Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: 3-Dimensional Analysis Using Cone-Beam Computed Tomography on the Outcomes of Non-Surgical Endodontic Treatment Using A-PRF+ and Calcium Hydroxide: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
- Generally healthy patients, both sexes, aged from 20 to 50 years old.
- Root canal treatments of apical periodontitis cases with both preoperative and 6-month follow-up CBCT images.
- Teeth with appropriate amount of hard tooth tissue to be restored.
- Patients who had an intact restoration at follow-up.
- Teeth with root fractures or perforations.
- Teeth which were previously endodontically treated.
2.2. Pain Assessment
2.3. Healing Assessment
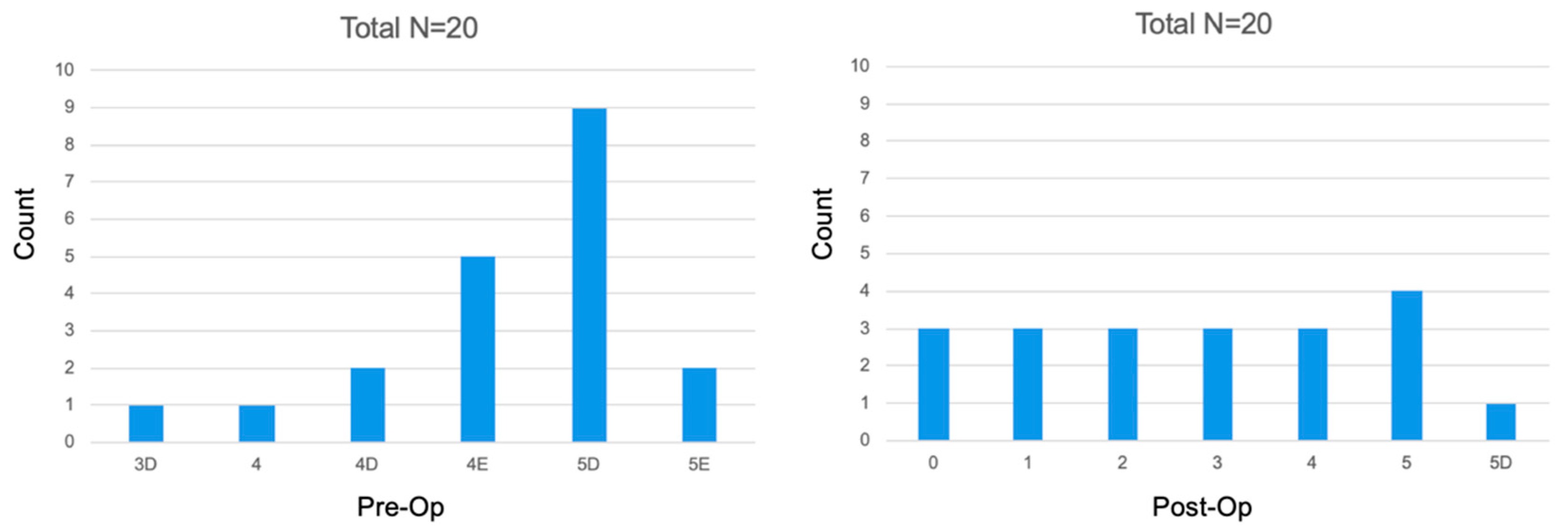
- Healed lesion: CBCT-PAI from 3, 4, 5 (+E/D) pre-operatively to CBCT-PAI 0, 1, 2 after 6-months.
- Healing lesion: CBCT-PAI from 3, 4, 5 (+E/D) pre-operatively improved, but is not 0, 1, 2 at a follow up CBCT.
- Diseased (not healed/healing): CBCT-PAI pre-operatively stays the same at follow up or is enlarged.
