Long-Term Prognosis Value of Paravalvular Leak and Patient–Prosthesis Mismatch following Transcatheter Aortic Valve Implantation: Insight from the France-TAVI Registry
Abstract
1. Introduction
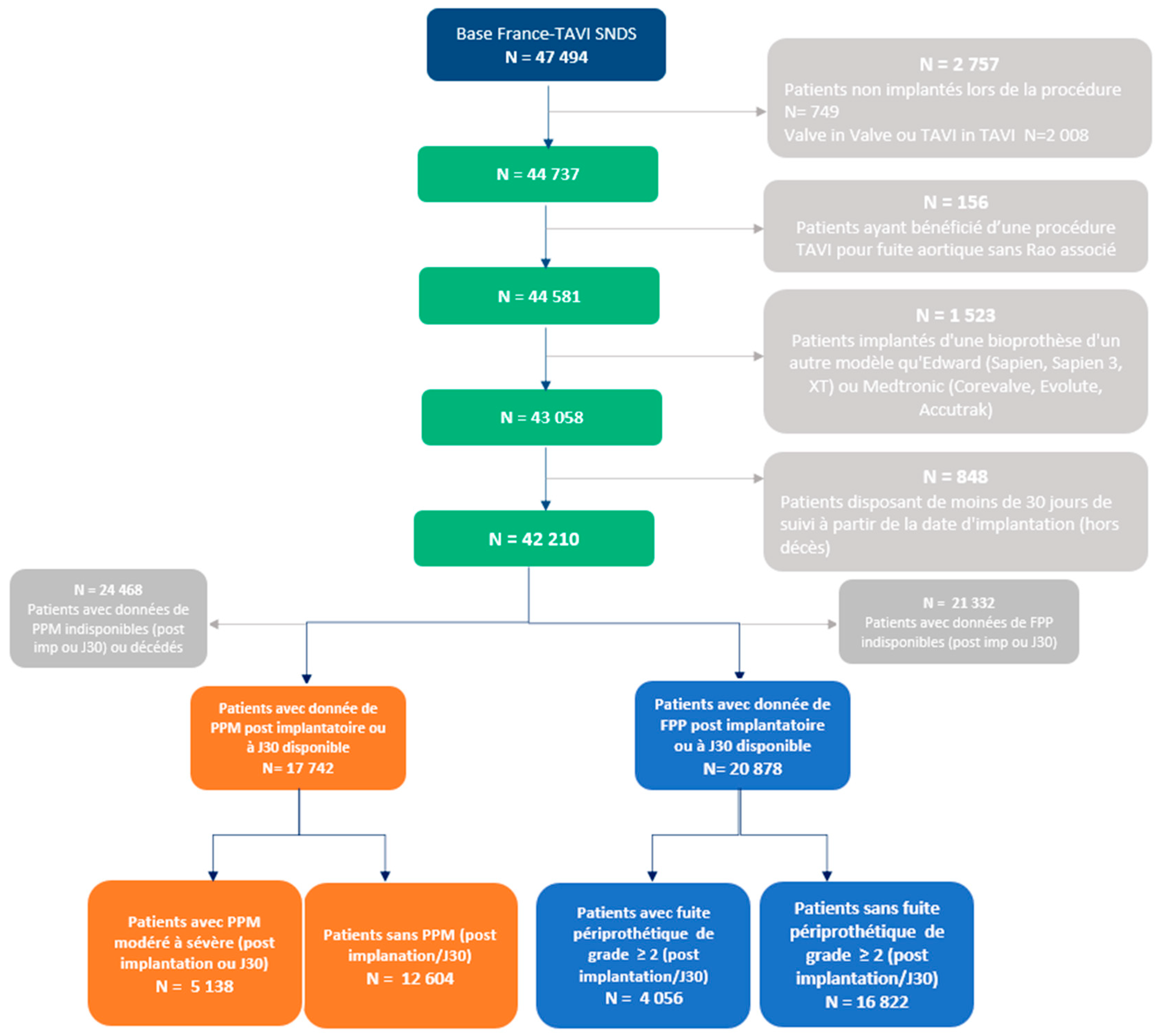
2. Methods
2.1. TAVI Registries
2.2. Data Collection
2.3. Study Design
2.3.1. Study Groups and PPM and PVL Definitions
2.3.2. Endpoints
- –
- The incidence and identification of the risk factors for moderate-to-severe PPM and PVL ≥ 2 after TAVI;
- –
- Rehospitalization for heart failure, stroke, aortic valve reintervention, pacemaker implantation at 30 days, or cardiac arrhythmia during follow-up;
- –
- Predictors of all-cause mortality.
2.3.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Incidence and Risk Factors for PPM and PVL
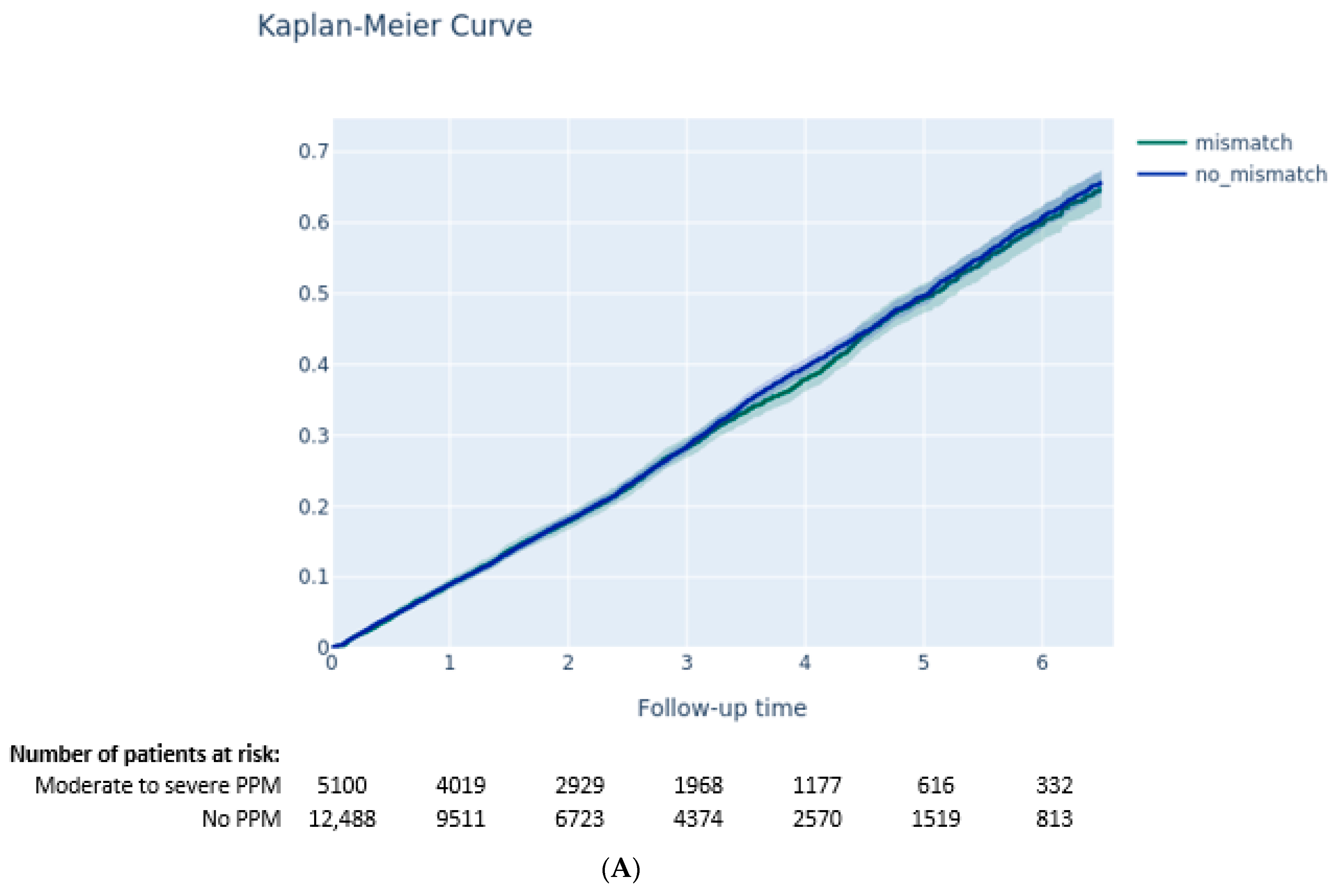
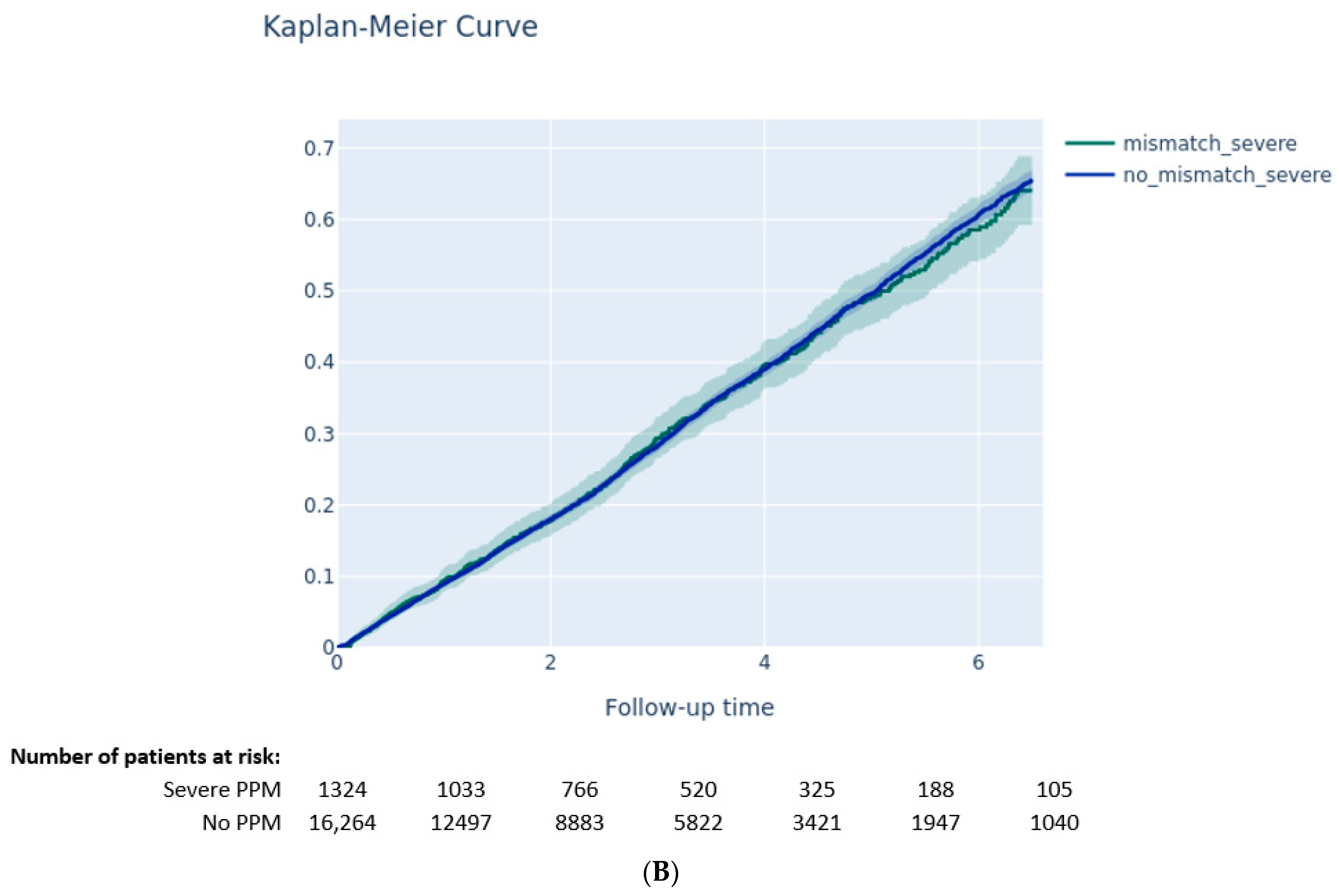
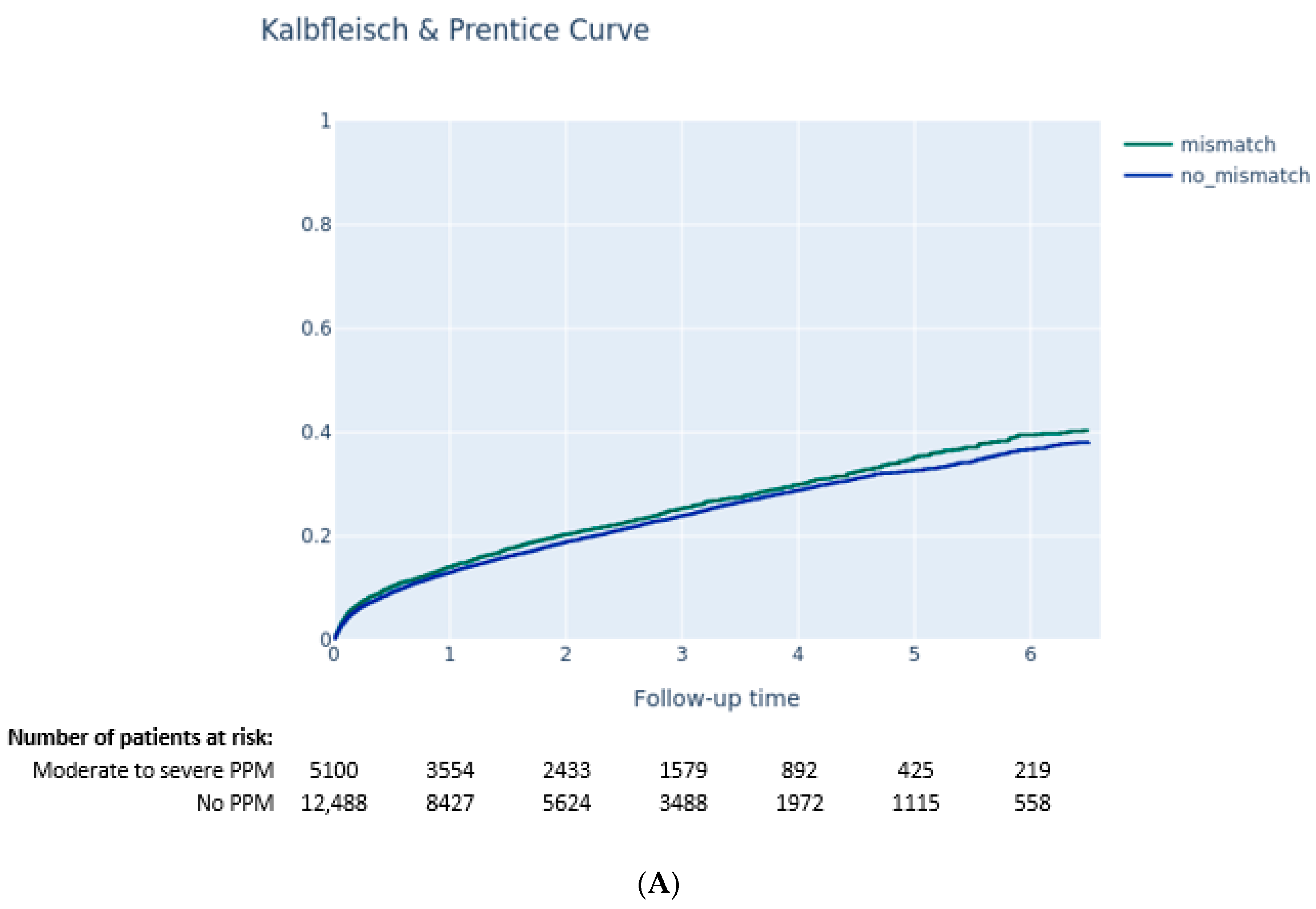
3.3. Outcomes according to PVL and PPM
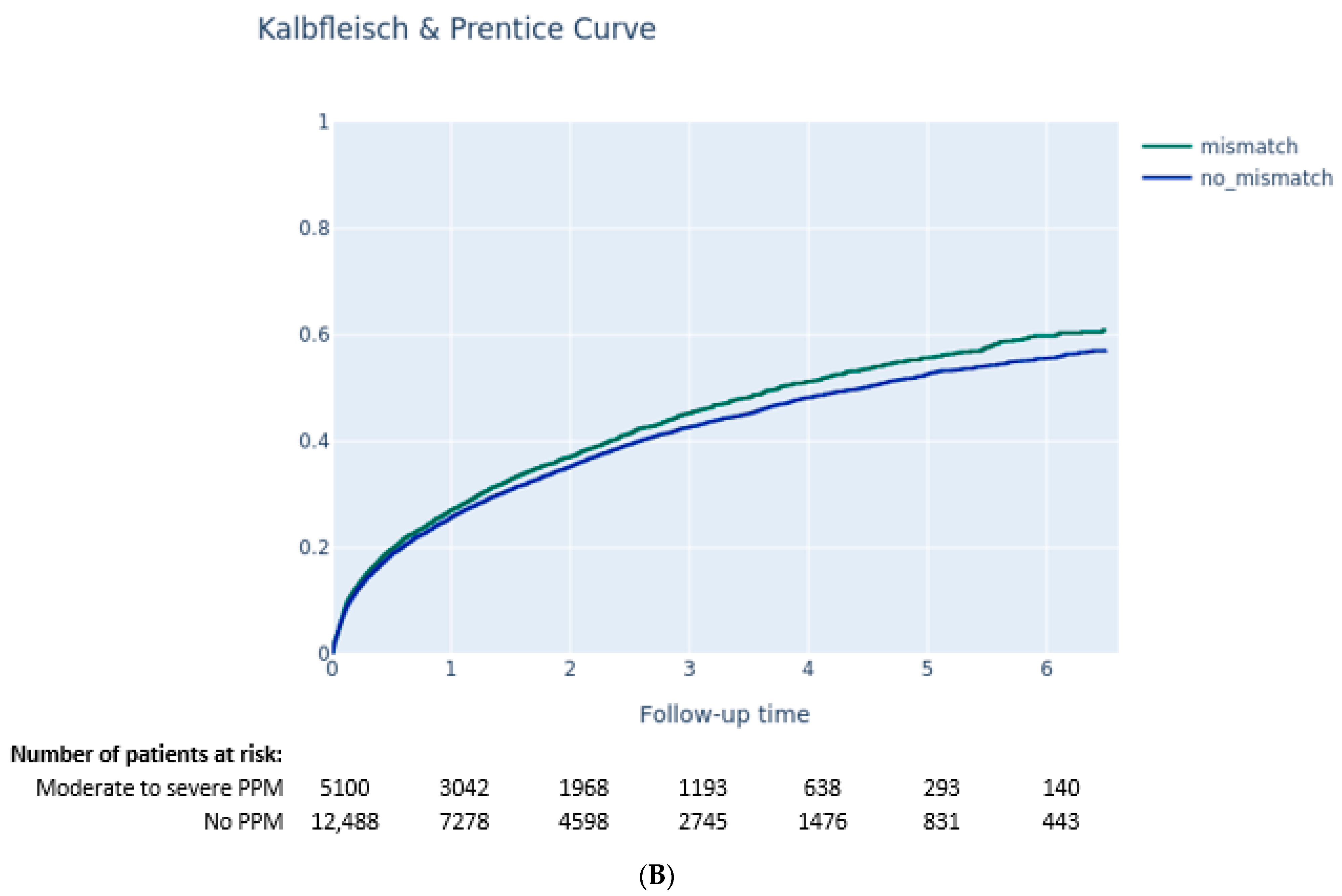
3.3.1. Impact of PPM on Clinical Outcomes
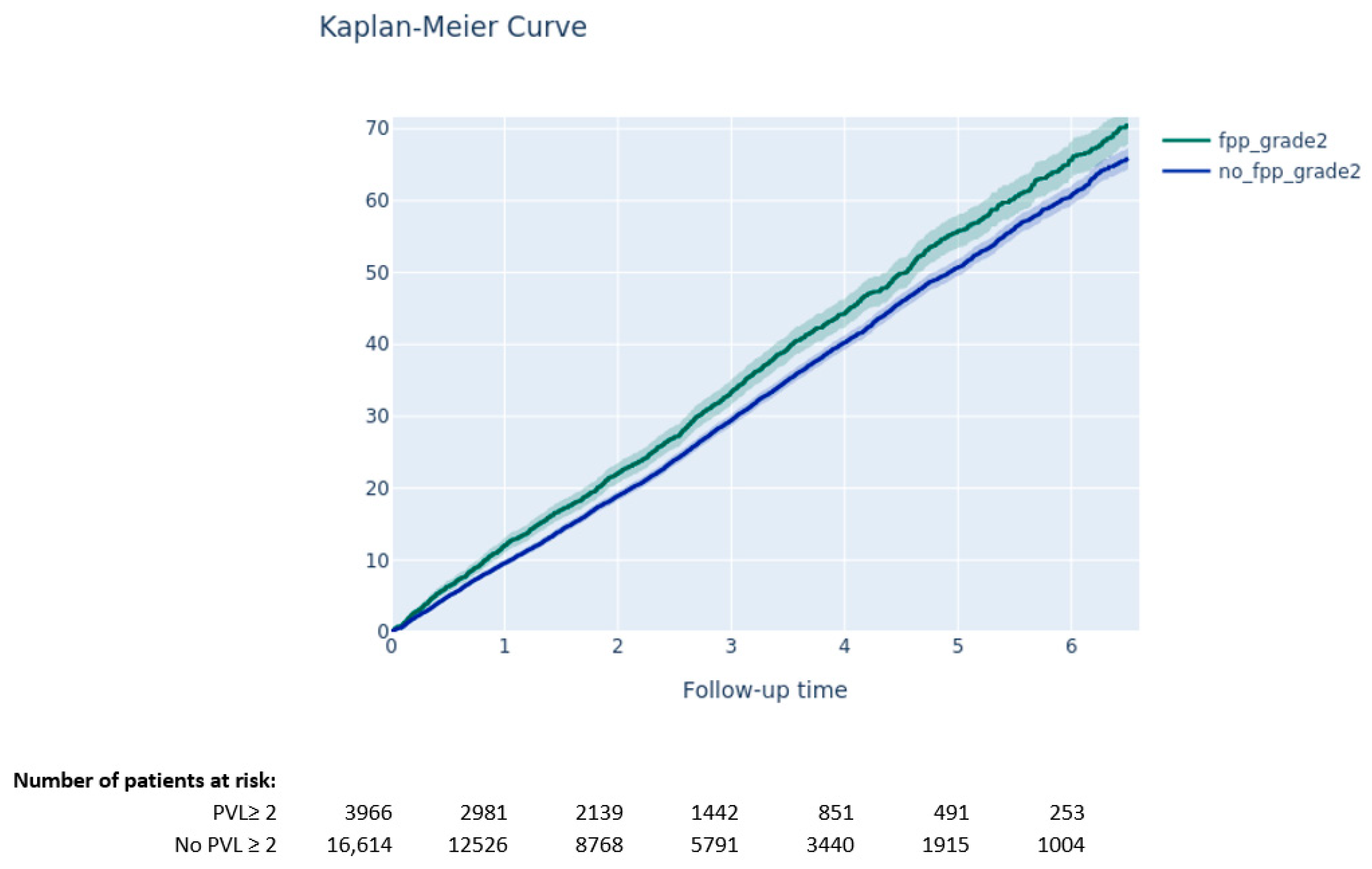
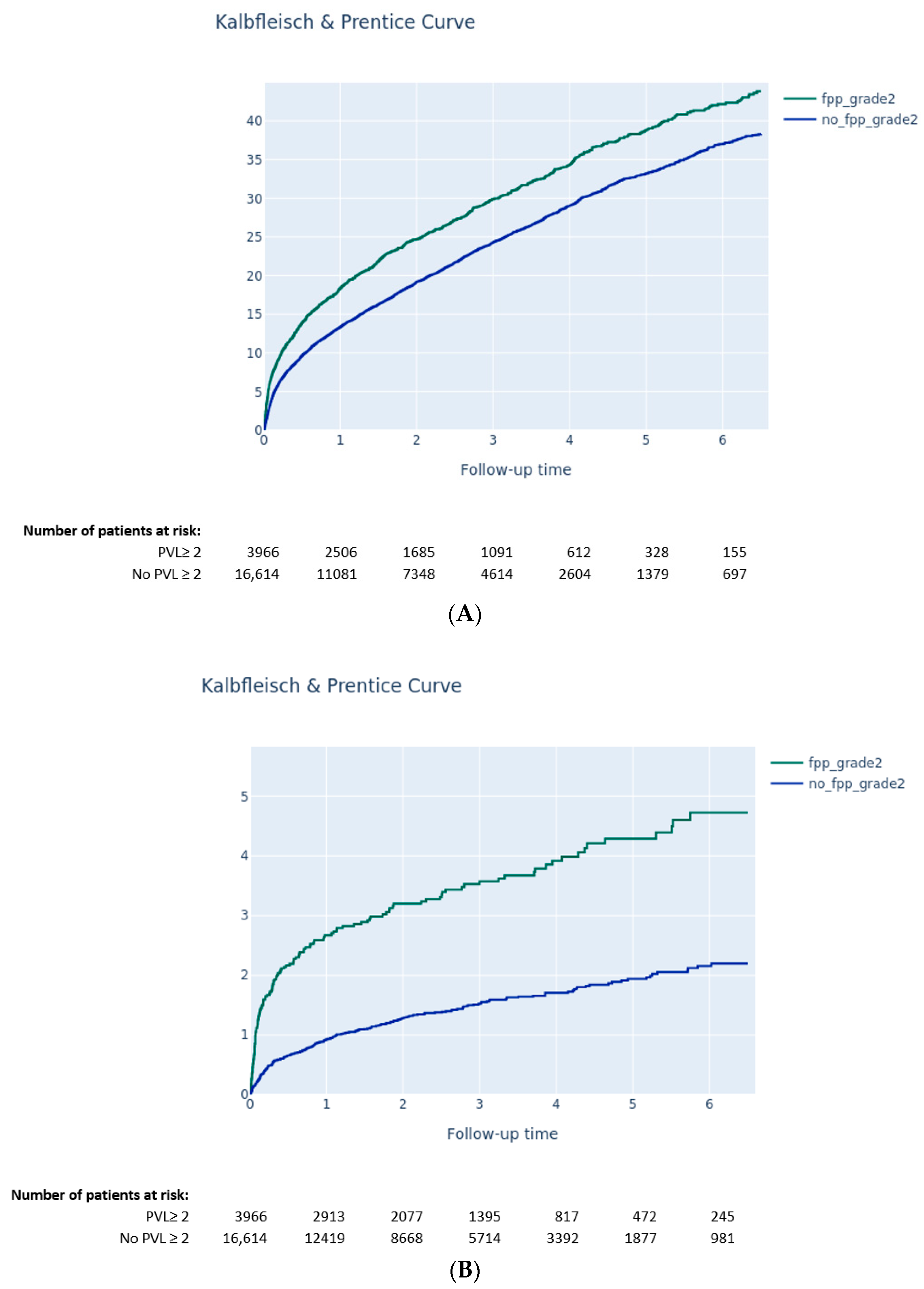
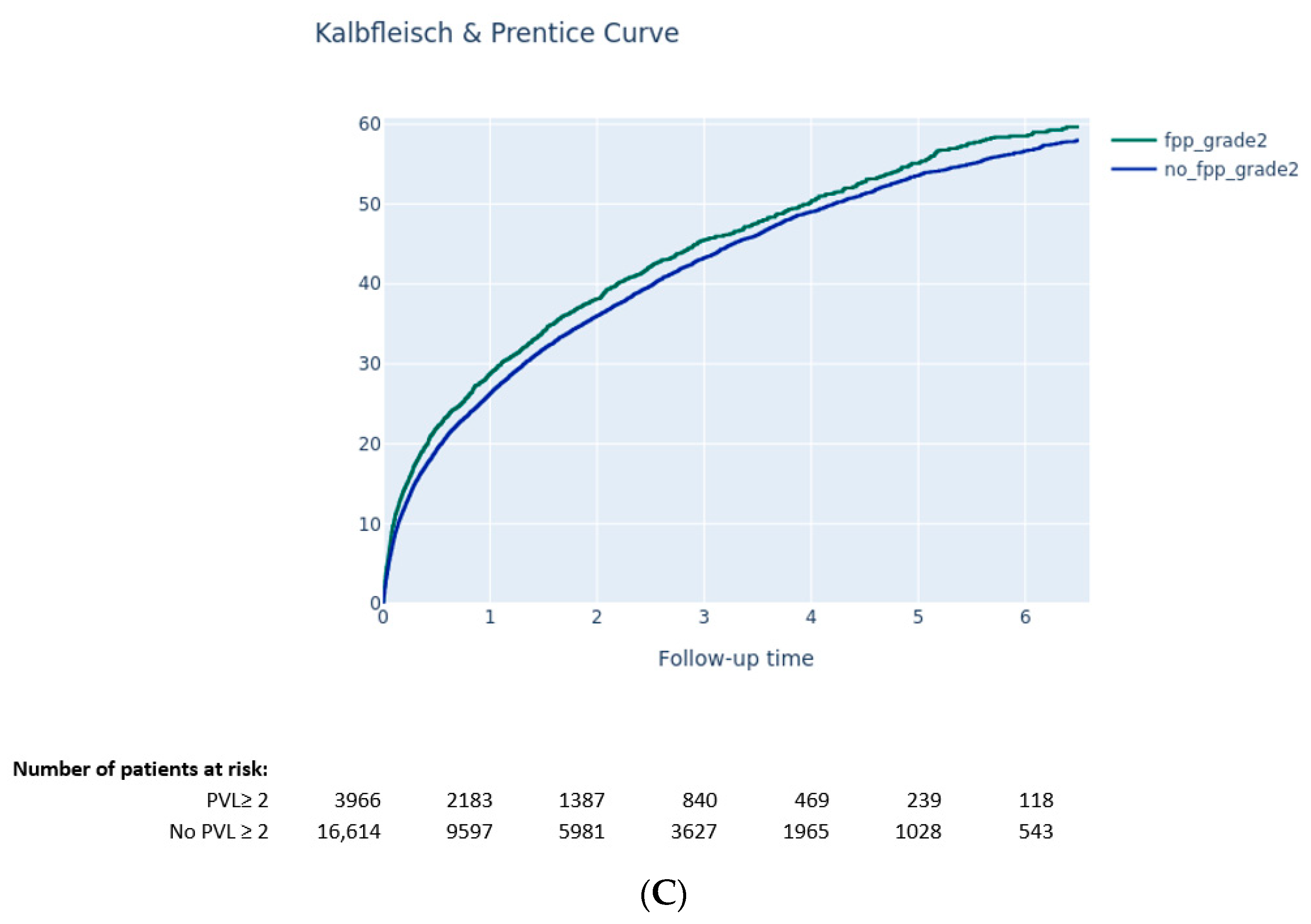
3.3.2. Impact of PVL on Clinical Outcomes
4. Discussion
- –
- Moderate-to-severe PPM and PVL ≥ 2 were reported in 29.0% and 19.4%, respectively, of TAVI patients in France between 2010 and 2019.
- –
- The main risk factors for moderate-to-severe PPM are a younger age, a lower BMI, a higher body surface area, a lower ejection fraction, a smaller aortic annulus, and a smaller diameter of TAVI device, while the main risk factors for PVL ≥ 2 are higher aortic gradients pre-TAVI, a lower BMI, a higher body surface area, a lower ejection fraction, a smaller diameter of TAVI, and the use of an SEV TAVI.
- –
- Moderate-to-severe PPM was not associated with a higher risk of long-term death, while PVL ≥ 2 after TAVI was associated with higher mortality.
4.1. Background
4.2. Physiopathology and PPM and PVL Risk Factors
4.3. BEV and SEV
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632, Erratum in Eur. Heart J. 2022, 43, 2022. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Van Belle, E.; Juthier, F.; Susen, S.; Vincentelli, A.; Iung, B.; Dallongeville, J.; Eltchaninoff, H.; Laskar, M.; Leprince, P.; Lievre, M.; et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: Analysis of predictors and impact on long-term mortality: Insights from the FRANCE2 Registry. Circulation 2014, 129, 1415–1427. