The Role of Innate Immune Cells in the Prediction of Early Renal Allograft Injury Following Kidney Transplantation
Abstract
1. Introduction
2. Methods
2.1. Study Population and Study Design
2.2. Clinical Parameters and Outcome Analysis
2.3. Measurements of Immunological Parameters
2.4. Sample Preparation
2.5. Flow Cytometric Analysis and Gating Strategy
2.6. Organ Procurement, Transplantation and Immunosuppression
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Peri- and Postoperative Outcome
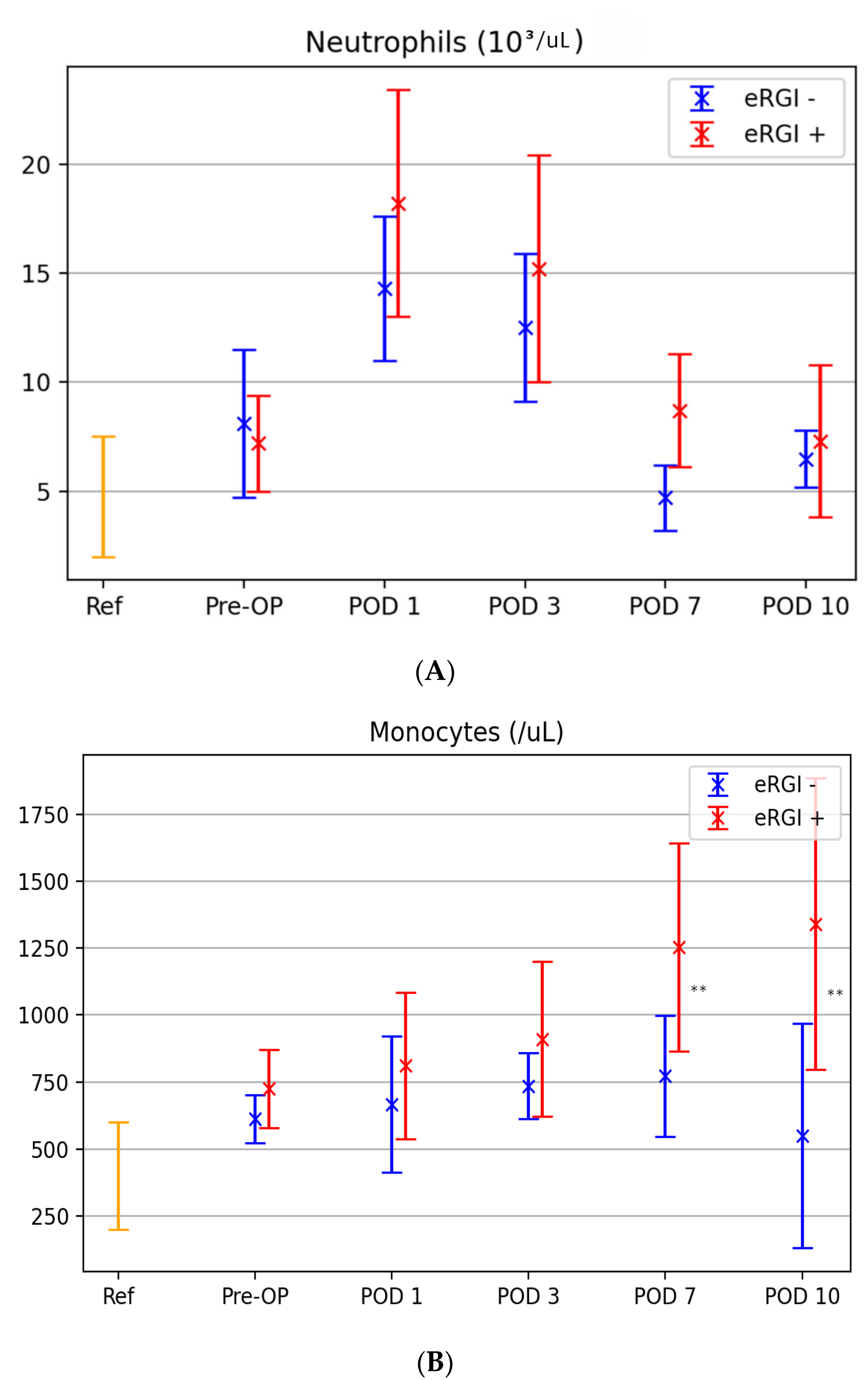
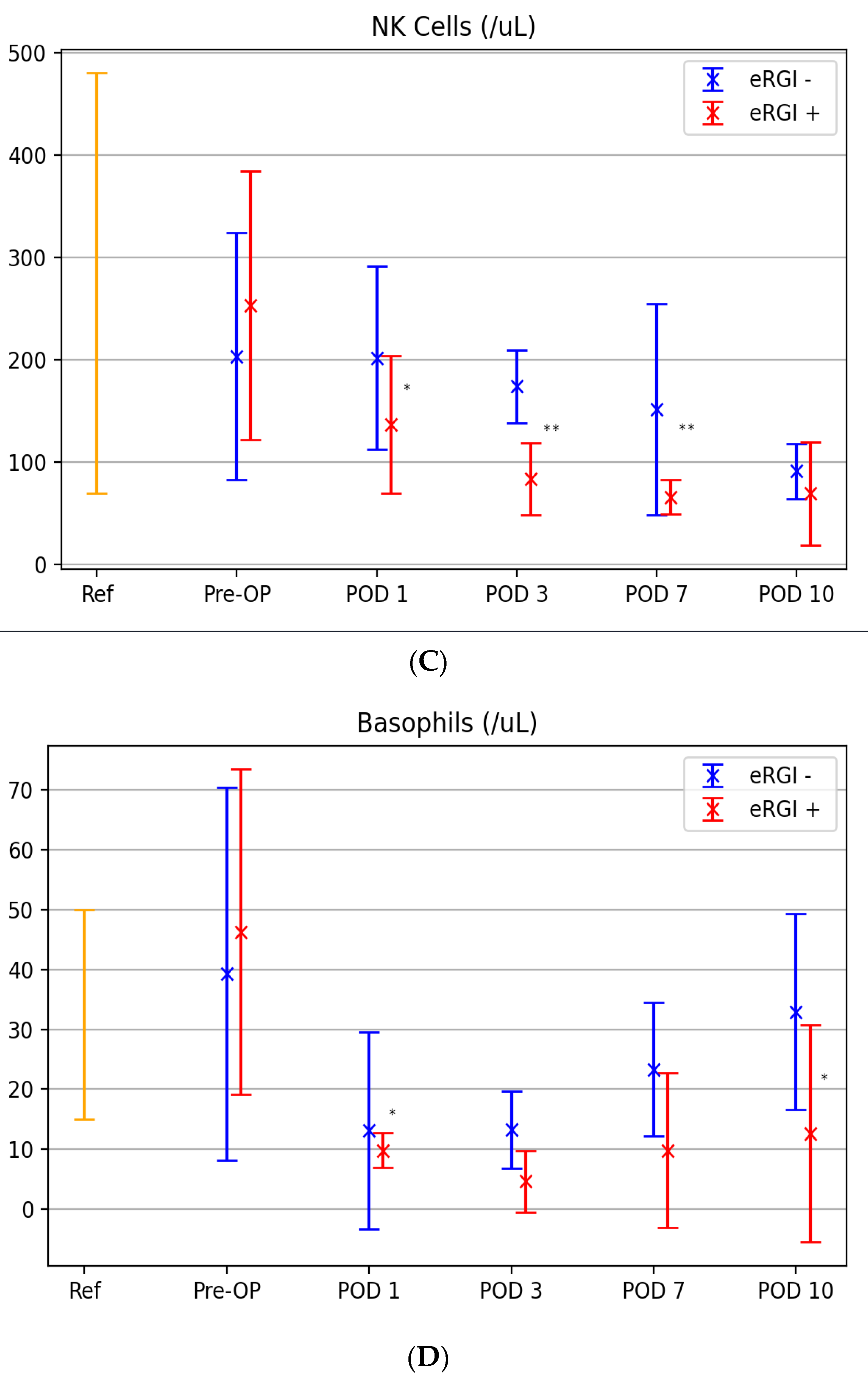
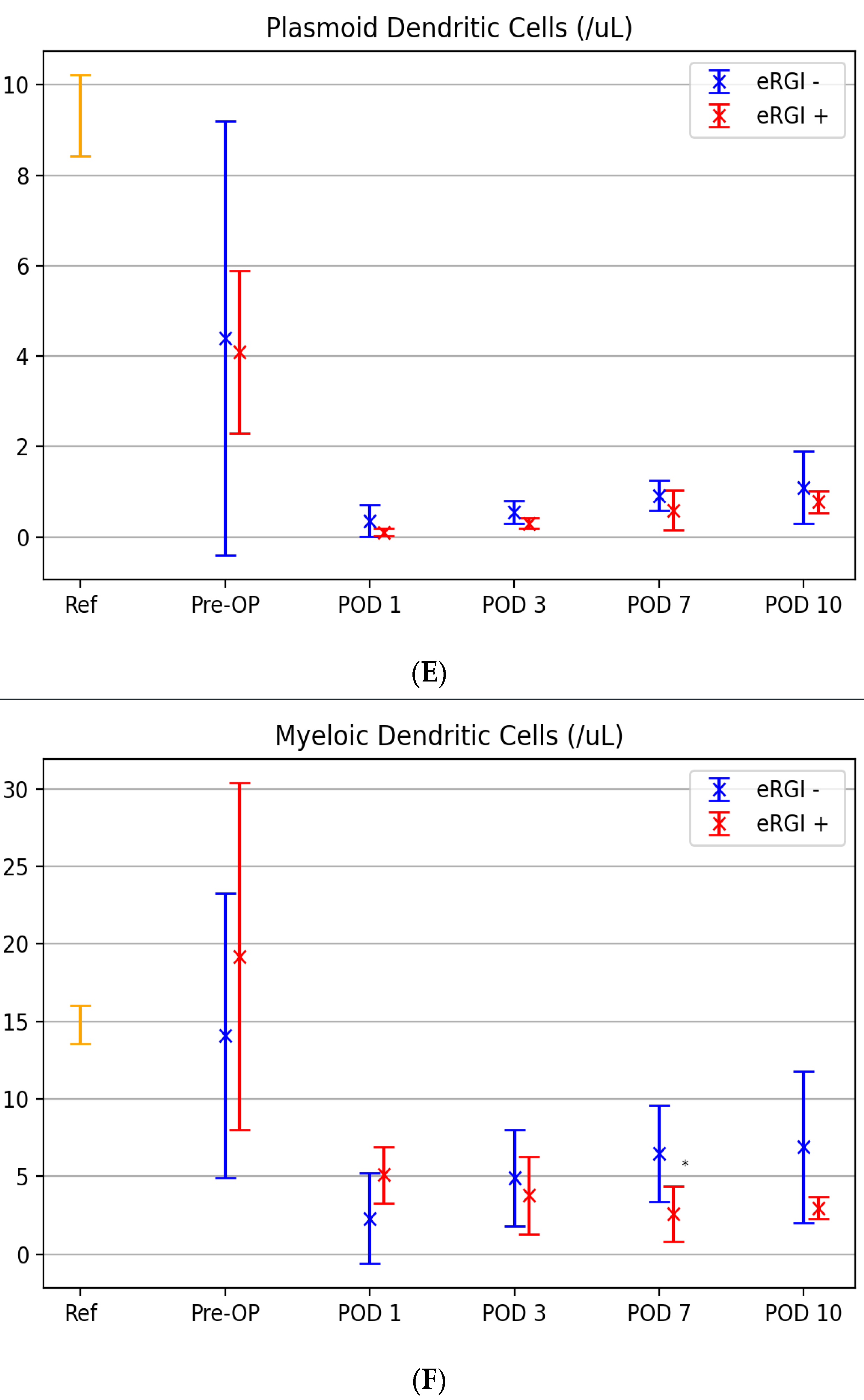
3.3. Kinetic Levels of Innate Immune Cells between Both Groups
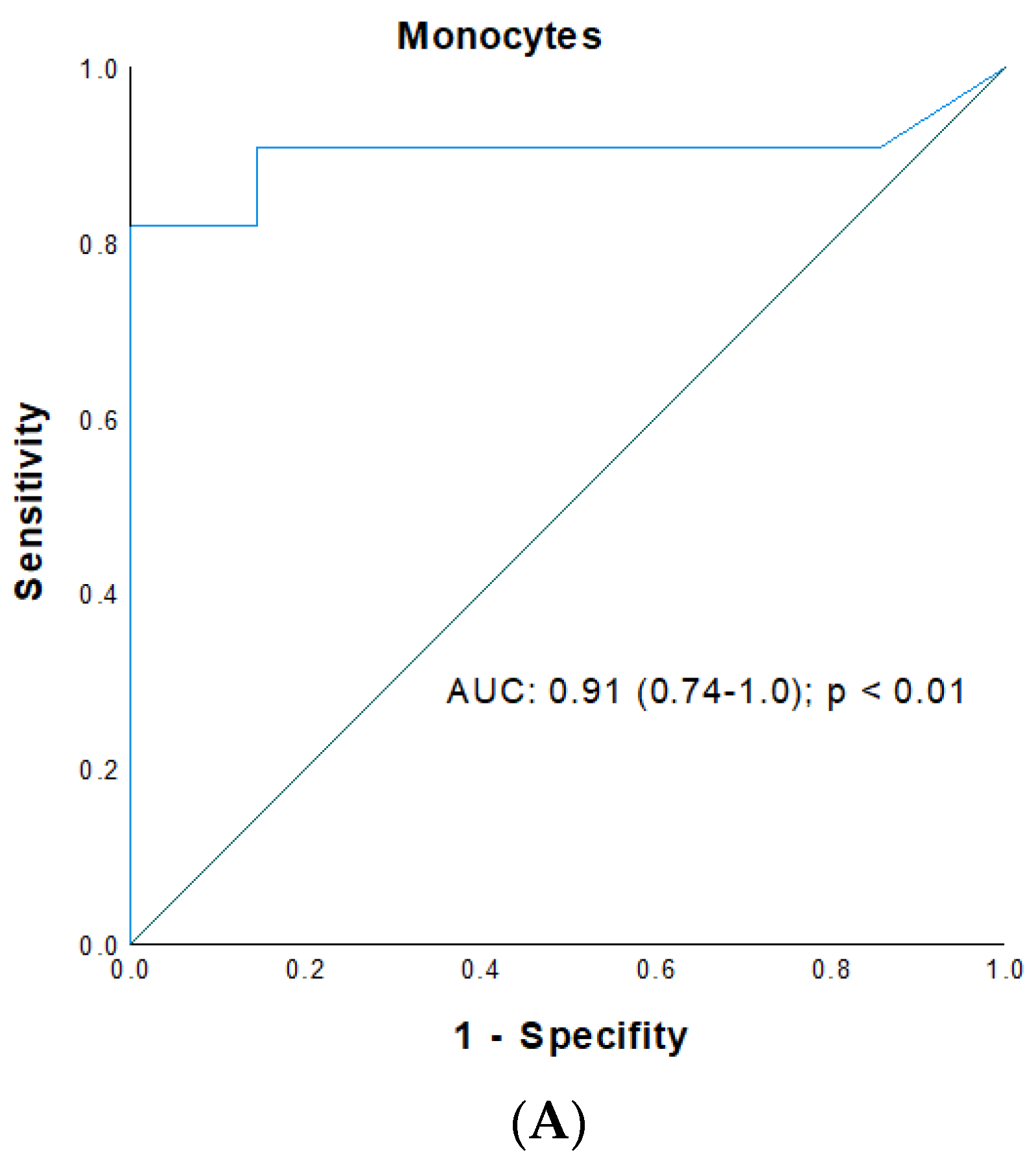
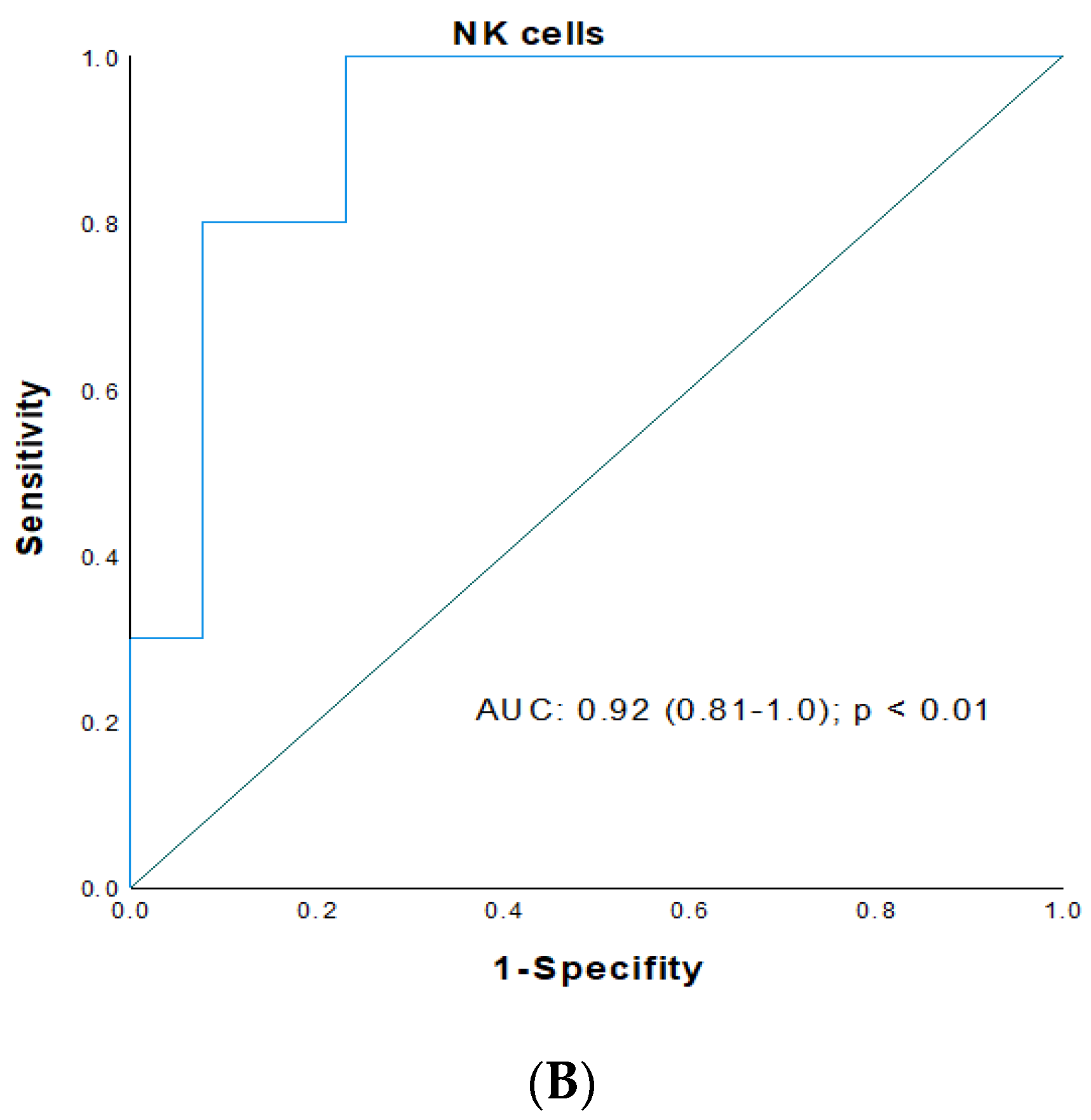
3.4. ROC Analysis for Prediction of Early Renal Graft Injury
3.5. Logistic Regression of IRI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| ATG | antithymocyte globulin |
| ATN | acute tubular necrosis |
| AUC | are under the curve |
| BMI | body mass index |
| CI | confidence interval |
| CIT | cold ischemia time |
| CMV | cytomegalovirus |
| CVD | cardiovascular disease |
| DC | dendritic cell |
| DCreg | regulatory dendritic cells |
| DGF | delayed graft function |
| eRGI | early renal graft in jury |
| ET | Eurotransplant |
| HLA | human leukocyte antigen |
| HLT | Hoshmer–Lemshow test |
| HTK | histidine–tryptophan–ketoglutarate |
| INF | initial nonfunction |
| IRI | ischemia reperfusion injury |
| KT | kidney transplantation |
| LT | liver transplantation |
| mDC | myeloid dendritic cells |
| Mreg | regulatory macrophages |
| NK | natural killer |
| OR | odds ratio |
| pDC | plasmacytoid dendritic cells |
| PRA | panel reactive antibodies |
| ROC | receiver operating characteristics |
| TAC | Tacrolimus |
| TCMR | T-cell mediated rejection |
| Treg | regulatory T cells |
| WIT | warm ischemia time |
References
- Nakamura, K.; Kageyama, S.; Kupiec-Weglinski, J.W. Innate immunity in ischemia-reperfusion injury and graft rejection. Curr. Opin. Organ Transpl. 2019, 24, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kezić, A.; Stajic, N.; Thaiss, F. Innate Immune Response in Kidney Ischemia/Reperfusion Injury: Potential Target for Therapy. J. Immunol. Res. 2017, 2017, 6305439. [Google Scholar] [CrossRef] [PubMed]
- Menke, J.; Sollinger, D.; Schamberger, B.; Heemann, U.; Lutz, J. The effect of ischemia/reperfusion on the kidney graft. Curr. Opin. Organ Transpl. 2014, 19, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Abou-Daya, K.I.; Dai, H.; Oberbarnscheidt, M.H.; Li, X.C.; Lakkis, F.G. Innate Allorecognition and Memory in Transplantation. Front. Immunol. 2020, 11, 918. [Google Scholar] [CrossRef]
- Béland, S.; Désy, O.; Vallin, P.; Basoni, C.; De Serres, S.A. Innate immunity in solid organ transplantation: An update and therapeutic opportunities. Expert Rev. Clin. Immunol. 2015, 11, 377–389. [Google Scholar] [CrossRef]
- Jo, S.-K.; Sung, S.-A.; Cho, W.-Y.; Go, K.-J.; Kim, H.-K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transpl. 2006, 21, 1231–1239. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Zuk, A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004, 66, 480–485. [Google Scholar] [CrossRef]
