Comparison of Corneal Higher-Order Aberrations Following Topography-Guided LASIK and SMILE for Myopic Correction: A Propensity Score Matching Analysis
Abstract
1. Introduction
2. Materials and Methods
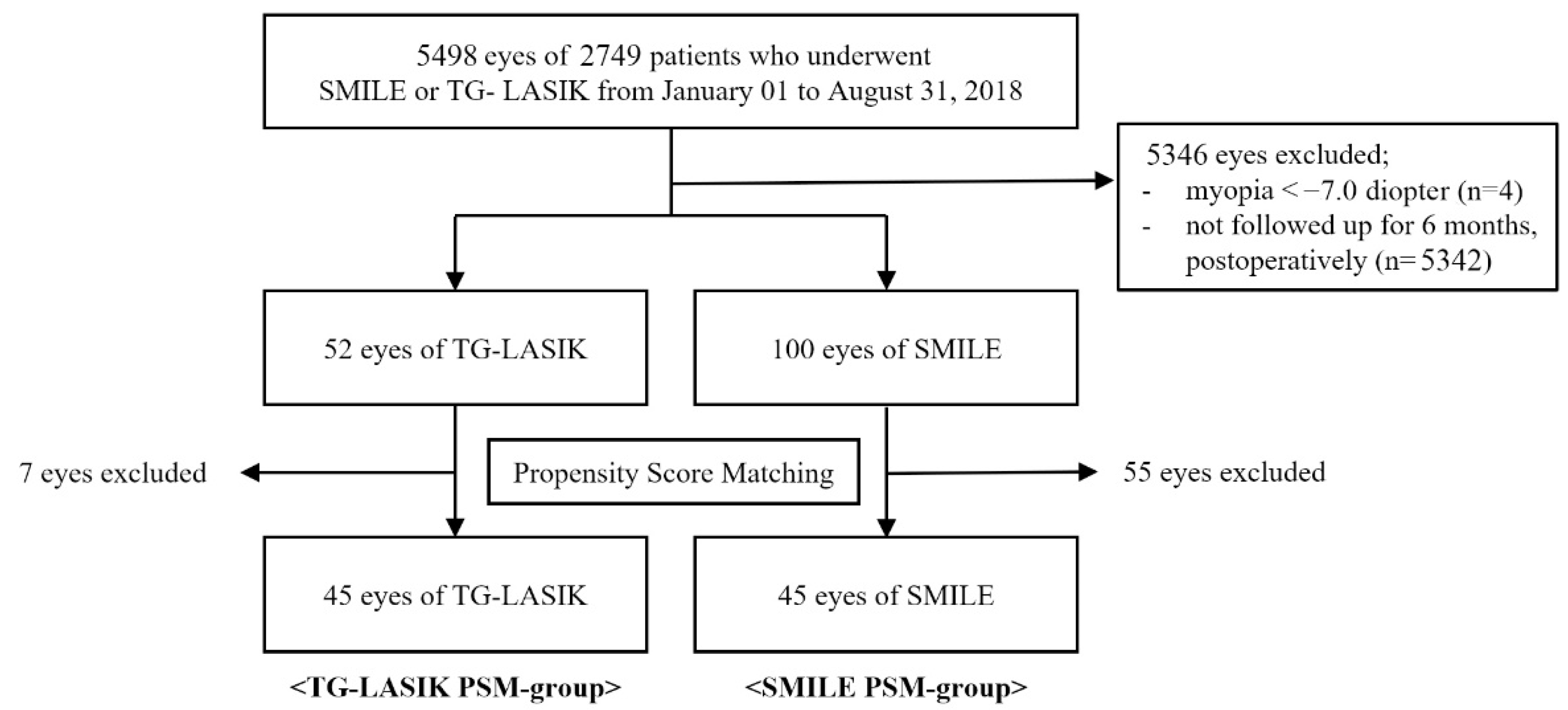
2.1. Patients
2.2. Preoperative Examination
2.3. Surgical Technique
2.3.1. TG-LASIK Procedure
2.3.2. SMILE Procedure
2.4. Postoperative Examination
2.5. Measurement of HOAs
2.6. Statistical Analysis
3. Results
3.1. Preoperative Characteristics of Both Surgical Groups Both Surgical Procedures after Propensity Score Matching
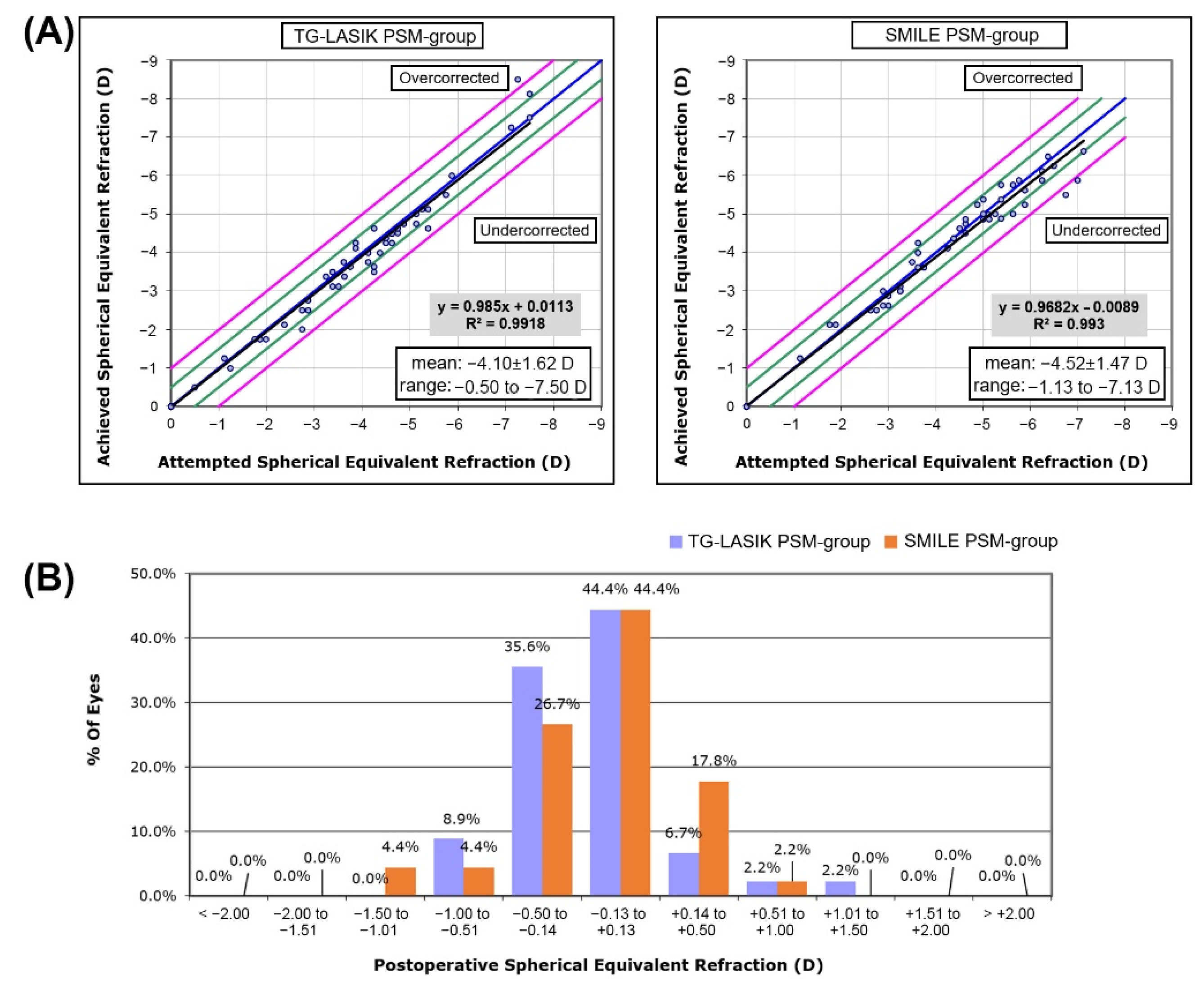
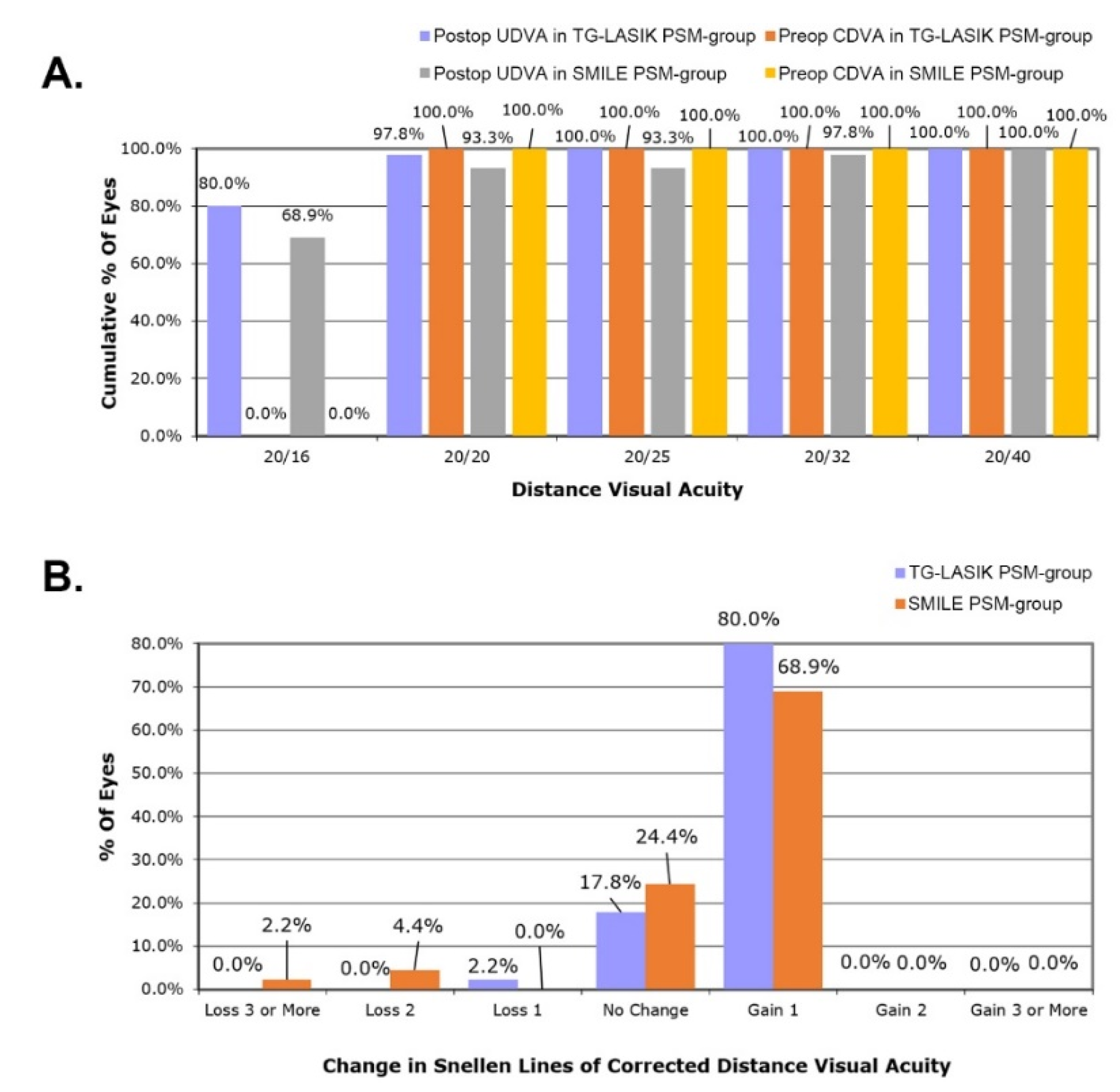
3.2. Comparison of Refractive Outcome and Visual Acuity between Two Propensity-Score-Matched Groups after Each Surgical Procedures
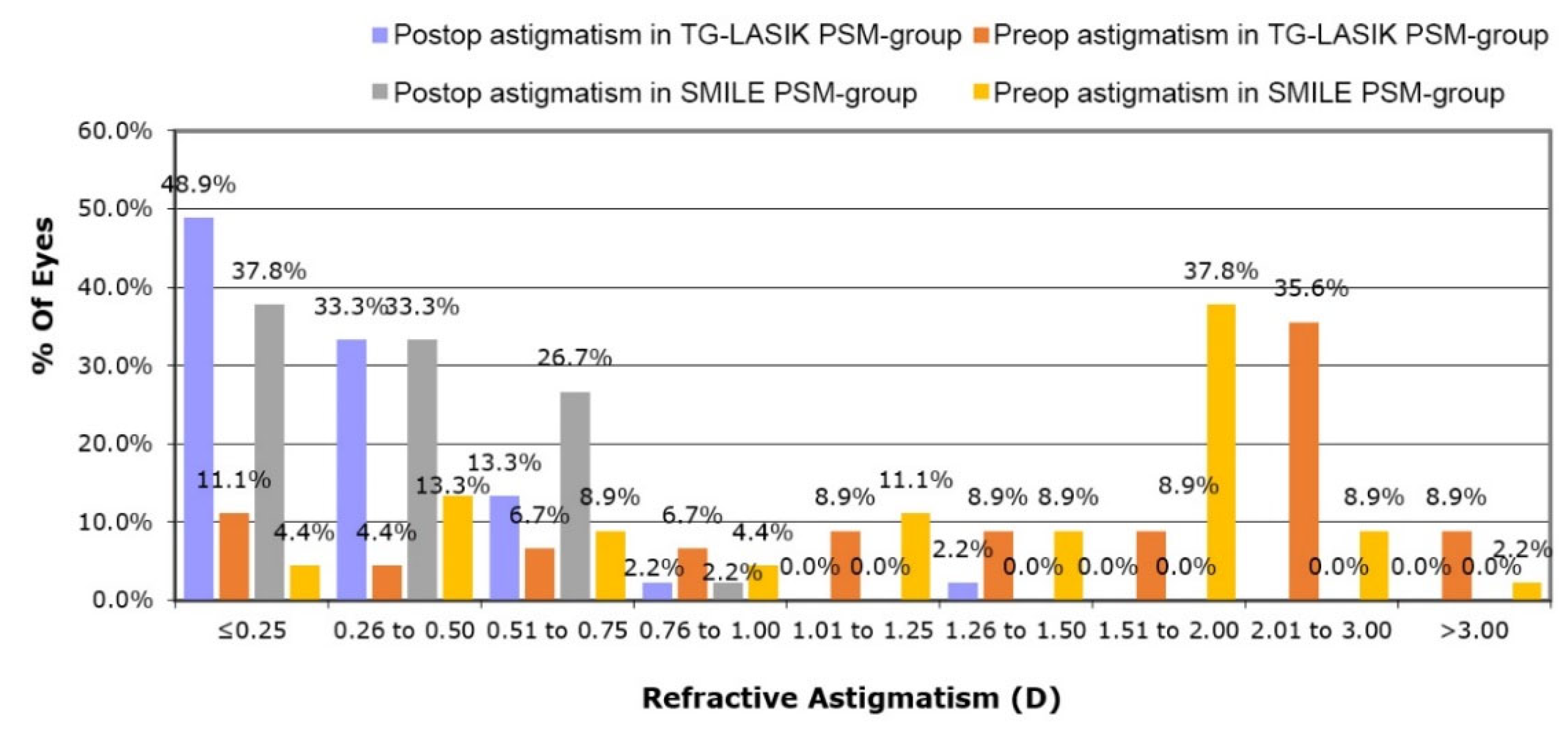
3.3. Changes in Astigmatism and Corneal Aberration after Surgical Procedures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atchison, D.A.; Suheimat, M.; Mathur, A.; Lister, L.J.; Rozema, J. Anterior Corneal, Posterior Corneal, and Lenticular Contributions to Ocular Aberrations. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5263–5270. [Google Scholar] [CrossRef]
- Tiwari, N.N.; Sachdev, G.S.; Ramamurthy, S.; Dandapani, R. Comparative analysis of visual outcomes and ocular aberrations following wavefront optimized and topography-guided customized femtosecond laser in situ keratomileusis for myopia and myopic astigmatism: A contralateral eye study. Indian J. Ophthalmol. 2018, 66, 1558–1561. [Google Scholar] [CrossRef]