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Theron, A.; Pinto, J.; Grisoli, D.; Griffiths, K.; Salaun, E.; Jaussaud, N.; Ravis, E.; Lambert, M.; Messous, L.; Amanatiou, C.; et al. Patient-prosthesis mismatch in new generation trans-catheter heart valves: A propensity score analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 225–233. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Daneshvar, S.A.; Fonarow, G.C.; Stebbins, A.; Vemulapalli, S.; Desai, N.D.; Malenka, D.J.; Thourani, V.H.; Rymer, J.; Kosinski, A.S. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2018, 72, 2701–2711. [Google Scholar] [CrossRef]
- Okuno, T.; Khan, F.; Asami, M.; Praz, F.; Heg, D.; Winkel, M.G.; Lanz, J.; Huber, A.; Gräni, C.; Räber, L.; et al. Prosthesis-Patient Mismatch Following Transcatheter Aortic Valve Replacement With Supra-Annular and Intra-Annular Prostheses. JACC Cardiovasc. Interv. 2019, 12, 2173–2182. [Google Scholar] [CrossRef]
- Gilard, M.; Eltchaninoff, H.; Iung, B.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N. Engl. J. Med. 2012, 366, 1705–1715. [Google Scholar] [CrossRef]
- Gilard, M.; Eltchaninoff, H.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; Teiger, E.; et al. Late Outcomes of Transcatheter Aortic Valve Replacement in High-Risk Patients: The FRANCE-2 Registry. J. Am. Coll. Cardiol. 2016, 68, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef]
- Didier, R.; Gouysse, M.; Eltchaninoff, H.; Le Breton, H.; Commeau, P.; Cayla, G.; Glatt, N.; Glatt, B.; Gabbas, M.; Tuppin, P.; et al. Successful linkage of French large-scale national registry populations to national reimbursement data: Improved data completeness and minimized loss to follow-up. Arch. Cardiovasc. Dis. 2020, 113, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A., Jr.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: A report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014, quiz 1082-4. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Rovin, J.D.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Sorajja, P.; Heiser, J.C.; et al. 2-Year Outcomes After Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J. Am. Coll. Cardiol. 2022, 79, 882–896. [Google Scholar] [CrossRef]
- Van Belle, E.; Vincent, F.; Labreuche, J.; Auffret, V.; Debry, N.; Lefèvre, T.; Eltchaninoff, H.; Manigold, T.; Gilard, M.; Verhoye, J.P.; et al. Balloon-Expandable Versus Self-Expanding Transcatheter Aortic Valve Replacement: A Propensity-Matched Comparison from the FRANCE-TAVI Registry. Circulation 2020, 141, 243–259. [Google Scholar] [CrossRef]