- Sepe, V.; Libetta, C.; Gregorini, M.; Rampino, T. The innate immune system in human kidney inflammaging. J. Nephrol. 2022, 35, 381–395. [Google Scholar] [CrossRef]
- Zhuang, Q.; Lakkis, F.G. Dendritic cells and innate immunity in kidney transplantation. Kidney Int. 2015, 87, 712–718. [Google Scholar] [CrossRef]
- Lin, J.; Wang, H.; Liu, C.; Cheng, A.; Deng, Q.; Zhu, H.; Chen, J. Dendritic Cells: Versatile Players in Renal Transplantation. Front. Immunol. 2021, 12, 654540. [Google Scholar] [CrossRef]
- Dai, H.; Thomson, A.W.; Rogers, N.M. Dendritic Cells as Sensors, Mediators, and Regulators of Ischemic Injury. Front. Immunol. 2019, 10, 2418. [Google Scholar] [CrossRef]
- Rowshani, A.T.; Vereyken, E.J.F. The Role of Macrophage Lineage Cells in Kidney Graft Rejection and Survival. Transplantation 2012, 94, 309–318. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Iovino, L.; Taddei, R.; Bindi, M.L.; Morganti, R.; Ghinolfi, D.; Petrini, M.; Biancofiore, G. Clinical use of an immune monitoring panel in liver transplant recipients: A prospective, observational study. Transpl. Immunol. 2019, 52, 45–52. [Google Scholar] [CrossRef]
- Smith, S.F.; Hosgood, S.A.; Nicholson, M.L. Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int. 2019, 95, 50–56. [Google Scholar] [CrossRef]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transpl. 2011, 11, 2279–2296. [Google Scholar] [CrossRef]
- Solez, K.; Colvin, R.B.; Racusen, L.C.; Haas, M.; Sis, B.; Mengel, M.; Halloran, P.F.; Baldwin, W.; Banfi, G.; Collins, A.B.; et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am. J. Transpl. 2008, 8, 753–760. [Google Scholar] [CrossRef]
- Racusen, L.C.; Solez, K.; Colvin, R.B.; Bonsib, S.M.; Castro, M.C.; Cavallo, T.; Croker, B.P.; Demetris, A.J.; Drachenberg, C.B.; Fogo, A.B.; et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell– and antibody-mediated rejection. Am. J. Transpl. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Boldt, A.; Borte, S.; Fricke, S.; Kentouche, K.; Emmrich, F.; Borte, M.; Kahlenberg, F.; Sack, U. Eight-color immunophenotyping of T-, B-, and NK-cell subpopulations for characterization of chronic immunodeficiencies. Cytom. Part B Clin. Cytom. 2014, 86, 191–206. [Google Scholar] [CrossRef]
- Ladurner, R.; Steurer, W. Technik der Multiorganentnahme. Viszeralchirurgie 2004, 39, 439–442. [Google Scholar] [CrossRef]
- Eurotransplant Chapter 9: The Donor. Available online: https://www.eurotransplant.org/wp-content/uploads/2020/01/H9-The-Donor-Februar-2020.pdf (accessed on 11 July 2021).
- Sollinger, H.W.; Odorico, J.S.; Knechtle, S.J.; D’Alessandro, A.M.; Kalayoglu, M.; Pirsch, J.D. Experience with 500 simultaneous pancreas-kidney transplants. Ann. Surg. 1998, 228, 284–296. [Google Scholar] [CrossRef]
- Sollinger, H.W.; Odorico, J.S.; Becker, Y.T.; D’Alessandro, A.M.; Pirsch, J.D. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann. Surg. 2009, 250, 618–630. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Assessing the Fit of the Model. In Applied Logistic Regression, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; pp. 143–200. [Google Scholar] [CrossRef]
