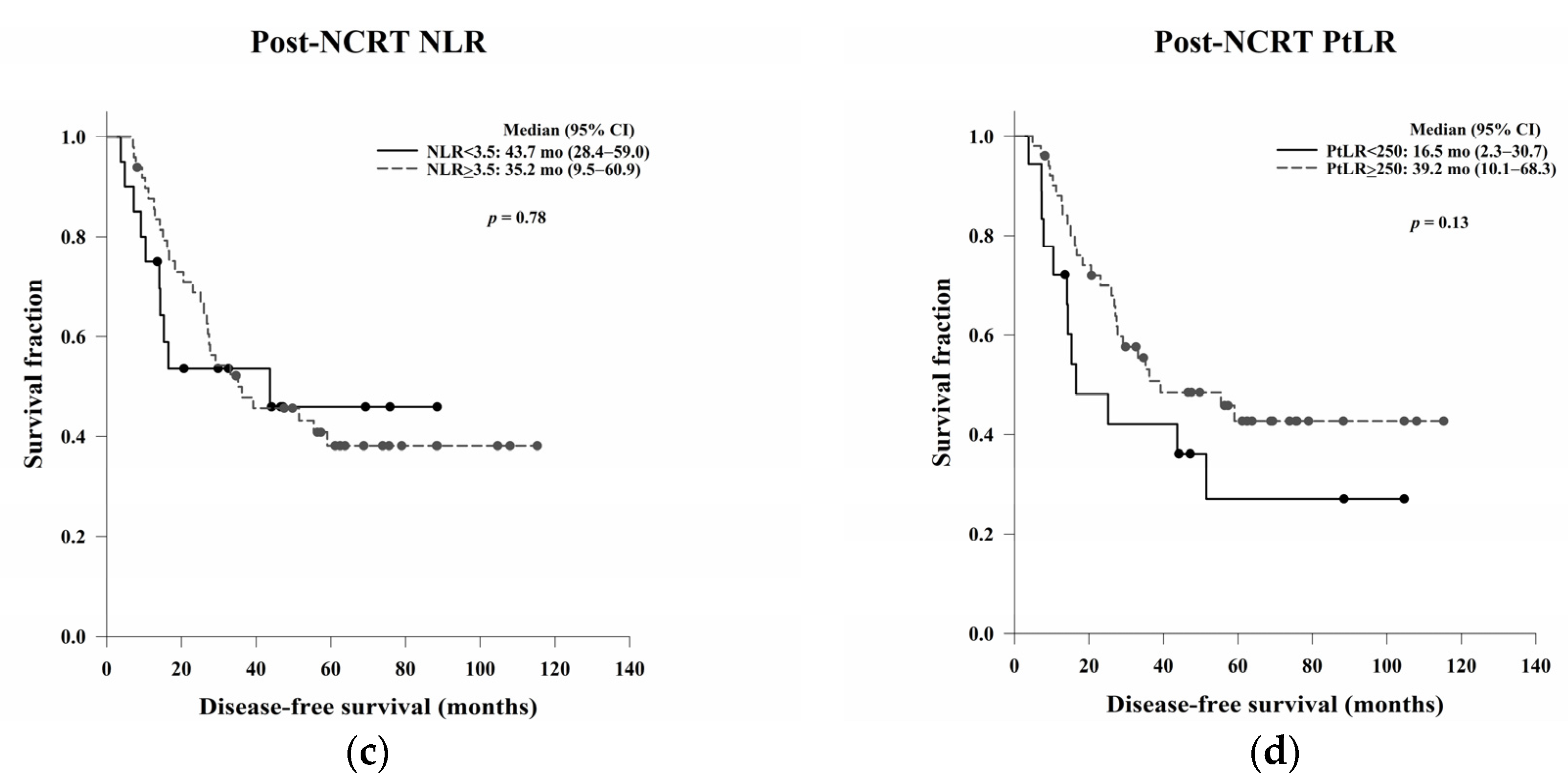
In this study, we aimed to evaluate the clinical, pathological, and blood count data of patients with LARC receiving NCRT without a complete response to identify prognostic predictors. In the survival analysis, LVI, ypN, and the post-NCRT NLR and PtLR were significant predictors of OS, and pathological stage, LVI, ypN, and the PLR were significant predictors of DFS. Univariate and multivariate analyses revealed that LVI, the post-NCRT neutrophil count, and the lymphocyte count significantly influenced OS and that LVI and PLR were independent predictors of DFS.
Previous studies have investigated the association between the ILR and PLR and survival in patients with CRC. Rozek et al. examined the clinical and pathological characteristics of survival in 2369 CRC patients and reported that a high ILR and PLR were associated with a statistically significant increase in OS and CRC-specific survival [
25]. Ogino et al. also proposed that increased overall lymphocytic reactions in CRC, including the ILR and PLR, were related to a significant improvement in OS and CRC-specific survival, independent of lymphocyte count and MSI phenotype [
26]. Väyrynen et al. demonstrated superior survival with high PLR density compared to low PLR density in 567 patients with CRC. Therefore, quantitative evaluation of the PLR is recommended as a prognostic indicator for CRC [
27].