- Rahimtoola, S.H. The problem of valve prosthesis-patient mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef]
- Pibarot, P.; Ternacle, J.; Jaber, W.A.; Salaun, E.; Dahou, A.; Asch, F.M.; Weissman, N.J.; Rodriguez, L.; Xu, K.; Annabi, M.S.; et al. Structural Deterioration of Transcatheter Versus Surgical Aortic Valve Bioprostheses in the PARTNER-2 Trial. J. Am. Coll. Cardiol. 2020, 76, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Ternacle, J.; Pibarot, P.; Herrmann, H.C.; Kodali, S.; Leipsic, J.; Blanke, P.; Jaber, W.; Mack, M.J.; Clavel, M.A.; Salaun, E.; et al. Prosthesis-Patient Mismatch After Aortic Valve Replacement in the PARTNER 2 Trial and Registry. JACC Cardiovasc. Interv. 2021, 14, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.L.; Sengupta, A.; Alexis, S.L.; Bapat, V.N.; Adams, D.H.; Sharma, S.K.; Kini, A.S.; Kodali, S.K.; Ramlawi, B.; Gada, H.; et al. Outcomes of Prosthesis-Patient Mismatch Following Supra-Annular Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. JACC Cardiovasc. Interv. 2021, 14, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Hoelschermann, F.; Tambor, G.; Yoon, S.H.; Neuss, M.; Butter, C. Predictors of Paravalvular Regurgitation After Transcatheter Aortic Valve Implantation for Aortic Stenosis Using New-Generation Balloon-Expandable SAPIEN 3. Am. J. Cardiol. 2017, 119, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tölg, R.; Zachow, D.; Guerra, E.; Massberg, S.; Schäfer, U.; et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA 2014, 311, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Saint Etienne, C.; Grammatico-Guillon, L.; Porto, A.; Collart, F.; Bourguignon, T.; Cuisset, T.; et al. Impact of Sapien 3 Balloon-Expandable Versus Evolut R Self-Expandable Transcatheter Aortic Valve Implantation in Patients With Aortic Stenosis: Data from a Nationwide Analysis. Circulation 2020, 141, 260–268. [Google Scholar] [CrossRef]
| All Patients | Moderate-to-Severe Mismatch (n = 5138) | No Moderate-to-Severe Mismatch (n = 12,604) | p | |
|---|---|---|---|---|
| Duration of follow-up (days since the date of procedure) | 816 (391.3–1360.8) | 867 (427–1414) | 795 (379–1341) | <0.001 |
| Demography | ||||
| Age (years) | 82.6 ± 6.8 | 82.0 ± 7.3 | 82.9 ± 6.6 | <0.001 |
| Female | 8686 (49%) | 2577 (50%) | 6109 (48%) | 0.04 |
| Male | 9056 (51%) | 2561 (50%) | 6495 (52%) | |
| BMI | 26.2 (23.4–29.6) | 26.6 (23.9–29.3) | 26 (23.1–29.8) | <0.001 |
| Body surface (m2) | 1.8 (1.65–1.94) | 1.82 (1.69–1.97) | 1.78 (1.63–1.94) | <0.001 |