- Leclerc, S.; Lamarche, C. Cellular therapies in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2021, 30, 584–592. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress-Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef]
- Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nava, R.G.; Zhu, J.; Huang, H.J.; Ibrahim, M.; Mohanakumar, T.; Miller, M.J.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J. Immunol. 2012, 189, 4221–4225. [Google Scholar] [CrossRef]
- Haug, C.E.; Colvin, R.B.; Delmonico, F.L.; Auchincloss, H.; Tolkoff-Rubin, N.; Preffer, F.I.; Rothlein, R.; Norris, S.; Scharschmidt, L.; Cosimi, A.B. A phase I trial of immunosuppression with anti-ICAM-1 (CD54) mAb in renal allograft recipients. Transplantation 1993, 55, 766–772. [Google Scholar] [CrossRef]
- Salmela, K.; Wramner, L.; Ekberg, H.; Hauser, I.; Bentdal, O.; Lins, L.E.; Isoniemi, H.; Bäckman, L.; Persson, N.; Neumayer, H.H.; et al. A randomized multicenter trial of the anti-ICAM-1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: A report of the European Anti-ICAM-1 Renal Transplant Study Gro. Transplantation 1999, 67, 729–736. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Zhou, Y.; Xie, M.; Qi, X.; Xu, Z.; Cai, Q.; Sheng, H.; Chen, E.; Zhao, B.; et al. Leukocyte cell population data from the blood cell analyzer as a predictive marker for severity of acute pancreatitis. J. Clin. Lab. Anal. 2021, 35, e23863. [Google Scholar] [CrossRef] [PubMed]
- Sekerkova, A.; Krepsova, E.; Brabcova, E.; Slatinska, J.; Viklicky, O.; Lanska, V.; Striz, I. CD14+CD16+ and CD14+CD163+ monocyte subpopulations in kidney allograft transplantation. BMC Immunol. 2014, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Švachová, V.; Krupičková, L.; Novotný, M.; Fialová, M.; Mezerová, K.; Čečrdlová, E.; Lánská, V.; Slavčev, A.; Viklický, O.; Viklický, O.; et al. Changes in phenotypic patterns of blood monocytes after kidney transplantation and during acute rejection. Physiol. Res. 2021, 70, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Tinckam, K.J.; Djurdjev, O.; Magil, A.B. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005, 68, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Dooper, I.M.; Hoitsma, A.J.; Maass, C.N.; Assmann, K.J.; Tax, W.J.; Koene, R.A.; Bogman, M.J. The extent of peritubular CD14 staining in renal allografts as an independent immunohistological marker for acute rejection. Transplantation 1994, 58, 820–827. [Google Scholar] [CrossRef]
- Girlanda, R.; Kleiner, D.E.; Duan, Z.; Ford, E.A.S.; Wright, E.C.; Mannon, R.B.; Kirk, A.D. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am. J. Transpl. 2008, 8, 600–607. [Google Scholar] [CrossRef]
- van den Bosch, T.P.P.; Hilbrands, L.B.; Kraaijeveld, R.; Litjens, N.H.R.; Rezaee, F.; Nieboer, D.; Steyerberg, E.W.; van Gestel, J.A.; Roelen, D.L.; Clahsen-van Groningen, M.C.; et al. Pretransplant Numbers of CD16+ Monocytes as a Novel Biomarker to Predict Acute Rejection After Kidney Transplantation: A Pilot Study. Am. J. Transpl. 2017, 17, 2659–2667. [Google Scholar] [CrossRef]
- Sablik, K.A.; Litjens, N.H.R.; Klepper, M.; Betjes, M.G.H. Increased CD16 expression on NK cells is indicative of antibody-dependent cell-mediated cytotoxicity in chronic-active antibody-mediated rejection. Transpl. Immunol. 2019, 54, 52–58. [Google Scholar] [CrossRef]