- Ye, M.J.; Liu, C.Y. SMILE and Wavefront-Guided LASIK Out-Compete Other Refractive Surgeries in Ameliorating the Induction of High-Order Aberrations in Anterior Corneal Surface. 2016, 2016, 8702162. J Ophthalmol . 2016, 2016, 8702162. [Google Scholar] [CrossRef]
- Gyldenkerne, A.; Ivarsen, A.; Hjortdal, J.O. Comparison of corneal shape changes and aberrations induced by FS-LASIK and SMILE for myopia. J. Refract. Surg. 2015, 31, 223–229. [Google Scholar] [CrossRef]
- Kamiya, K.; Shimizu, K.; Igarashi, A.; Kobashi, H.; Komatsu, M. Comparison of visual acuity, higher-order aberrations and corneal asphericity after refractive lenticule extraction and wavefront-guided laser-assisted in situ keratomileusis for myopia. Br. J. Ophthalmol. 2013, 97, 968–975. [Google Scholar] [CrossRef]
- El Awady, H.E.; Ghanem, A.A.; Saleh, S.M. Wavefront-optimized ablation versus topography-guided customized ablation in myopic LASIK: Comparative study of higher order aberrations. Ophthalmic Surg. Lasers Imaging Retin. 2011, 42, 314–320. [Google Scholar] [CrossRef]
- McAlinden, C.; Moore, J.E. The change in internal aberrations following myopic corneal laser refractive surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 775–781. [Google Scholar] [CrossRef]