There is a relative lack of data concerning the TME as a prognostic predictor for LARC patients receiving NCRT. Chen et al. reviewed a series of 126 LARC patients treated with NCRT and found that the post-NCRT stromal-infiltrating CD8+ T cell density corresponded with tumour regression grades, distant metastasis rates, and DFS [
28]. Zhang et al. documented 109 LARC patients treated with NCRT and concluded that high levels of CD4+, CD8+, and PD-L1+ TILs were associated with a favourable response to NCRT, whereas high levels of FOXP3+ TILs were associated with a poor response [
29]. Akiyoshi et al. noted that CD8+ T cells may be a key element in the response to NCRT, whereas immune checkpoint molecules could be therapeutic targets to enhance tumour regression [
30]. Our data showed that the PLR may be a prognostic predictor of DFS in LARC patients receiving NCRT, which is consistent with the results of previous studies assessing NCRT-induced cytotoxic immune response in the TME of rectal cancer [
31].
The NLR and PtLR are indicators of systemic inflammation and are easily affected by factors such as trauma, local or systemic infection, acute coronary syndromes, and malignancies. Some studies have examined the poor pathological response, OS, and DFS associated with an elevated pre- and post-NCRT NLR and PtLR in patients with LARC receiving NCRT [
32,
33,
34,
35,
36]. However, an increased post-NCRT NLR and PtLR significantly correlated with improved OS in our study. This indicates that NCRT-induced systemic inflammation could be a positive prognostic indicator.
Neutrophils secrete various cytokines to stimulate tumour growth, capillary proliferation, and metastasis. High neutrophil counts can upregulate growth factors such as chemokines to induce tumour progression [
37]. Platelets secrete platelet-derived growth factor, vascular endothelial growth factor, platelet factor 4, and transforming growth factor-β to increase angiogenesis, microvascular invasion, and cancer cell extravasation to promote tumour progression and metastasis [
38]. Lymphocytes can induce cell death and inhibit cancer cell proliferation and migration. Low lymphocyte counts are associated with a reduced antitumour immune response, which leads to tumour growth and disease progression [
39]. Therefore, high neutrophil and platelet counts and low lymphocyte counts are negative prognostic predictors in several malignancies [
40,
41,
42]. In our study, high post-NCRT neutrophil levels were associated with poor OS. However, patients with low post-NCRT lymphocyte counts had better OS than those with high lymphocyte counts, which differed from the results of previous studies [
43,
44,
45]. This implies that decreased lymphocyte counts after NCRT may be associated with a better prognosis.
Few studies have focused on decreases in lymphocyte counts and their impact on response to NCRT in LARC. Wu et al. conducted a prospective phase III study that indicated that decreased lymphocyte counts during NCRT were an independent predictor of tumour regression [
46]. Heo et al. assessed the correlation between lymphocyte subpopulation counts during NCRT and tumour responses in LARC and reported that decreased lymphocyte counts during NCRT were related to good treatment responses, especially decreases in natural killer cells [
47]. Ishihara et al. verified the effects of radiation-induced apoptosis in peripheral blood lymphocytes, which was associated with the extent of tumour regression in patients with LARC after NCRT [
48]. From these studies, we may assume that the intrinsic radiosensitivity of cancer cells and circulating lymphocytes could be correlated, which has been validated by previous studies of RT in various cancer types [
49,
50,
51,
52]. The increases of NLR and PtLR after NCRT may be related to systemic inflammation, bone marrow suppression with decreases in lymphocyte counts, or intrinsic radiosensitivity of cancer cells.
In the Kaplan–Meier analysis, we reported that high post-NCRT NLR and PtLR were significantly associated with better OS. Then, we wanted to do a further analysis for the impacts of neutrophil, lymphocyte, and platelet counts separately, not only the ratio. Therefore, the counts not the ratio were used in the following univariate and multivariate Cox regression analyses. In the multivariate analysis, LVI, post-NCRT neutrophil count, and lymphocyte count were independent predictors of OS, and LVI and PLR were independent predictors of DFS. LVI has been considered as a well-known and strong stage-independent prognostic predictor of OS and DFS in LARC. Neutrophils possess an intrinsic ability to secrete cytokines to stimulate tumour growth, so higher post-NCRT neutrophil counts may be related to a poorer OS. In the literature, decreased lymphocyte counts during NCRT have been shown to be an independent predictor of treatment responses. The lower lymphocyte counts may be associated with the better radiosensitivity of cancer cells and circulating lymphocytes and better OS. The PLR is a local not systemic NCRT responder, which may be related to DFS.
Our study had some limitations. First, CD4+, CD8+, PD-L1+, and FOXP3+ TILs were not analysed separately. If additional stains had been done with immunohistochemistry data available, we may have been able to assess the prognostic effects of these individual lymphocyte types. In this study, we found that a high PLR without special stains significantly related to better DFS. This preliminary result may be a practical indicator for clinical use. Second, different lymphocyte subpopulations, i.e., T lymphocytes, B lymphocytes, and natural killer cells were not studied. Different immune functions and treatment responses are observed in each subpopulation; therefore, these subpopulations should be independently inspected. Third, the number of patients in our retrospective study was too small to draw firm conclusions. Fourth, the causal relationship between local immune response, systemic inflammation, and good prognosis remained undetermined and may require further investigation. Fifth, quantitative evaluation of the ILR and PLR density should be performed in future research. Sixth, the ILR and PLR were assessed by two pathologists according to the cancer reporting protocol released by the College of American Pathologists in 2013. The bias of interobserver variability should be examined. Finally, local or systemic inflammation may have influenced our results. All possible aetiologies that could affect the TME or circulating lymphocytes should be surveyed in the future.