- Guillén-Gómez, E.; Guirado, L.; Belmonte, X.; Maderuelo, A.; Santín, S.; Juarez, C.; Ars, E.; Facundo, C.; Ballarín, J.A.; Vidal, S.; et al. Monocyte implication in renal allograft dysfunction. Clin. Exp. Immunol. 2014, 175, 323–331. [Google Scholar] [CrossRef]
- Cho, J.-H.; Yoon, Y.-D.; Jang, H.M.; Kwon, E.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, Y.-L.; Kim, H.-K.; Huh, S.; et al. Immunologic Monitoring of T-Lymphocyte Subsets and Hla-Dr-Positive Monocytes in Kidney Transplant Recipients: A Prospective, Observational Cohort Study. Medicine 2015, 94, e1902. [Google Scholar] [CrossRef]
- Pontrelli, P.; Rascio, F.; Castellano, G.; Grandaliano, G.; Gesualdo, L.; Stallone, G. The Role of Natural Killer Cells in the Immune Response in Kidney Transplantation. Front. Immunol. 2020, 11, 1454. [Google Scholar] [CrossRef]
- Xu, X.; Han, Y.; Huang, H.; Bi, L.; Kong, X.; Ma, X.; Shi, B.; Xiao, L. Circulating NK cell subsets and NKT-like cells in renal transplant recipients with acute T-cell-mediated renal allograft rejection. Mol. Med. Rep. 2019, 19, 4238–4248. [Google Scholar] [CrossRef]
- López-Botet, M.; Vilches, C.; Redondo-Pachón, D.; Muntasell, A.; Pupuleku, A.; Yélamos, J.; Pascual, J.; Crespo, M. Dual Role of Natural Killer Cells on Graft Rejection and Control of Cytomegalovirus Infection in Renal Transplantation. Front. Immunol. 2017, 8, 166. [Google Scholar] [CrossRef]
- Jung, H.R.; Kim, M.J.; Wee, Y.-M.; Kim, J.Y.; Choi, M.Y.; Choi, J.Y.; Kwon, H.; Jung, J.H.; Cho, Y.M.; Go, H.; et al. CD56+CD57+ infiltrates as the most predominant subset of intragraft natural killer cells in renal transplant biopsies with antibody-mediated rejection. Sci. Rep. 2019, 9, 16606. [Google Scholar] [CrossRef]
- Dendle, C.; Mulley, W.R.; Holdsworth, S. Can immune biomarkers predict infections in solid organ transplant recipients? A review of current evidence. Transpl. Rev. 2019, 33, 87–98. [Google Scholar] [CrossRef]
- Dendle, C.; Gan, P.-Y.; Polkinghorne, K.R.; Ngui, J.; Stuart, R.L.; Kanellis, J.; Thursky, K.; Mulley, W.R.; Holdsworth, S. Natural killer cell function predicts severe infection in kidney transplant recipients. Am. J. Transpl. 2019, 19, 166–177. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Silva, J.T.; López-Medrano, F.; Allende, L.M.; San Juan, R.; Cambra, F.; Justo, I.; Paz-Artal, E.; Jiménez, C.; Aguado, J.M. Post-transplant monitoring of NK cell counts as a simple approach to predict the occurrence of opportunistic infection in liver transplant recipients. Transpl. Infect. Dis. 2016, 18, 552–565. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; López-Medrano, F.; San Juan, R.; Allende, L.M.; Paz-Artal, E.; Aguado, J.M. Low Natural Killer Cell Counts and Onset of Invasive Fungal Disease After Solid Organ Transplantation. J. Infect. Dis. 2016, 213, 873–874. [Google Scholar] [CrossRef]
- Podestà, M.A.; Cucchiari, D.; Ponticelli, C. The diverging roles of dendritic cells in kidney allotransplantation. Transpl. Rev. 2015, 29, 114–120. [Google Scholar] [CrossRef]