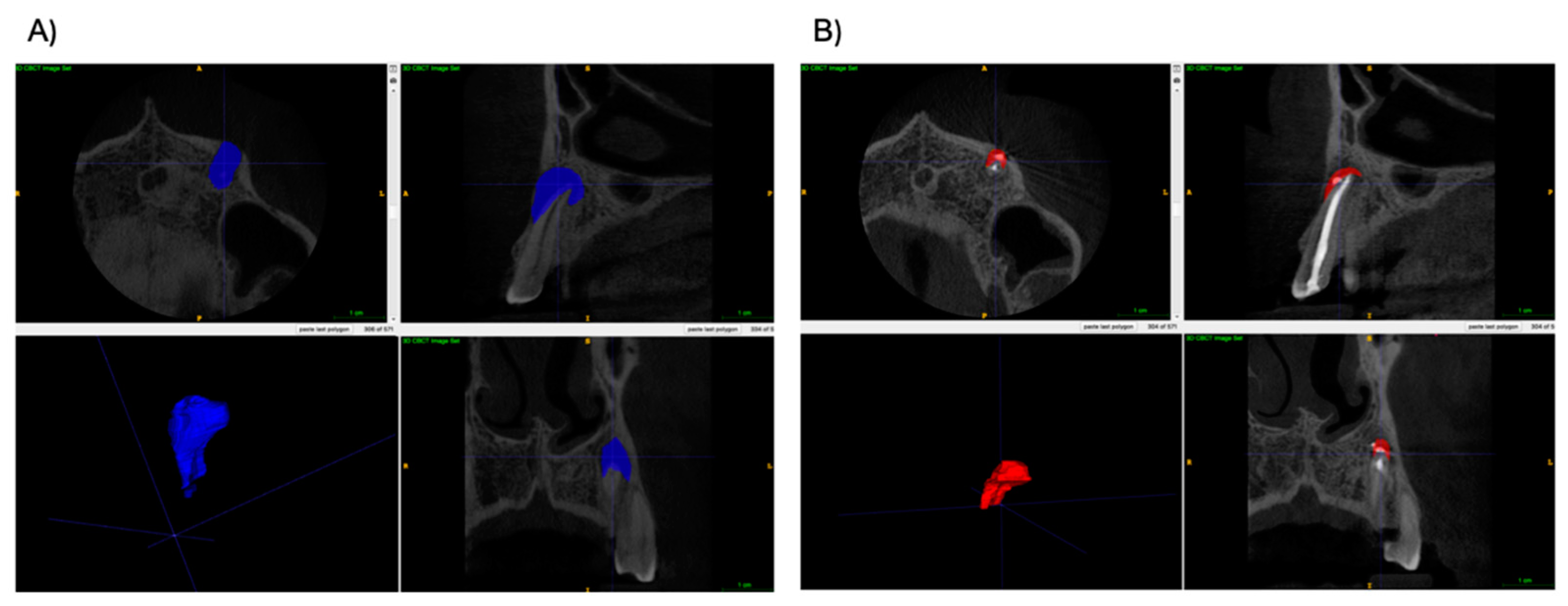
2.4. Calculation of Lesion Volume in CBCT
2.5. Statistical Analysis
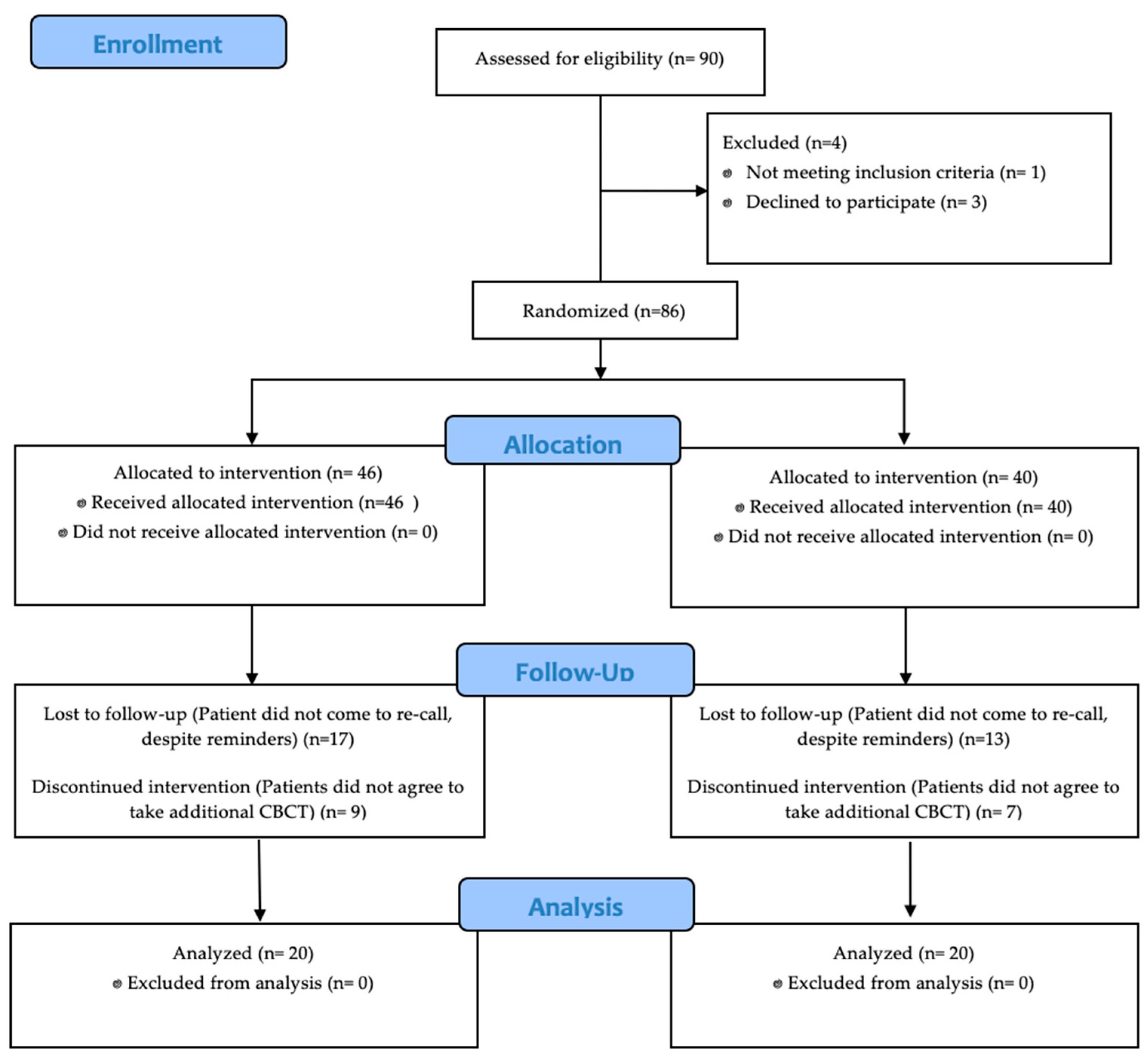
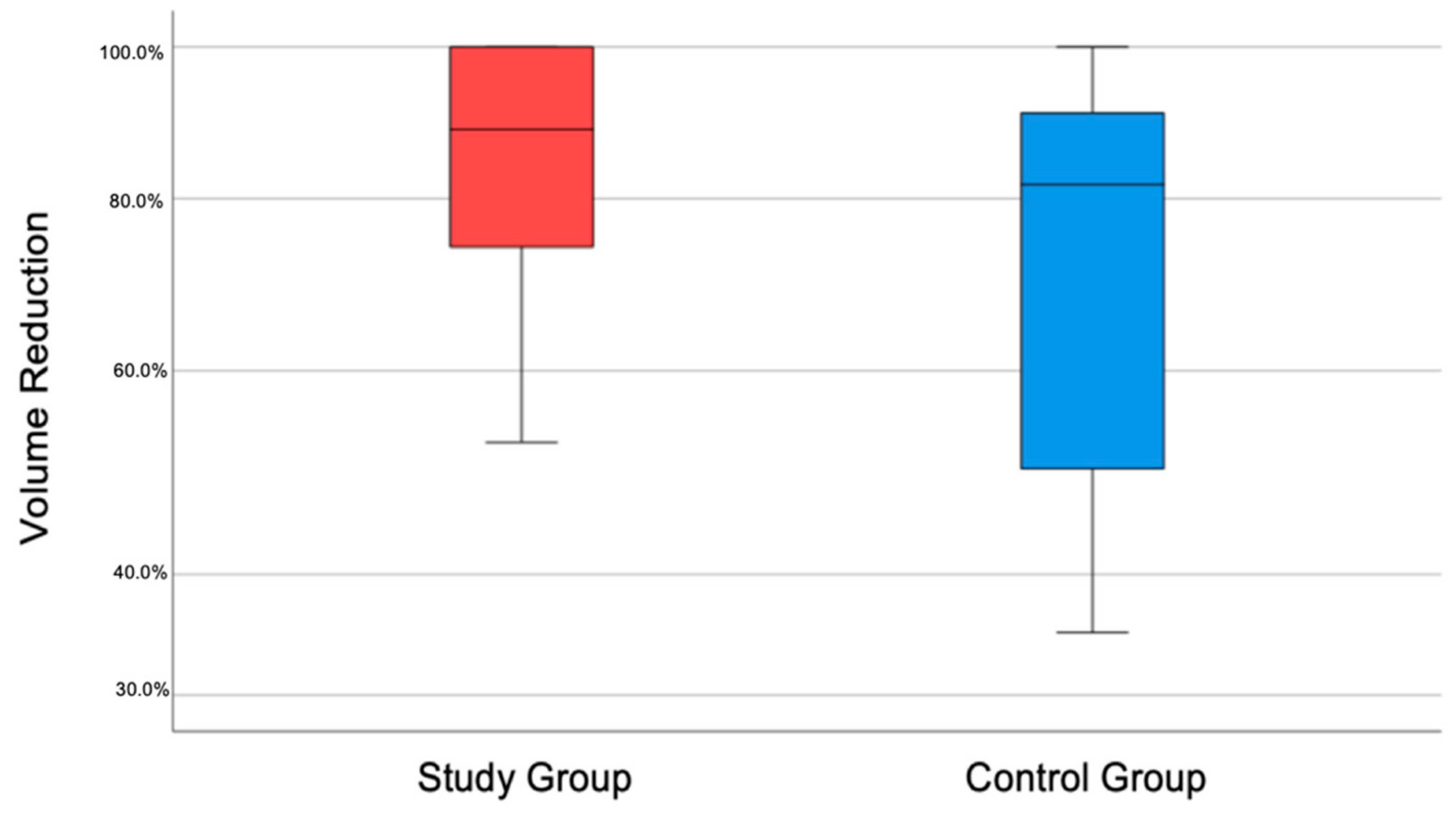
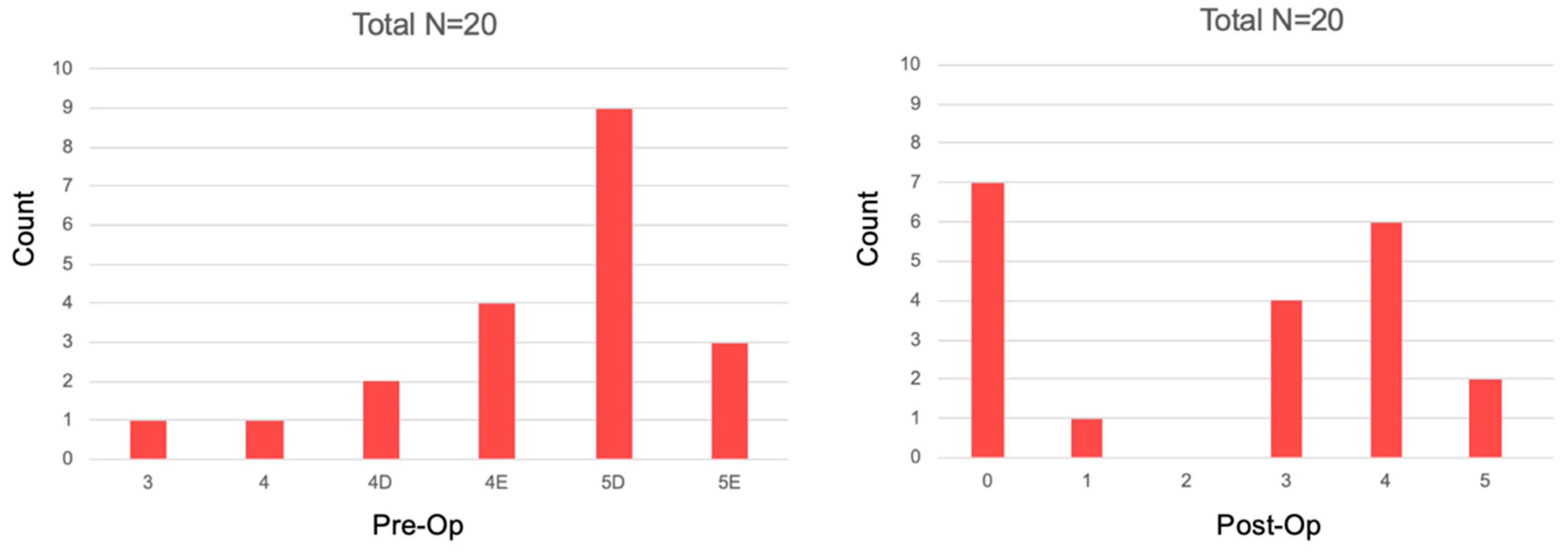
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbott, P.V. Classification, diagnosis and clinical manifestation of apical periodontitis. Endod. Top. 2004, 8, 36–54. [Google Scholar] [CrossRef]
- Nair, P.N.R. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed]
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; de Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol Scand. 2019, 77, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, T.; Bowles, W.R.; Rohrer, M. Frequency and distribution of radiolucent jaw lesions: A retrospective analysis of 9723 cases. J. Endod. 2012, 38, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Akinyamoju, A.O.; Gbadebo, S.O.; Adeyemi, B.F. Periapical lesions of the jaws: A review of 104 cases in ibadan. Ann. Ib. Postgrad. Med. 2014, 12, 115–119. [Google Scholar] [PubMed]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef]
- Fernandes, M.; de Ataide, I. Nonsurgical management of periapical lesions. J. Conserv. Dent. 2010, 13, 240–245. [Google Scholar] [CrossRef]
- Zoltowska, A.; Machut, K.; Pawlowska, E.; Derwich, M. Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: A Narrative Review. Pharmaceuticals 2021, 14, 1041. [Google Scholar] [CrossRef]
- Johns, D.A.; Varughese, J.M.; Thomas, K.; Abraham, A.; James, E.P.; Maroli, R.K. Clinical and radiographical evaluation of the healing of large periapical lesions using triple antibiotic paste, photo activated disinfection and calcium hydroxide when used as root canal disinfectant. J. Clin. Exp. Dent. 2014, 6, 230–236. [Google Scholar] [CrossRef]
- Demiriz, L.; Bodrumlu, E.H. Retrospective Evaluation of Healing of Periapical Lesions after Unintentional Extrusion of Mineral Trioxide Aggregate. J. Appl. Biomater. Funct. Mater. 2017, 15, 382–386. [Google Scholar] [CrossRef]
- Nair, P.N.R.; Henry, S.; Cano, V.; Vera, J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after ‘one-visit’ endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, N.; Alghamdi, F. Effect of Chemical Debridement and Irrigant Activation on Endodontic Treatment Outcomes: An Updated Overview. Cureus 2022, 14, 21525. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.T.; Karygianni, L.; Attin, T.; Thurnheer, T. Antibacterial Effect of Sodium Hypochlorite and EDTA in Combination with High-Purity Nisin on an Endodontic-like Biofilm Model. Antibiotics 2021, 10, 1141. [Google Scholar] [CrossRef]