- Schallhorn, J.M.; Seifert, S.; Schallhorn, S.C. SMILE, Topography-Guided LASIK, and Wavefront-Guided LASIK: Review of Clinical Outcomes in Premarket Approval FDA Studies. J. Refract. Surg. 2019, 35, 690–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y. A Randomized Comparative Study of Topography-Guided Versus Wavefront-Optimized FS-LASIK for Correcting Myopia and Myopic Astigmatism. J. Refract. Surg. 2019, 35, 575–582. [Google Scholar] [CrossRef]
- Motwani, M. The use of WaveLight® Contoura to create a uniform cornea: The LYRA Protocol. Part 3: The results of 50 treated eyes. Clin. Ophthalmol. 2017, 11, 915–921. [Google Scholar] [CrossRef][Green Version]
- Moshirfar, M.; Shah, T.J.; Skanchy, D.F.; Linn, S.H.; Kang, P.; Durrie, D.S. Comparison and analysis of FDA reported visual outcomes of the three latest platforms for LASIK: Wavefront guided Visx iDesign, topography guided WaveLight Allegro Contoura, and topography guided Nidek EC-5000 CATz. Clin. Ophthalmol. 2017, 11, 135–147. [Google Scholar] [CrossRef]
- Shetty, R.; Shroff, R.; Deshpande, K.; Gowda, R.; Lahane, S.; Jayadev, C. A Prospective Study to Compare Visual Outcomes Between Wavefront-optimized and Topography-guided Ablation Profiles in Contralateral Eyes with Myopia. J. Refract. Surg. 2017, 33, 6–10. [Google Scholar] [CrossRef]
- Kanellopoulos, A.J. Topography-Guided LASIK versus Small Incision Lenticule Extraction: Long-term Refractive and Quality of Vision Outcomes. Ophthalmology 2018, 125, 1658–1659. [Google Scholar] [CrossRef]