- Zhuang, J.; Hou, J. The Role of Regulatory Myeloid Cell Therapy in Renal Allograft Rejection. Front. Immunol. 2021, 12, 625998. [Google Scholar] [CrossRef]
- Hackstein, H.; Renner, F.C.; Bohnert, A.; Nockher, A.; Frommer, T.; Bein, G.; Weimer, R. Dendritic cell deficiency in the blood of kidney transplant patients on long-term immunosuppression: Results of a prospective matched-cohort study. Am. J. Transpl. 2005, 5, 2945–2953. [Google Scholar] [CrossRef]
- Nikoueinejad, H.; Amirzargar, A.; Sarrafnejad, A.; Einollahi, B.; Nafar, M.; Ahmadpour, P.; Pour-Reze-Gholi, F.; Sehat, O.; Lesanpezeshki, M. Dynamic changes of regulatory T cell and dendritic cell subsets in stable kidney transplant patients: A prospective analysis. Iran. J. Kidney Dis. 2014, 8, 130–138. [Google Scholar] [PubMed]
- Hesselink, D.A.; Vaessen, L.M.B.; Hop, W.C.J.; Schoordijk, W.; Ijzermans, J.N.M.; Baan, C.C.; Weimar, W. The effects of renal transplantation on circulating dendritic cells. Clin. Exp. Immunol. 2005, 140, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Batal, I.; Mohan, S.; De Serres, S.A.; Vasilescu, E.-R.; Tsapepas, D.; Crew, R.J.; Patel, S.S.; Serban, G.; McCune, K.; Husain, S.A.; et al. Analysis of dendritic cells and ischemia-reperfusion changes in postimplantation renal allograft biopsies may serve as predictors of subsequent rejection episodes. Kidney Int. 2018, 93, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Y.; Wu, J.; Xu, X.; Liu, F.; Feng, L.; Xie, Z.; Tang, Y.; Sun, W.; Guo, H.; et al. Changes in dendritic cells and dendritic cell subpopulations in peripheral blood of recipients during acute rejection after kidney transplantation. Chin. Med. J. 2014, 127, 1469–1473. [Google Scholar]
- Loverre, A.; Capobianco, C.; Stallone, G.; Infante, B.; Schena, A.; Ditonno, P.; Palazzo, S.; Battaglia, M.; Crovace, A.; Castellano, G.; et al. Ischemia-reperfusion injury-induced abnormal dendritic cell traffic in the transplanted kidney with delayed graft function. Kidney Int. 2007, 72, 994–1003. [Google Scholar] [CrossRef]
- Sun, Q.; Hall, E.C.; Huang, Y.; Chen, P.; Dibadj, K.; Murawski, M.; Shraybman, R.; Van Kirk, K.; Tang, V.; Peng, R.; et al. Pre-transplant myeloid dendritic cell deficiency associated with cytomegalovirus infection and death after kidney transplantation. Transpl. Infect. Dis. 2012, 14, 618–625. [Google Scholar] [CrossRef]
- Thomson, A.W.; Ezzelarab, M.B. Generation and functional assessment of nonhuman primate regulatory dendritic cells and their therapeutic efficacy in renal transplantation. Cell. Immunol. 2020, 351, 104087. [Google Scholar] [CrossRef]
- Thomson, A.W.; Metes, D.M.; Ezzelarab, M.B.; Raïch-Regué, D. Regulatory dendritic cells for human organ transplantation. Transpl. Rev. 2019, 33, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef]
- Baci, D.; Bosi, A.; Parisi, L.; Buono, G.; Mortara, L.; Ambrosio, G.; Bruno, A. Innate Immunity Effector Cells as Inflammatory Drivers of Cardiac Fibrosis. Int. J. Mol. Sci. 2020, 21, 7165. [Google Scholar] [CrossRef]
- He, Z.; Ma, C.; Yu, T.; Song, J.; Leng, J.; Gu, X.; Li, J. Activation mechanisms and multifaceted effects of mast cells in ischemia reperfusion injury. Exp. Cell Res. 2019, 376, 227–235. [Google Scholar] [CrossRef]