| Indicators at inclusion | ||||
| NYHA III/IV | 10,335 (62%) | 3020 (63%) | 7315 (62%) | 0.14 |
| Euroscore 2 | 3.72 (2.24–6.15) | 3.86 (2.24–6.79) | 3.69 (2.24–6) | <0.001 |
| Logistic Euroscore | 13.57 (8.9–21.43) | 13.83 (8.78–21.69) | 13.57 (8.97–21.3) | 0.28 |
| History and comorbidities | ||||
| Dyslipidemia | 5487 (31%) | 1624 (32%) | 3863 (31%) | 0.2 |
| Hypertension | 13,320 (75%) | 3847 (75%) | 9473 (75%) | 0.71 |
| CCS Class IV angina | 549 (4%) | 138 (3%) | 411 (4%) | 0.05 |
| Coronary angioplasty | 4661 (30%) | 1316 (29%) | 3345 (30%) | 0.17 |
| Coronary bypass | 1604 (9%) | 502 (10%) | 1102 (9%) | 0.04 |
| Diabetes | 4624 (26%) | 1371 (27%) | 3253 (26%) | 0.21 |
| Myocardial Infarction < 90 days | 244 (2%) | 86 (2%) | 158 (1%) | 0.04 |
| History of stroke | 1951 (11%) | 564 (11%) | 1387 (11%) | 0.99 |
| Chronic renal failure | 7702 (50%) | 2239 (50%) | 5463 (50%) | 0.23 |
| Dialysis | 309 (2%) | 80 (2%) | 229 (2%) | 0.3 |
| Creatinine (µmol/L) | 92 (74–118) | 93 (75–119.85) | 91 (74–117) | <0.001 |
| Chronic obstructive pulmonary disease | 3426 (19%) | 996 (19%) | 2430 (19%) | 0.9 |
| Pacemaker | 1961 (13%) | 548 (12%) | 1413 (13%) | 0.3 |
| Peripheral arterial disease | 3793 (24%) | 1112 (24%) | 2681 (23%) | 0.8 |
| Preimplantation examination | ||||
| Severe pulmonary hypertension (>60 mmHg) | 1452 (11%) | 472 (12%) | 980 (10%) | <0.001 |
| Aortic valve area (cm2) | 0.7 (0.58–0.8) | 0.7 (0.55–0.8) | 0.7 (0.6–0.82) | <0.001 |
| Mean gradient (mmHg) | 47 (40–57) | 47 (39–57) | 47 (40–56) | 0.24 |
| Ejection fraction (%) | 60 (50–65) | 60 (45–65) | 60 (50–65) | <0.001 |
| Aortic annulus diameter (mm) | 23.5 (22–25.7) | 23 (22–25.4) | 23.6 (22–25.9) | <0.001 |
| Aortic regurgitation ≥ 2 | 2695 (18%) | 863 (20%) | 1832 (18%) | <0.001 |
| Mitral regurgitation ≥ 2 | 3345 (23%) | 1011 (23%) | 2334 (22%) | 0.2 |
| Coronary stenosis (>50%) | 6901 (41%) | 1952 (41%) | 4949 (42%) | 0.4 |
| Procedure | ||||
| Programmed predilatation | 4464 (37%) | 1302 (36%) | 3162 (37%) | 0.5 |
| Number of valves implanted > 1 | 200 (1%) | 51 (1%) | 149 (1%) | 0.3 |
| Access (iliofemoral) | 15,329 (87%) | 4410 (86%) | 10,919 (87%) | 0.15 |
| Valve type | <0.001 | |||
| SEV | 5638 (32%) | 1308 (25%) | 4330 (34%) | |
| Corevalve and Corevalve Evolut | 2229 (12%) | 619 (12%) | 1601 (12%) | |
| Corevalve Evolut Pro | 897 (5%) | 138 (3%) | 759 (6%) | |
| Corevalve Evolut R | 2512 (14%) | 542 (11%) | 1970 (16%) | |
| BEV | 12,104 (68%) | 3830 (75%) | 8274 (66%) | |
