Left Ventricular Mass with Delayed Enhancement as a Predictor of Major Events in Patients with Myocarditis with Preserved Ejection Fraction
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Biohumoral Evaluation
2.3. Transthoracic Echocardiography
2.4. Cardiac Magnetic Resonance
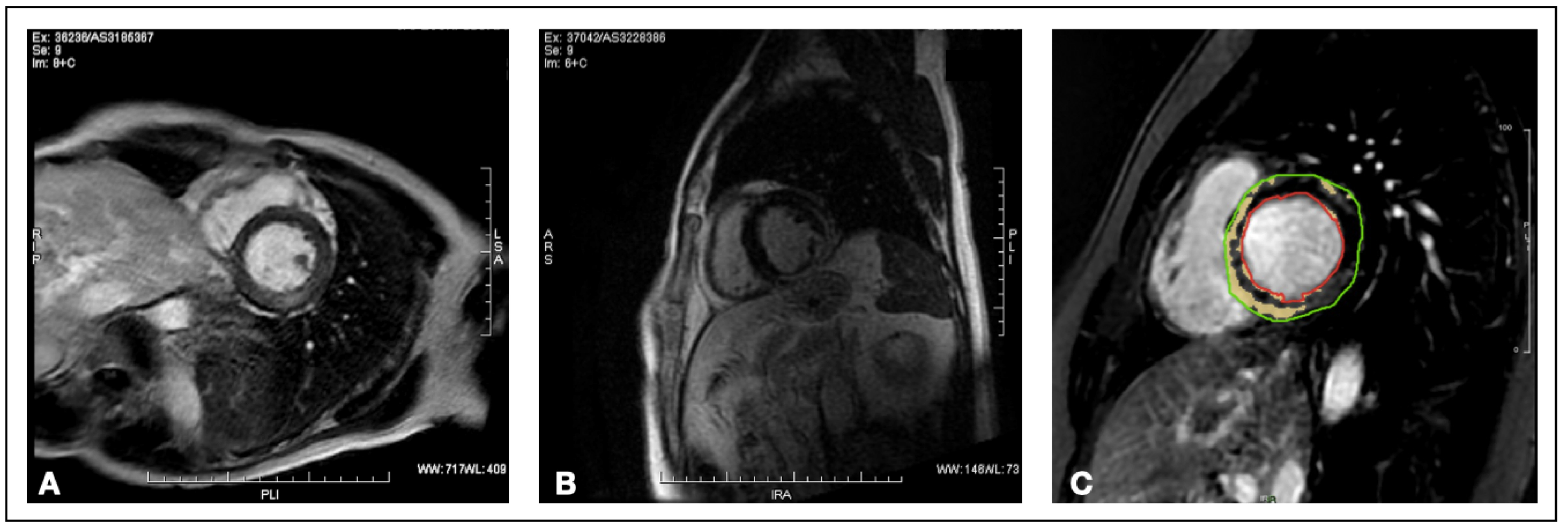
2.5. Late Gadolinium Enhancement Quantification
2.6. Follow-Up Evaluation
2.7. Statistical Methods
3. Results
3.1. Baseline Features
3.2. Biochemical Findings
3.3. Baseline Echocardiographic Findings
3.4. Cardiac Magnetic Resonance Findings
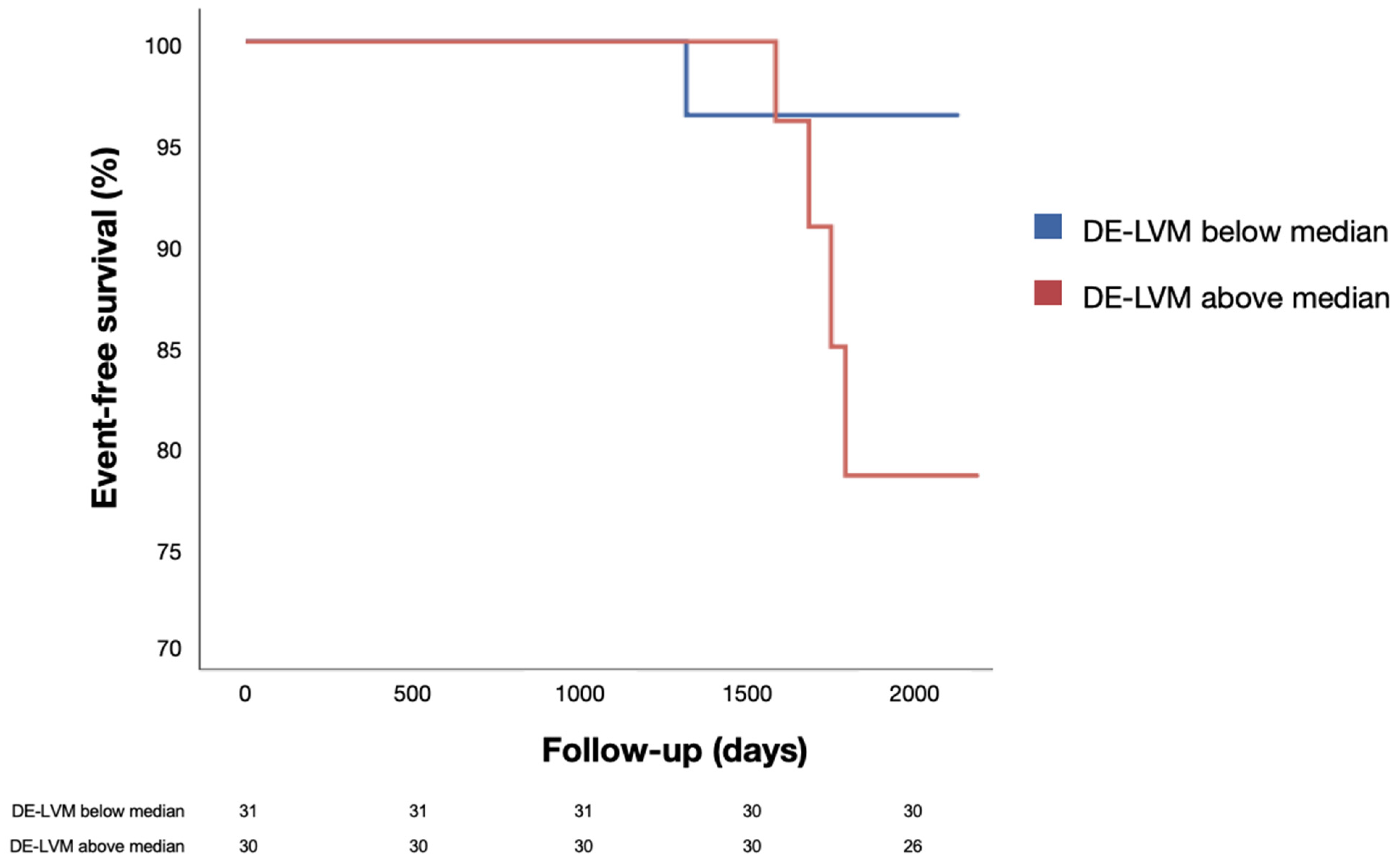
3.5. Events at Follow-up
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMpEF | acute myocarditis with preserved ejection fraction |
| CMR | cardiac magnetic resonance |
| DE-LVM | delayed enhancement-left ventricular mass |
| HF | heart failure |
| LGE | late gadolinium enhancement |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| NYHA | New York Heart Association |
| TAPSE | tricuspid annular plane systolic excursion |
References
- Richardson, P.; McKenna, W.; Bristow, M.; Rossi, V. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Sheppard, M.N. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart 2006, 92, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Caforio, A.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Marcotte, F. Cardiac magnetic resonance assessment of myocarditis. Circ. Cardiovasc. Imaging 2013, 6, 833–839. [Google Scholar] [CrossRef]