- Yang, M.; Ma, Y.; Ding, J.; Li, J. The role of mast cells in ischemia and reperfusion injury. Inflamm. Res. 2014, 63, 899–905. [Google Scholar] [CrossRef]
- Yang, M.; Ma, Y.; Tao, S.; Ding, J.; Rao, L.; Jiang, H.; Li, J. Mast cell degranulation promotes ischemia-reperfusion injury in rat liver. J. Surg. Res. 2014, 186, 170–178. [Google Scholar] [CrossRef]
- El-Shitany, N.; El-desoky, K. Cromoglycate, not ketotifen, ameliorated the injured effect of warm ischemia/reperfusion in rat liver: Role of mast cell degranulation, oxidative stress, proinflammatory cytokine, and inducible nitric oxide synthase. Drug Des. Devel. Ther. 2015, 9, 5237–5246. [Google Scholar] [CrossRef][Green Version]
- Egido, J.; Sánchez Crespo, M.; Lahoz, C.; García, M.; de la Concha, G.; García, R.; Hernando, L. Evidence of sensitized basophils in renal transplanted patients. Transplantation 1980, 29, 435–438. [Google Scholar] [CrossRef]
- Colvin, R.B.; Dvorak, H.F. Letter: Basophils and mast cells in renal allograft rejection. Lancet 1974, 1, 212–214. [Google Scholar] [CrossRef]
- Dvorak, H.F. Role of the basophilic leukocyte in allograft rejection. J. Immunol. 1971, 106, 279–281. [Google Scholar]
- Kho, M.M.L.; Bouvy, A.P.; Cadogan, M.; Kraaijeveld, R.; Baan, C.C.; Weimar, W. The effect of low and ultra-low dosages Thymoglobulin on peripheral T, B and NK cells in kidney transplant recipients. Transpl. Immunol. 2012, 26, 186–190. [Google Scholar] [CrossRef]
- Krepsova, E.; Tycova, I.; Sekerkova, A.; Wohlfahrt, P.; Hruba, P.; Striz, I.; Sawitzki, B.; Viklicky, O. Effect of induction therapy on the expression of molecular markers associated with rejection and tolerance. BMC Nephrol. 2015, 16, 146. [Google Scholar] [CrossRef]
- Womer, K.L.; Peng, R.; Patton, P.R.; Murawski, M.R.; Bucci, M.; Kaleem, A.; Schold, J.; Efron, P.A.; Hemming, A.W.; Srinivas, T.; et al. The effects of renal transplantation on peripheral blood dendritic cells. Clin. Transpl. 2005, 19, 659–667. [Google Scholar] [CrossRef]
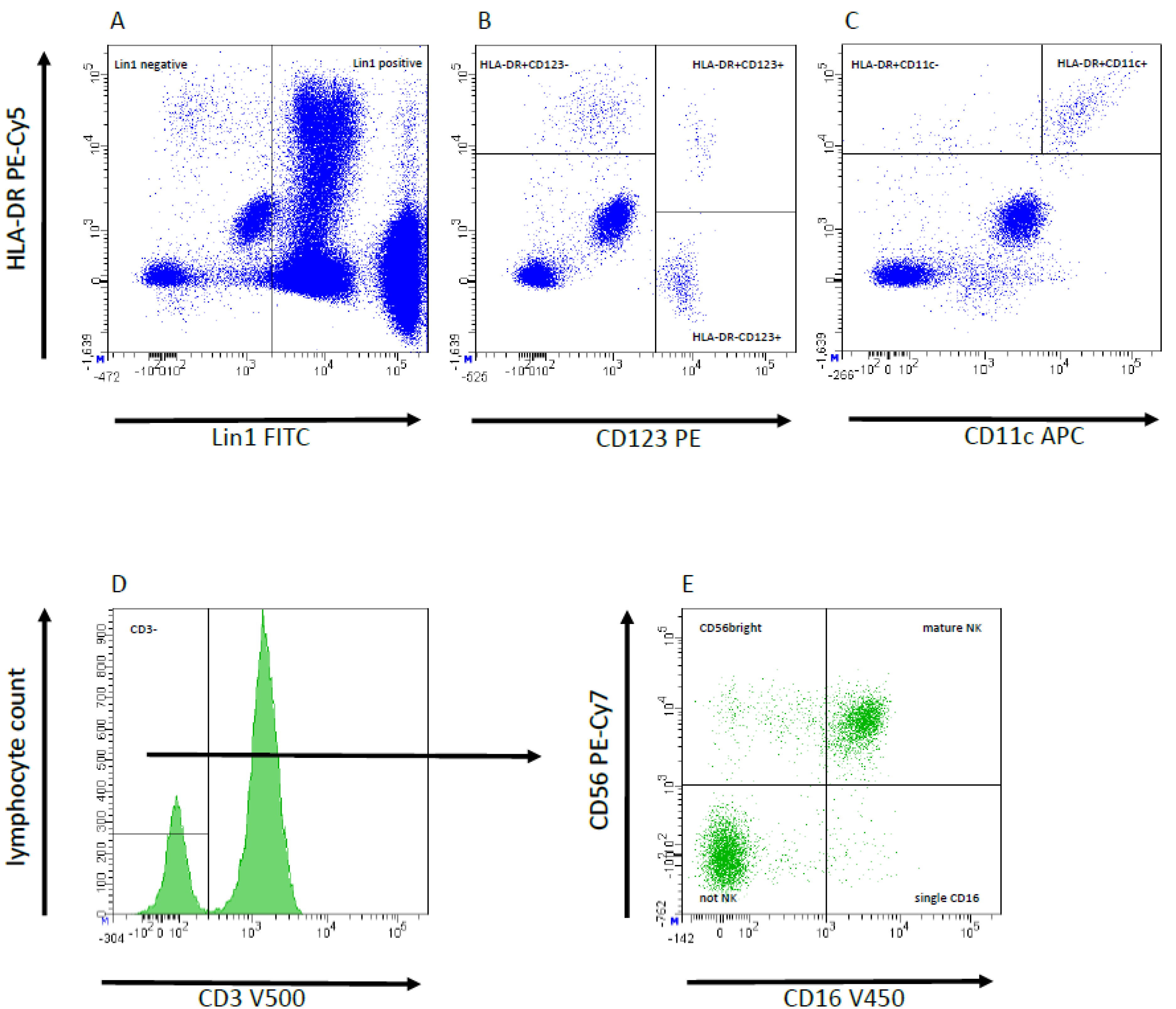
| Variables | Total (n = 50 Patients) | eRGI− (n = 27 Patients) | eRGI+ (n = 23 Patients) | p-Value |
|---|---|---|---|---|
| Donor | ||||
| Age, years | 54.2 ± 15.9 | 51.4 ± 15.4 | 59.9 ± 16.5 | 0.01 |
| Gender, male/female | 25 (50)/25 (50) | 12 (44)/15 (56) | 13 (57)/10 (43) | 0.395 |
| BMI, kg/m2 | 26.2 ± 4.3 | 25.3 ± 2.9 | 27.4 ± 4.8 | 0.029 |
| Hypertension (yes/no) | 18 (36)/32 (64) | 7 (26)/20 (74) | 11 (48)/12 (52) | 0.108 |
| Diabetes mellitus (yes/no) | 3 (6)/47 (94) | 1 (4)/26 (96) | 2 (9)/21 (91) | 0.459 |
| Recipient | ||||
| Age, years | 52.62 ± 14.3 | 48.85 ± 14.9 | 56.1 ± 13.3 | 0.042 |
| Gender, male/female | 34 (68)/16 (32) | 15 (56)/12 (44) | 19 (83)/4 (17) | 0.09 |
| BMI, kg/m2 | 25.5 ± 3.4 | 24.7 ± 3.4 | 26.1 ± 3.5 | 0.07 |
| Diabetes | 7 (14) | 3 (6) | 4 (8) | 0.624 |
| Peripheral Arterial Disease | 3 (6) | 2 (4) | 1 (2) | 0.650 |
| Previous Dialysis | 40 (80) | 21 (78) | 19 (83) | 0.670 |
| Dialysis modality | ||||
| Hemodialysis/peritoneal | 34 (85)/6 (15) | 17 (81)/4 (19) | 17 (90)/2 (10) | 0.451 |
| Duration of dialysis, months | 59.9 ± 7.9 | 34.9 ± 5.9 | 30.1 ± 3.8 | 0.175 |
| Waiting time, months | 28.04 ± 30.3 | 21.9 ± 28.2 | 24.7 ± 31.9 | 0.145 |