- Kanellopoulos, A.J. Topography-Guided LASIK Versus Small Incision Lenticule Extraction (SMILE) for Myopia and Myopic Astigmatism: A Randomized, Prospective, Contralateral Eye Study. J. Refract. Surg. 2017, 33, 306–312. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.H.; Lim, D.H.; Yang, C.M.; Yoon, G.J.; Chung, T.Y. Topography-guided versus wavefront-optimized laser in situ keratomileusis for myopia: Surgical outcomes. J. Cataract Refract. Surg. 2019, 45, 959–965. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Hashemi, M.; Modarres, M.; Sanjari, M.S.; Darvish, N.; Gordiz, A. Topography-Guided vs Wavefront-Optimized Surface Ablation for Myopia Using the WaveLight Platform: A Contralateral Eye Study. J. Refract. Surg. 2011, 27, 13–17. [Google Scholar] [CrossRef]
- Kim, T.I.; Alio Del Barrio, J.L.; Wilkins, M.; Cochener, B.; Ang, M. Refractive surgery. Lancet 2019, 393, 2085–2098. [Google Scholar] [CrossRef]
- Raevdal, P.; Grauslund, J.; Vestergaard, A.H. Comparison of corneal biomechanical changes after refractive surgery by noncontact tonometry: Small-incision lenticule extraction versus flap-based refractive surgery—A systematic review. Acta Ophthalmol. 2019, 97, 127–136. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Zhang, L.; Wei, S.; Tang, X. Corneal biomechanical effects: Small-incision lenticule extraction versus femtosecond laser-assisted laser in situ keratomileusis. J. Cataract Refract. Surg. 2014, 40, 954–962. [Google Scholar] [CrossRef]
- Ivarsen, A.; Asp, S.; Hjortdal, J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology 2014, 121, 822–828. [Google Scholar] [CrossRef]