- Hosoya, N.; Takahashi, G.; Arai, T.; Nakamura, J. Calcium concentration and pH of the periapical environment after applying calcium hydroxide into root canals in vitro. J. Endod. 2001, 27, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.S.; Bhuyan, A.C.; Kalita, C.; Das, L.; Kataki, R.; Bhuyan, D. Single Versus Multi-visit Endodontic Treatment of Teeth with Apical Periodontitis: An in vivo Study with 1-year Evaluation. Ann. Med. Health Sci. Res. 2016, 6, 19–26. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial activity of calcium hydroxide in endodontics: A review. Chonnam Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Kumar, K.; Venkatesan, R.; Krishnamoorthy, S.; Manoharan, V.; Marimuthu, S. Assessment of Coronal Leakage with Two Intracanal Medicaments After Exposure to Human Saliva-An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2018, 11, 406–411. [Google Scholar] [CrossRef]
- Wong, A.W.Y.; Zhang, C.; Chu, C.-H. A systematic review of nonsurgical single-visit versus multiple-visit endodontic treatment. Clin. Cosmet. Investig. Dent. 2014, 6, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Göstemeyer, G. Single-visit or multiple-visit root canal treatment: Systematic review, meta-analysis and trial sequential analysis. BMJ Open 2017, 7, 013115. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 37–44. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Canellas, J.V.D.S.; Medeiros, P.J.D.; Figueredo, C.M.D.S.; Fischer, R.G.; Ritto, F.G. Platelet-rich fibrin in oral surgical procedures: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2018, 29, 48–55. [Google Scholar] [CrossRef]
- Lektemur Alpan, A.; Torumtay Cin, G. PRF improves wound healing and postoperative discomfort after harvesting subepithelial connective tissue graft from palate: A randomized controlled trial. Clin. Oral Investig. 2020, 24, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Sterczała, B.; Chwiłkowska, A.; Szwedowicz, U.; Kobielarz, M.; Chwiłkowski, B.; Dominiak, M. Impact of APRF+ in Combination with Autogenous Fibroblasts on Release Growth Factors, Collagen, and Proliferation and Migration of Gingival Fibroblasts: An In Vitro Study. Materials 2022, 15, 796. [Google Scholar] [CrossRef]