| Sapien | 1650 (9%) | 497 (10%) | 1153 (9%) | |
| Sapien 3 | 8971 (51%) | 2931 (57%) | 6040 (48%) | |
| Sapien XT | 1483 (8%) | 402 (8%) | 1081 (9%) | |
| Diameter of the prosthesis (mm) | 26 (23–29) | 26 (23–26) | 26 (26–29) | <0.001 |
| Postimplant examination | ||||
| Ejection fraction (%) | 60 (50–65) | 60 (50–65) | 60 (52–65) | <0.001 |
| Mean gradient (mmHg) | 10 (7–13) | 12 (9–15) | 9 (6.7–12) | <0.001 |
| Aortic valve area (cm2) | 1.78 (1.47–2.1) | 1.31 (1.17–1.5) | 1.9 (1.7–2.29) | <0.001 |
| Indexed aortic valve area (cm2/m2) | 0.99 (0.81–1.2) | 0.74 (0.64–0.83) | 1.08 (0.94–1.28) | <0.001 |
| Mitral regurgitation ≥2 | 2361 (16%) | 748 (17%) | 1613 (16%) | 0.02 |
| Severe pulmonary hypertension (>60 mmHg) | 638 (5%) | 217 (6%) | 421 (5%) | 0.02 |
| Events during hospitalization | ||||
| Pacemaker | 2386 (15%) | 640 (13%) | 1746 (15%) | 0.01 |
| Infection | 596 (4%) | 182 (4%) | 414 (4%) | 0.4 |
| ST+ infarction | 22 (0%) | <11 (<0.2%) * | 13 (0%) | 0.3 |
| Stroke | 272 (2%) | 78 (2%) | 194 (2%) | 0.95 |
| Major bleeding | 889 (5%) | 242 (5%) | 647 (6%) | 0.23 |
| Death | 154 (1%) | 38 (1%) | 116 (1%) | 0.31 |
| Duration of index hospitalization (days) | 8 (6–13) | 8 (6–13) | 8 (6–12) | <0.001 |
| Grade ≥ 2 periprosthetic aortic leak | 1055 (16%) | 317 (16%) | 738 (16%) | 0.44 |
| Variable | All Patients | PVL ≥ 2 (n = 4056) | PVL < 2 (n = 16,822) | p |
|---|---|---|---|---|
| Duration of follow-up (days since the date of procedure) | 781 (356–1341) | 803 (350–1351) | 777 (357–1339) | 0.95 |
| Demography | ||||
| Age (years) | 84 (80–87) | 84 (81–88) | 84 (80–87) | <0.001 |
| Female | 10,313 (49%) | 2034 (50%) | 8531 (51%) | 0.53 |
| Male | 10,565 (51%) | 2022 (50%) | 8291 (49%) | |
| BMI | 25.7 (23–29) | 25.2 (22.5–28.4) | 25.8 (23.1–29.1) | <0.001 |
| Body surface (m2) | 1.79 (1.63–1.93) | 1.77 (1.62–1.91) | 1.79 (1.63–1.94) | <0.001 |
| Indicators at inclusion | ||||
| NYHA III/IV | 12,126 (63%) | 2295 (61%) | 9831 (63%) | 0.02 |
| Euroscore 2 | 4 (2.39–6.86) | 3.75 (2.3–6) | 4 (2.4–7) | <0.001 |
| Logistic Euroscore | 13.57 (8.97–21.71) | 13.8 (9–21.56) | 13.57 (8.9–21.82) | 0.36 |
| History and comorbidities | ||||
| Dyslipidemia | 6207 (30%) | 1132 (28%) | 5075 (30%) | 0.01 |
| Hypertension | 15,425 (74%) | 2927 (72%) | 12,498 (74%) | 0.01 |
| Class IV angina | 611 (3%) | 104 (3%) | 507 (3%) | 0.24 |
| Coronary angioplasty | 5802 (31%) | 1014 (29%) | 4788 (32%) | <0.001 |
| Coronary bypass | 1865 (9%) | 317 (8%) | 1548 (9%) | <0.001 |