- Sinha Roy, A.; Dupps, W.J., Jr.; Roberts, C.J. Comparison of biomechanical effects of small-incision lenticule extraction and laser in situ keratomileusis: Finite-element analysis. J. Cataract Refract. Surg. 2014, 40, 971–980. [Google Scholar] [CrossRef]
- Yan, H.; Gong, L.-Y.; Huang, W.; Peng, Y.-L. Clinical outcomes of small incision lenticule extraction versus femtosecond laser-assisted LASIK for myopia: A Meta-analysis. Int. J. Ophthalmol. 2017, 10, 1436. [Google Scholar]
- Liu, M.; Chen, Y.; Wang, D.; Zhou, Y.; Zhang, X.; He, J.; Zhang, T.; Sun, Y.; Liu, Q. Clinical Outcomes After SMILE and Femtosecond Laser-Assisted LASIK for Myopia and Myopic Astigmatism: A Prospective Randomized Comparative Study. Cornea 2016, 35, 210–216. [Google Scholar] [CrossRef]
- Klokova, O.A.; Sakhnov, S.N.; Geydenrikh, M.S.; Damashauskas, R.O. Quality of life after refractive surgery: ReLEx SMILE vs Femto-LASIK. Clin. Ophthalmol. 2019, 13, 561–570. [Google Scholar] [CrossRef]
- Lin, F.; Xu, Y.; Yang, Y. Comparison of the visual results after SMILE and femtosecond laser-assisted LASIK for myopia. J. Refract. Surg. 2014, 30, 248–254. [Google Scholar] [CrossRef]
- Wan, X.H.; Li, S.M.; Xiong, Y.; Liang, Y.B.; Li, J.; Wang, F.H.; Li, J.; Jhanji, V.; Wang, N.L. Ocular monochromatic aberrations in a rural Chinese adult population. Optom. Vis. Sci. 2014, 91, 68–75. [Google Scholar] [CrossRef]
- Buhren, J.; Nagy, L.; Yoon, G.; MacRae, S.; Kohnen, T.; Huxlin, K.R. The effect of the asphericity of myopic laser ablation profiles on the induction of wavefront aberrations. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2805–2812. [Google Scholar] [CrossRef]