| Cardiovascular disease | 10 (20) | 3 (6) | 7 (14) | 0.09 |
| Type of Donation | ||||
| Deceased Donor | 35 (70) | 15 (66) | 20 (87) | 0.018 |
| Living Donation | 15 (30) | 12 (44) | 3 (13) | |
| Original renal disease | 0.686 | |||
| Glomerulonephritis | 18 (36) | 8 (16) | 10 (20) | |
| Diabetes mellitus | 5 (10) | 2 (4) | 3 (6) | |
| Vascular/hypertension | 10 (20) | 7 (14) | 3 (6) | |
| Zystic | 7 (14) | 4 (8) | 3 (6) | |
| Other | 10 (20) | 6 (12) | 4 (8) | |
| Number of transplants | 0.145 | |||
| First/Retransplant | 43(86)/7 (14) | 25 (93)/2 (7) | 18 (78)/5 (22) | |
| HLA- mismatches > 3 | 26 (52) | 13 (48) | 13 (56) | 0.554 |
| Panel reactive antibodies | 8.1 ± 16.9 | 4.1 ± 8.1 | 12.8 ± 22.8 | 0.06 |
| Variables | Total (n = 50 Patients) | eRGI− (n = 27 Patients) | eRGI+ (n = 23 Patients) | p-Value |
|---|---|---|---|---|
| Warm ischemia time, minutes | 43.2 ± 8.1 | 42.1 ± 9.8 | 44.6 ± 6.1 | 0.495 |
| Cold ischemia time, minutes | 511 ± 253 | 411 ± 198 | 612 ± 212 | 0.01 |
| Operating time, minutes | 206 ± 59.5 | 196.8 ± 49.1 | 219 ± 70.3 | 0.211 |
| Hospital stay, days | 27 ± 19.3 | 23 +/− 16.2 | 32 +/− 21.5 | <0.01 |
| Induction therapy (ATG/IL-2 RA/none) | 5 (10)/27 (54)/18 (36) | 2 (4)/14 (28)/11 (22) | 3 (6)/13 (26)/7 (14) | 0.667 |
| CMV-state; D+/R− | 18 (36) | 11 (22) | 7 (14) | 0.449 |
| Average tacrolimus levels, ng/ml | 8.19 ± 4.6 | 8.3 ± 3.4 | 7.99 ± 5.2 | 0.08 |
| Bacterial infections | 17 (34) | 6 (19) | 11 (43) | 0.06 |
| Viral infections | 13 (26) | 4 (14) | 9 (39) | 0.05 |
| Fungal infections | 2 (4%) | 1 (3.7) | 1 (4.3) | 0.901 |
| Days | Neutrophils | Monocytes | NK Cells | |||||||||
| Cutoff | AUC (CI) | Sensitivity/ Specificity | p-Value | Cutoff | AUC (CI) | Sensitivity/ Specificity | p-Value | Cutoff | AUC (CI) | Sensitivity/ Specificity | p-Value | |
| 0 | 7.7 | 0.43 (0.38–0.79) | 56/62 | 0.165 | 550 | 0.64 (0.34–0.94) | 85/75 | 0.335 | 129 | 0.60 (0.34–0.85) | 80/39 | 0.439 |
| 1 | 12.5 | 0.83 (0.66–0.99) | 67/88 | 0.011 | 725 | 0.75 (0.51–0.98) | 72/78 | 0.151 | 177 | 0.72 (0.49–0.95) | 76/81 | 0.078 |
| 3 | 14.1 | 0.79 (0.61–0.99) | 75/90 | 0.028 | 850 | 0.66 (0.41–0.90) | 78/62 | 0.236 | 125 | 0.84 (0.66–1.0) | 91/73 | <0.01 |
| 7 | 9.4 | 0.89 (0.72–0.99) | 83/86 | <0.01 | 1150 | 0.91 (0.74–1.0) | 82/94 | <0.01 | 91 | 0.92 (0.81–1.0) | 99/77 | <0.01 |
| 10 | 8.2 | 0.64 (0.59–0.79) | 54/78 | 0.456 | 1340 | 0.89 (0.71–0.98) | 78/91 | <0.01 | 66 | 0.67 (0.42–0.92) | 62/91 | 0.181 |
| Days | Basophils | mDC | pDC | |||||||||
| Cutoff | AUC (CI) | Sensitivity/ Specificity | p-Value | Cutoff | AUC (CI) | Sensitivity/ Specificity | p-Value | Cutoff | AUC (CI) | Sensitivity/ Specificity% | p-Value | |
| 0 | 11.7 | 0.54 (0.21–0.88) | 83/42 | 0.775 | 9.8 | 0.51 (0.16–0.83) | 83/49 | 0.865 | 3.89 | 0.52 (0.18–0.87) | 83/43 | 0.886 |
| 1 | 7.15 | 0.79 (0.54–1.0) | 67/75 | 0.071 | 5.5 | 0.74 (0.13–0.89) | 66/88 | 0.123 | 0.76 | 0.46 (0.25–0.58) | 93/51 | 0.247 |
| 3 | 6.73 | 0.69 (041–0.98) | 86/59 | 0.214 | 3.8 | 0.63 (0.46–0.95) | 62/73 | 0.465 | 0.26 | 0.61 (0.48–0.74) | 58/49 | 0.257 |
| 7 | 12.2 | 0.77 (0.49–0.97) | 71/88 | 0.08 | 4.7 | 0.80 (0.55–0.92.) | 93/82 | 0.032 | 0.55 | 0.77 (0.56–0.98) | 86/57 | 0.09 |