| Diabetes | 5126 (25%) | 850 (21%) | 4276 (26%) | <0.001 |
| Myocardial Infarction < 90 days | 284 (2%) | 50 (1%) | 234 (2%) | 0.64 |
| History of stroke | 2288 (11%) | 436 (11%) | 1852 (11%) | 0.6 |
| Chronic renal failure | 8874 (48%) | 1586 (46%) | 7288 (49%) | <0.001 |
| Dialysis | 385 (2%) | 68 (2%) | 317 (2%) | |
| Creatinine (µmol/L) | 92 (74–119) | 92 (74–119) | 92 (74–118) | >0.99 |
| Chronic obstructive pulmonary disease | 3668 (18%) | 685 (17%) | 2983 (18%) | 0.18 |
| Pacemaker | 2455 (13%) | 474 (14%) | 1981 (13%) | 0.58 |
| Peripheral arterial disease | 4700 (25%) | 846 (23%) | 3854 (25%) | 0.05 |
| Preimplantation examination | ||||
| Valve surface (cm2) | 0.7 (0.57–0.8) | 0.7 (0.56–0.8) | 0.7 (0.58–0.8) | 0.07 |
| Mean gradient (mmHg) | 47 (40–57) | 48 (40–60) | 46 (39–56) | <0.001 |
| Ejection fraction (%) | 60 (49–65) | 60 (48–65) | 60 (50–65) | 0.27 |
| Annulus diameter (mm) | 24 (22–26) | 24 (22–26) | 24 (22–26) | 0.06 |
| Severe pulmonary hypertension (>60 mmHg) | 1807 (11%) | 335 (10%) | 1472 (11%) | 0.17 |
| Aortic regurgitation ≥ 2 | 3511 (20%) | 927 (27%) | 2584 (19%) | <0.001 |
| Mitral regurgitation ≥ 2 | 4138 (24%) | 1003 (29%) | 3135 (23%) | <0.001 |
| Coronary stenosis (>50%) | 7995 (41%) | 1506 (40%) | 6489 (41%) | 0.19 |
| Procedure | ||||
| Programmed predilatation | 4166 (28%) | 814 (29%) | 3352 (28%) | 0.65 |
| Number of valves implanted > 1 | 286 (1%) | 83 (2%) | 203 (1%) | <0.001 |
| Access (iliofemoral) | 18,184 (87%) | 3581 (89%) | 14,603 (87%) | 0.01 |
| Valve type | <0.001 | |||
| SEV | 8583 (41%) | 2148 (53%) | 6435 (38%) | |
| Corevalve and Corevalve Evolut | 3182 (16%) | 885 (20%) | 2526 (16%) | |
| Corevalve Evolut Pro | 1227 (6%) | 269 (7%) | 958 (6%) | |
| Corevalve Evolut R | 4174 (20%) | 994 (25%) | 3180 (19%) | |
| BEV | 12,295 (59%) | 1908 (47%) | 10,387 (62%) | |
| Sapien | 1923 (9%) | 321 (8%) | 1602 (10%) | |
| Sapien 3 | 8889 (43%) | 1240 (31%) | 7649 (45%) | |
| Sapien XT | 1483 (7%) | 347 (9%) | 1136 (7%) | |
| Diameter of the prosthesis (mm) | 26 (23–29) | 26 (23–29) | 26 (23–29) | <0.001 |
| Postimplantation examination | ||||
| Ejection fraction (%) | 60 (50–65) | 60 (50–65) | 60 (50–65) | 0.25 |
| Mean gradient (mmHg) | 9.7 (7–13) | 10 (7–13) | 9.25 (7–13) | <0.001 |
| Aortic valve area (cm2) | 1.8 (1.5–2.1) | 1.8 (1.5–2.1) | 1.79 (1.5–2.1) | 0.78 |
| Indexed aortic valve area (cm2/m2) | 1 (0.83–1.21) | 1.01 (0.84–1.23) | 1 (0.82–1.21) | 0.05 |
| Aortic paravalvular leak ≥ 2 | 2982 (15%) | 2982 (80%) | 0 (0%) | |