- Orstavik, D.; Kerekes, K.; Eriksen, H.M. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Endod. Dent. Traumatol. 1986, 2, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ha, S.W.; Kim, U.; Kim, S.; Kim, E. A One-Year Radiographic Healing Assessment after Endodontic Microsurgery Using Cone-Beam Computed Tomographic Scans. J. Clin. Med. 2020, 9, 3714. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, R.; Nicita, F.; Puleio, F.; Alibrandi, A.; Cervino, G.; Lizio, A.S.; Pantaleo, G. Accuracy of Periapical Radiography and CBCT in Endodontic Evaluation. Int. J. Dent. 2018, 2018, 2514243. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Jin, L.; Han, J.; Li, Y.; Zhang, L.; Qi, S. Comparison of periapical radiography and cone beam computed tomography for the diagnosis of anterior maxillary trauma in children and adolescents. Dent. Traumatol. 2022, 38, 62–70. [Google Scholar] [CrossRef]
- Shukla, S.; Chug, A.; Afrashtehfar, K.I. Role of Cone Beam Computed Tomography in Diagnosis and Treatment Planning in Dentistry: An Update. J. Int. Soc. Prev. Community Dent. 2017, 7, 125–136. [Google Scholar] [CrossRef]
- Patel, S.; Brown, J.; Semper, M.; Abella, F.; Mannocci, F. European Society of Endodontology position statement: Use of cone beam computed tomography in Endodontics: European Society of Endodontology (ESE) developed by. Int. Endod. J. 2019, 52, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Patel, R.; Foschi, F.; Mannocci, F. The impact of different diagnostic imaging modalities on the evaluation of root canal anatomy and endodontic residents’ stress levels: A clinical study. J. Endod. 2019, 45, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Bueno, M.R.; Azevedo, B.C.; Azevedo, J.R.; Pécora, J.D. A new periapical index based on cone beam computed tomography. J. Endod. 2008, 34, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Graunaite, I.; Lodiene, G.; Maciulskiene, V. Pathogenesis of apical periodontitis: A literature review. J. Oral Maxillofac. Res. 2012, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Bracks, I.V.; Armada, L.; Gonçalves, L.S.; Pires, F.R. Distribution of mast cells and macrophages and expression of interleukin-6 in periapical cysts. J. Endod. 