- Calvo, R.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M.; Patel, S.V. Corneal aberrations and visual acuity after laser in situ keratomileusis: Femtosecond laser versus mechanical microkeratome. Am. J. Ophthalmol. 2010, 149, 785–793. [Google Scholar] [CrossRef][Green Version]
- Stulting, R.D.; Fant, B.S.; Bond, W.; Chotiner, B.; Durrie, D.; Gordon, M.; Milauskas, A.; Moore, C.; Slade, S.; Randleman, J.B.; et al. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J. Cataract Refract. Surg. 2016, 42, 11–18. [Google Scholar] [CrossRef]
- Hu, P.C.; Li, L.; Wu, X.H.; Li, Y.Q.; Li, K.W. Visual differences in topography-guided versus wavefront-optimized LASIK in the treatment of myopia: A Meta-analysis. Int. J. Ophthalmol. 2021, 14, 1602–1609. [Google Scholar] [CrossRef]
- Wallerstein, A.; Caron-Cantin, M.; Gauvin, M.; Adiguzel, E.; Cohen, M. Primary Topography-Guided LASIK: Refractive, Visual, and Subjective Quality of Vision Outcomes for Astigmatism 2.00 Diopters. J. Refract. Surg. 2019, 35, 78–86. [Google Scholar] [CrossRef] [PubMed]
| SMILE PSM-Group (n = 45) | TG-LASIK PSM-Group (n = 45) | p-Value | |
|---|---|---|---|
| Sex, male, % | 44.4 | 46.7 | 0.832 |
| Age, years | 24.9 ± 4.86 | 25.9 ± 5.34 | 0.307 |
| Sphere, diopter | −3.80 ± 1.47 | −3.23 ± 1.66 | 0.092 |
| Cylinder, diopter | −1.44 ± 0.68 | −1.73 ± 1.09 | 0.096 |
| SEQ, diopter | −4.52 ± 1.47 | −4.10 ± 1.62 | 0.178 |
| CDVA, logMAR | 0.00 ± 0.00 | 0.0011 ± 0.0075 | 0.323 |