| Mitral regurgitation ≥ 2 | 3047 (18%) | 891 (27%) | 2156 (16%) | <0.001 |
| Severe pulmonary hypertension (>60 mmHg) | 841 (6%) | 233 (8%) | 608 (5%) | <0.001 |
| Events during hospitalization | ||||
| Pacemaker | 2888 (14%) | 601 (15%) | 2287 (14%) | 0. |
| Infection | 722 (4%) | 144 (4%) | 578 (3%) | 0.8 |
| ST+ infarction | 25 (0%) | <11 (<0.3%) | ≥11 (<1%) | 0.41 |
| Stroke | 324 (2%) | 74 (2%) | 250 (2%) | 0.15 |
| Major bleeding | 1240 (6%) | 255 (7%) | 985 (6%) | 0.35 |
| Death | 298 (1%) | 90 (2%) | 208 (1%) | <0.001 |
| Duration of index hospitalization (days) | 8 (6–13) | 9 (6–14) | 8 (6–13) | <0.001 |
| Follow-up data at D30 | ||||
| Mismatch | 1602 (24%) | 376 (23%) | 1226 (24%) | 0.39 |
| Moderate | 1289 (19%) | 309 (19%) | 980 (19%) | 0.43 |
| Severe | 313 (5%) | 67 (4%) | 246 (5%) |
| Moderate-to-Severe PPM | ||||
|---|---|---|---|---|
| Variable | OR | IC 95 Lower | IC 95 Upper | p-Value |
| BMI (per 1 unit increase) | 0.958 | 0.947 | 0.968 | <0.001 |
| Age (per 1 year increase) | 0.992 | 0.987 | 0.997 | 0.003 |
| BSA (per 1 m2 increase) | 8.881 | 6.718 | 11.742 | <0.001 |
| Baseline ejection fraction (per 1% increase) | 0.985 | 0.982 | 0.988 | <0.001 |
| Diameter size of TAVI valve (per 1 mm increase) | 0.848 | 0.832 | 0.864 | <0.001 |
| Variable | OR | IC 95 Lower | IC 95 Upper | p-Value |
|---|---|---|---|---|
| BMI (per 1 unit increase) | 0.962 | 0.951 | 0.973 | <0.001 |
| Body Surface | 1.357 | 1.038 | 1.774 | 0.03 |
| Baseline mean gradient (per 1 mmHg increase) | 1.009 | 1.007 | 1.011 | <0.001 |
| Baseline ejection fraction (per 1 mmHg increase) | 0.995 | 0.992 | 0.998 | <0.001 |
| Diameter size of TAVI valve (per 1 mm increase) | 0.972 | 0.959 | 0.986 | <0.001 |
| Type of valve (BEV vs. SEV) | 0.509 | 0.469 | 0.552 | <0.001 |
| Moderate-to-Severe PPM | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio | IC 95 Lower | IC 95 Upper | p-Value |
| BMI (per 1 unit increase) | 0.992 | 0.986 | 0.998 | 0.009 |
| Age (per 1 year increase) | 1.008 | 1.003 | 1.012 | 0.001 |
| NYHA 3/4 (vs. 1/2) | 1.225 | 1.150 | 1.305 | <0.001 |
| Euroscore (per 1% increase) | 1.009 | 1.006 | 1.011 | <0.001 |
| COPD | 1.189 | 1.110 | 1.273 | <0.001 |
| Baseline mean gradient (per 1 mmHg increase) | 0.991 | 0.989 | 0.993 | <0.001 |
| Diameter size of TAVI valve (per 1 mm increase) | 1.021 | 1.009 | 1.034 | 0.001 |
| Major bleeding | 1.229 | 1.100 | 1.374 | <0.001 |
| Hospital stay duration (per 1 day increase) | 1.020 | 1.017 | 1.023 | <0.001 |