| 10 | 18.16 | 0.85 (0.63–0.99) | 83/89 | 0.024 | 5.1 | 0.89 (0.71–0.99) | 99/78 | 0.013 | 0.95 | 0.69 (0.45–0.94) | 88/68 | 0.127 |
| Variables | UVA | MVA | p-Value |
|---|---|---|---|
| p-Value | OR (95% CI) | ||
| Recipient factors | |||
| Recipient age, years | <0.01 | 1.53 (1.003–2.350) | 0.040 |
| Recipient gender (female versus male) | 0.047 | NS | |
| Recipient BMI, <25 versus >25 | 0.027 | 5.6 (1.36–23.9) | 0.015 |
| Pre-emptive transplantation (no versus yes) | 0.027 | ||
| Recipient cardiovascular disease (yes versus no) | <0.01 | 8.17 (1.28–52.16) | 0.026 |
| Donor factors | |||
| Donor age, years | 0.004 | 1.068 (1.011–1.128) | 0.012 |
| Donor BMI, per 5 kg/m2 | 0.08 | NS | |
| Donor type—DD versus LD | 0.004 | 2.18 (1.091–4.112 | 0.027 |
| Transplant-related factors | |||
| Cold ischemia time | 0.003 | 1.005 (1.001–1.01) | 0.019 |
| HLA- mismatches >3 versus <3 | 0.08 | ||
| Operating time | 0.03 | NS | |
| Number of transplant (re versus first) | 0.110 | ||
| Average tacrolimus levels; > versus < 8 ng/mL | 0.07 | ||
| Fungal infections | 0.908 | ||
| Viral infections | 0.06 | ||
| Bacterial infections | 0.05 | ||
| Immunological parameters | |||
| Neutrophils POD 1; >12.5 × 103/μL | 0.011 | NS | |
| Neutrophils POD 3; >14.1 × 103/μL | 0.031 | NS | |
| Neutrophils POD 7; >9.4 × 103/μL | 0.022 | 16.1 (1.31–195.6) | 0.031 |
| Monocytes POD 7; >1150 cells/μL | 0.018 | 7.81 (1.97–63.18) | 0.048 |
| Monocytes POD 10; >1340 cells/μL | 0.012 | NS | |
| NK cells POD 3; <125 cells/μL | <0.01 | 6.97 (3.81–12.7) | <0.01 |
| NK cells POD 7; <91 cells/μL | <0.01 | NS | |
| Basophils POD 10; <18.1 cells/μL | 0.019 | 3.45 (1.37–12.3) | 0.024 |
| mDC POD 7; <4.7 cells/μL | 0.029 | 11.68 (1.85–73.4) | <0.01 |
| mDC POD 10; <5.1 cells/μL | <0.01 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahn, N.; Sack, U.; Stehr, S.; Vöelker, M.T.; Laudi, S.; Seehofer, D.; Atay, S.; Zgoura, P.; Viebahn, R.; Boldt, A.; et al. The Role of Innate Immune Cells in the Prediction of Early Renal Allograft Injury Following Kidney Transplantation. J. Clin. Med. 2022, 11, 6148. https://doi.org/10.3390/jcm11206148
Jahn N, Sack U, Stehr S, Vöelker MT, Laudi S, Seehofer D, Atay S, Zgoura P, Viebahn R, Boldt A, et al. The Role of Innate Immune Cells in the Prediction of Early Renal Allograft Injury Following Kidney Transplantation. Journal of Clinical Medicine. 2022; 11(20):6148. https://doi.org/10.3390/jcm11206148
Chicago/Turabian StyleJahn, Nora, Ulrich Sack, Sebastian Stehr, Maria Theresa Vöelker, Sven Laudi, Daniel Seehofer, Selim Atay, Panagiota Zgoura, Richard Viebahn, Andreas Boldt, and et al. 2022. "The Role of Innate Immune Cells in the Prediction of Early Renal Allograft Injury Following Kidney Transplantation" Journal of Clinical Medicine 11, no. 20: 6148. https://doi.org/10.3390/jcm11206148
APA StyleJahn, N., Sack, U., Stehr, S., Vöelker, M. T., Laudi, S., Seehofer, D., Atay, S., Zgoura, P., Viebahn, R., Boldt, A., & Hau, H.-M. (2022). The Role of Innate Immune Cells in the Prediction of Early Renal Allograft Injury Following Kidney Transplantation. Journal of Clinical Medicine, 11(20), 6148. https://doi.org/10.3390/jcm11206148