2014, 40, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Altaie, A.M.; Venkatachalam, T.; Samaranayake, L.P.; Soliman, S.S.M.; Hamoudi, R. Comparative Metabolomics Reveals the Microenvironment of Common T-Helper Cells and Differential Immune Cells Linked to Unique Periapical Lesions. Front. Immunol. 2021, 12, 707267. [Google Scholar] [CrossRef]
- Metzger, Z. Macrophages in periapical lesions. Endod. Dent. Traumatol. 2000, 16, 1–8. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth Factor and Pro-Inflammatory Cytokine Contents in Platelet-Rich Plasma (PRP), Plasma Rich in Growth Factors (PRGF), Advanced Platelet-Rich Fibrin (A-PRF), and Concentrated Growth Factors (CGF). Int. J. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stähli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Alsalleeh, F.; Stephenson, L.; Lyons, N.; Young, A.; Williams, S. Human Periodontal Ligament Cells Response to Commercially Available Calcium Hydrox- ide Pastes. Int. J. Dent. Oral Sci. 2014, 2, 6–9. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, L.; Jiang, J.; Gui, J.; Zhang, L.; Huang, Y.; Chen, X.; Ji, J.; Fan, Y. Calcium Hydroxide-Induced Proliferation, Migration, Osteogenic Differentiation, and Mineralization via the Mitogen-Activated Protein Kinase Pathway in Human Dental Pulp Stem Cells. J. Endod. 2016, 42, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Patil, S.; Boreak, N.; Chohan, H.; AbuMelha, A.S.; Alkahtany, M.F.; Almadi, K.H.; Vinothkumar, T.S.; Raj, A.T.; Testarelli, L. Effect of Different Intracanal Medicaments on the Viability and Survival of Dental Pulp Stem Cells. J. Pers. Med. 2022, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Talpos-Niculescu, R.M.; Popa, M.; Rusu, L.C.; Pricop, M.O.; Nica, L.M.; Talpos-Niculescu, S. Conservative Approach in the Management of Large Periapical Cyst-Like Lesions. A Report of Two Cases. Medicina 2021, 57, 497. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lagares, D.; Segura-Egea, J.J.; Rodríguez-Caballero, A.; Llamas-Carreras, J.M.; Gutiérrez-Pérez, J.L. Treatment of a large maxillary cyst with marsupialization, decompression, surgical endodontic therapy and enucleation. J. Can. Dent. Assoc. 2011, 77, 87. [Google Scholar]