| PVL ≥ 2 | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio | IC 95 Lower | IC 95 Upper | p-Value |
| PVL ≥ 2 | 1.159 | 1.087 | 1.237 | <0.001 |
| Age (per 1 year increase) | 1.012 | 1.008 | 1.017 | <0.001 |
| BMI (per 1 unit increase) | 0.991 | 0.985 | 0.996 | 0.001 |
| NYHA (3/4 vs. 1/2) | 1.220 | 1.149 | 1.293 | <0.001 |
| Euroscore (per 1 unit increase) | 1.009 | 1.007 | 1.011 | <0.001 |
| COPD | 1.257 | 1.178 | 1.342 | <0.001 |
| Baseline mean gradient (per 1 mmHg increase) | 0.990 | 0.988 | 0.992 | <0.001 |
| Diameter of TAVI valve (per 1 mm increase) | 1.022 | 1.010 | 1.033 | <0.001 |
| Major bleeding | 1.178 | 1.068 | 1.300 | 0.001 |
| Hospital stay duration (per 1 day increase) | 1.018 | 1.016 | 1.021 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deharo, P.; Leroux, L.; Theron, A.; Ferrara, J.; Vaillier, A.; Jaussaud, N.; Porto, A.; Morera, P.; Gariboldi, V.; Iung, B.; et al. Long-Term Prognosis Value of Paravalvular Leak and Patient–Prosthesis Mismatch following Transcatheter Aortic Valve Implantation: Insight from the France-TAVI Registry. J. Clin. Med. 2022, 11, 6117. https://doi.org/10.3390/jcm11206117
Deharo P, Leroux L, Theron A, Ferrara J, Vaillier A, Jaussaud N, Porto A, Morera P, Gariboldi V, Iung B, et al. Long-Term Prognosis Value of Paravalvular Leak and Patient–Prosthesis Mismatch following Transcatheter Aortic Valve Implantation: Insight from the France-TAVI Registry. Journal of Clinical Medicine. 2022; 11(20):6117. https://doi.org/10.3390/jcm11206117
Chicago/Turabian StyleDeharo, Pierre, Lionel Leroux, Alexis Theron, Jérome Ferrara, Antoine Vaillier, Nicolas Jaussaud, Alizée Porto, Pierre Morera, Vlad Gariboldi, Bernard Iung, and et al. 2022. "Long-Term Prognosis Value of Paravalvular Leak and Patient–Prosthesis Mismatch following Transcatheter Aortic Valve Implantation: Insight from the France-TAVI Registry" Journal of Clinical Medicine 11, no. 20: 6117. https://doi.org/10.3390/jcm11206117
APA StyleDeharo, P., Leroux, L., Theron, A., Ferrara, J., Vaillier, A., Jaussaud, N., Porto, A., Morera, P., Gariboldi, V., Iung, B., Lefevre, T., Commeau, P., Gouysse, M., du Chayla, F., Glatt, N., Cayla, G., Le Breton, H., Benamer, H., Beurtheret, S., ... Cuisset, T., on behalf of France-TAVI and STOP-AS Investigators. (2022). Long-Term Prognosis Value of Paravalvular Leak and Patient–Prosthesis Mismatch following Transcatheter Aortic Valve Implantation: Insight from the France-TAVI Registry. Journal of Clinical Medicine, 11(20), 6117. https://doi.org/10.3390/jcm11206117