| IOP, mmHg | 14.2 ± 2.77 | 14.8 ± 1.91 | 0.235 |
| Pupil size, mm | 6.79 ± 0.58 | 6.79 ± 0.65 | 0.955 |
| CCT, μm | 554 ± 25.8 | 545 ± 24.4 | 0.079 |
| Flat K, diopter | 42.4 ± 1.39 | 42.1 ± 1.59 | 0.523 |
| Steep K, diopter | 44.4 ± 1.39 | 44.3 ± 1.77 | 0.836 |
| Optical zone, mm | 6.43 ± 0.18 | 6.5 | |
| Vertical coma, μm | 0.0392 ± 0.206 | 0.0129 ± 0.294 | 0.655 |
| Horizontal coma, μm | 0.0401 ± 0.152 | 0.0087 ± 0.170 | 0.331 |
| Spherical aberration, μm | 0.197 ± 0.0970 | 0.204 ± 0.0756 | 0.644 |
| Total HOAs, μm | 0.476 ± 0.145 | 0.577 ± 0.477 | 0.165 |
| SMILE PSM-Group (n = 45) | TG-LASIK PSM-Group (n = 45) | p-Value | |
|---|---|---|---|
| Sphere, diopter | 0.139 ± 0.383 | 0.100 ± 0.378 | 0.653 |
| Cylinder, diopter | −0.472 ± 0.234 | −0.439 ± 0.262 | 0.562 |
| SEQ, diopter | −0.0974 ± 0.369 | −0.119 ± 0.353 | 0.782 |
| UDVA, logMAR | −0.0367 ± 0.121 | −0.0722 ± 0.0627 | 0.087 |
| Steep K, diopter | 39.1 ± 1.56 | 39.4 ± 2.05 | 0.527 |
| Flat K, diopter | 40.1 ± 1.70 | 40.1 ± 2.24 | 0.929 |
| Preoperative | Postoperative | Induced HOAs | p-Value | |
|---|---|---|---|---|
| SMILE PSM-group | ||||
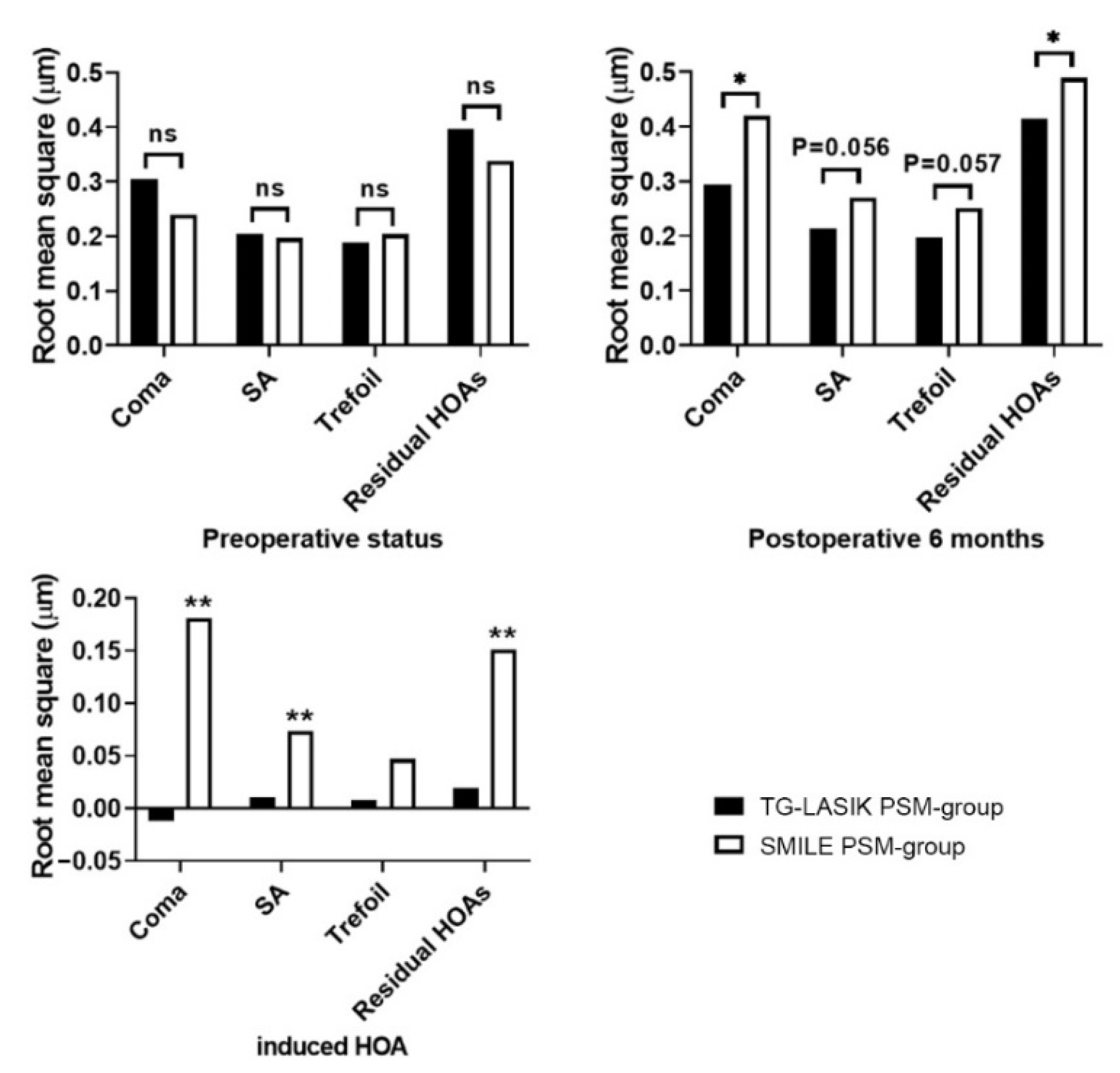
| Coma | 0.239 ± 0.104 | 0.420 ± 0.216 a | 0.181 ± 0.233 | <0.001 |
| SA | 0.197 ± 0.097 | 0.270 ± 0.133 b | 0.0736 ± 0.162 | 0.004 |
| Trefoil | 0.204 ± 0.134 | 0.251 ± 0.131 c | 0.047 ± 0.170 | 0.071 |
| Residual HOAs | 0.338 ± 0.132 | 0.489 ± 0.182 d | 0.151 ± 0.178 | <0.001 |
| TG-LASIK PSM-group | ||||
| Coma | 0.305 ± 0.144 | 0.293 ± 0.214 | −0.0118 ± 0.250 | 0.754 |