- Orstavik, D. Time-course and risk analyses of the development and healing of chronic apical periodontitis in man. Int. Endod. J. 1996, 29, 150–155. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, R.K.; Tikku, A.P.; Chandra, A.; Verma, P.; Bharti, R.; Shakya, V.K. A randomized controlled trial of endodontic treatment using ultrasonic irrigation and laser activated irrigation to evaluate healing in chronic apical periodontitis. J Clin Exp Dent. 2020, 12, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Mostafapoor, M.; Hemmatian, S. Evaluation of the accuracy values of cone-beam CT regarding apical periodontitis: A systematic review and meta-analysis. Oral Radiol. 2022, 38, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cotti, E.; Schirru, E. Present status and future directions: Imaging techniques for the detection of periapical lesions. Int. Endod. J. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Rice, D.D.; Maktabi, O.; Oyoyo, U.; Abramovitch, K. Prevalence and Size of Periapical Radiolucencies Using Cone-beam Computed Tomography in Teeth without Apparent Intraoral Radiographic Lesions: A New Periapical Index with a Clinical Recommendation. J. Endod. 2018, 44, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Konagala, R.K.; Mandava, J.; Pabbati, R.K.; Anupreeta, A.; Borugadda, R.; Ravi, R. Effect of pretreatment medication on postendodontic pain: A double-blind, placebo-controlled study. J. Conserv. Dent. 2019, 22, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Amit, A.; Ashkenazi, M. Post-operative pain and use of analgesic agents following various dental procedures. Am. J. Dent. 2006, 19, 245–247. [Google Scholar] [PubMed]
- Seltezer, S.; Naidorf, I.J. Flare-ups in endodontics: I. Etiological factors. J. Endod. 2004, 30, 476–481. [Google Scholar] [CrossRef]
- Al-Nahlawi, T.; Alabdullah, A.; Othman, A.; Sukkar, R.; Doumani, M. Postendodontic pain in asymptomatic necrotic teeth prepared with different rotary instrumentation techniques. J. Fam. Med. Prim. Care 2020, 9, 3474–3479. [Google Scholar] [CrossRef]
- Vishwanathaiah, S.; Maganur, P.C.; Khanagar, S.B.; Chohan, H.; Testarelli, L.; Mazzoni, A.; Gupta, A.A.; Raj, A.T.; Bhandi, S.; Mehta, D.; et al. The Incidence and Intensity of Postendodontic Pain and Flareup in Single and Multiple Visit Root Canal Treatments: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 3358. [Google Scholar] [CrossRef]




| Periapical Index | |
|---|---|
| 1 | Normal periapical structures |
| 2 | Small changes in bone structures |
| 3 | Change in bone structure with mineral loss |
| 4 | Periodontitis with well-defined radiolucent area |
| 5 | Severe periodontitis with exacerbating features |
| Score | Quantitative Radiolucency in Alveolar Bone Structures |
|---|---|
| 0 | Undisturbed periapical bone structures |
| 1 | Diameter of periapical radiolucency > 0.5–1 mm |
| 2 | Diameter of periapical radiolucency > 1–2 mm |
| 3 | Diameter of periapical radiolucency > 2–4 mm |
| 4 | Diameter of periapical radiolucency > 4–8 mm |
| 5 | Diameter of periapical radiolucency > 8 mm |
| Score (n) + E | Expansion of periapical cortical bone |
| Score (n) + D | Destruction of periapical cortical bone |
| Study Group | Control Group |
|---|---|
| All patients were treated by one investigator according to a standard regimen including elements of access, rubber dam, and establishment of asepsis. Local anesthesia (4% articaine with 1:100,000 epinephrine) was administered to the patient. | |
| Initial canal working length was established by using the electronic apex locator and a stainless-steel K-file (Endostar, Poldent LLC, Warsaw, Poland). Working length was confirmed by using radiographs. Canals were chemomechanically prepared with the modified crown-down technique using NiTi 0.04 rotary instruments (K3, Kerr, Glendora, CA, USA). Canals were irrigated with 5% sodium hypochlorite after each instrumentation cycle. All canals were irrigated with 40% citric acid (CA) for 1 min followed by a final irrigation with 5% sodium hypochlorite, and distillated water with the manual dynamic activation (MDA) and gutta-percha cone. Canals were then dried with sterile paper points. | |
| Application of A-PRF below the cementodentinal junction. Final obturation by the thermoplastic method with calibrated gutta-percha cone and AH-plus sealer, using the combination of a down-pack heat source with a Backfill extruder. | The canal was temporarily filled with non-hardening calcium hydroxide for 2 weeks. At the second appointment, the canal was obturated at the same appointment by using the thermoplastic method with a calibrated gutta-percha cone and AH-plus sealer, using the combination of a down-pack heat source with a Backfill extruder. |
| Score | Healing | Description |