| SA | 0.204 ± 0.0756 | 0.214 ± 0.130 | 0.0100 ± 0.151 | 0.658 |
| Trefoil | 0.188 ± 0.091 | 0.196 ± 0.116 | 0.008 ± 0.154 | 0.721 |
| Residual HOAs | 0.396 ± 0.494 | 0.415 ± 0.117 | 0.0195 ± 0.506 | 0.797 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, E.M.; Ryu, I.H.; Lee, I.S.; Kim, J.K.; Kim, S.W.; Ji, Y.W. Comparison of Corneal Higher-Order Aberrations Following Topography-Guided LASIK and SMILE for Myopic Correction: A Propensity Score Matching Analysis. J. Clin. Med. 2022, 11, 6171. https://doi.org/10.3390/jcm11206171
Kang EM, Ryu IH, Lee IS, Kim JK, Kim SW, Ji YW. Comparison of Corneal Higher-Order Aberrations Following Topography-Guided LASIK and SMILE for Myopic Correction: A Propensity Score Matching Analysis. Journal of Clinical Medicine. 2022; 11(20):6171. https://doi.org/10.3390/jcm11206171
Chicago/Turabian StyleKang, Eun Min, Ik Hee Ryu, In Sik Lee, Jin Kuk Kim, Sun Woong Kim, and Yong Woo Ji. 2022. "Comparison of Corneal Higher-Order Aberrations Following Topography-Guided LASIK and SMILE for Myopic Correction: A Propensity Score Matching Analysis" Journal of Clinical Medicine 11, no. 20: 6171. https://doi.org/10.3390/jcm11206171
APA StyleKang, E. M., Ryu, I. H., Lee, I. S., Kim, J. K., Kim, S. W., & Ji, Y. W. (2022). Comparison of Corneal Higher-Order Aberrations Following Topography-Guided LASIK and SMILE for Myopic Correction: A Propensity Score Matching Analysis. Journal of Clinical Medicine, 11(20), 6171. https://doi.org/10.3390/jcm11206171