|---|---|---|
| 1 | Complete |
|
| 2 | Limited |
|
| 3 | Uncertain |
|
| 4 | Unsatisfactory |
|
| Study Group | Control Group | |||
|---|---|---|---|---|
| Sex | female | 52.6% (n = 10) | 45.0% (n = 9) | |
| male | 47.4% (n = 9) | 55.0% (n = 11) | ||
| Age (average) ** | 33.7 | 30.0 | ||
| API (average) [%] *** | 54.3 | 65.0 | ||
| Tooth | maxillary | incisors | N = 10 | N = 8 |
| canines | N = 2 | N = 2 | ||
| premolars | N = 3 | N = 4 | ||
| molars | N = 0 | N = 0 | ||
| mandibular | incisors | N = 1 | N = 1 | |
| canines | N = 1 | N = 0 | ||
| premolars | N = 1 | N = 2 | ||
| molars | N = 2 | N = 3 | ||
| Healing Assessment | Study Group | Control Group |
|---|---|---|
| Completely healed | N = 9 | N = 6 |
| Good healing | N = 8 | N = 5 |
| Limited healing | N = 3 | N = 4 |
| Uncertain healing | N = 0 | N = 5 |
| Healed | N = 8 | N = 9 |
| Healing | N = 12 | N = 9 |
| Not Healed/No Healing | N = 0 | N = 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machut, K.; Żółtowska, A. Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: 3-Dimensional Analysis Using Cone-Beam Computed Tomography on the Outcomes of Non-Surgical Endodontic Treatment Using A-PRF+ and Calcium Hydroxide: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 6092. https://doi.org/10.3390/jcm11206092
Machut K, Żółtowska A. Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: 3-Dimensional Analysis Using Cone-Beam Computed Tomography on the Outcomes of Non-Surgical Endodontic Treatment Using A-PRF+ and Calcium Hydroxide: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(20):6092. https://doi.org/10.3390/jcm11206092
Chicago/Turabian StyleMachut, Katarzyna, and Agata Żółtowska. 2022. "Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: 3-Dimensional Analysis Using Cone-Beam Computed Tomography on the Outcomes of Non-Surgical Endodontic Treatment Using A-PRF+ and Calcium Hydroxide: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 20: 6092. https://doi.org/10.3390/jcm11206092
APA StyleMachut, K., & Żółtowska, A. (2022). Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: 3-Dimensional Analysis Using Cone-Beam Computed Tomography on the Outcomes of Non-Surgical Endodontic Treatment Using A-PRF+ and Calcium Hydroxide: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(20), 6092. https://doi.org/10.3390/jcm11206092