- Cipriani, M.; Merlo, M.; Gabrielli, D.; Ammirati, E.; Autore, C.; Basso, C.; Caldarola, P.; Camici, P.; di Lenarda, A.; Frustaci, A.; et al. ANMCO/SIC Consensus document on the management of myocarditis [article in Italian]. G. Ital. Di Cardiol. 2006, 21, 969–989. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; Di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2021, 14, e011492. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. (Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology). Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Shephard, D.A. The 1975 Declaration of Helsinki and consent. Can. Med. Assoc. J. 1976, 115, 1191–1192. [Google Scholar] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Sechtem, U.; Pflugfelder, P.W.; Gould, R.G.; Cassidy, M.M.; Higgins, C.B. Measurement of Right and Left Ventricular Volumes in Healthy Individuals with Cine MR Imaging. Radiology 1987, 163, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.M.; Prasad, S.K.; Khan, M.; Pennell, D.J. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur. Heart J. 2006, 27, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.M.; Prasad, S.K.; Khan, M.; Pennell, D.J. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2006, 8, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int. J. Cardiovasc. Imaging 2002, 18, 539–542. [Google Scholar] [PubMed]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Coelho-Filho, O.R.; Danik, S.B.; Shah, R.V.; Dodson, J.A.; Verdini, D.J.; Tokuda, M.; Daly, C.; Tedrow, U.B.; Stevenson, W.G.; et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc. Imaging 2013, 6, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Schumm, J.; Greulich, S.; Wagner, A.; Grün, S.; Ong, P.; Bentz, K.; Klingel, K.; Kandolf, R.; Bruder, O.; Schneider, S.; et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J. Cardiovasc. Magn. Reson. 2014, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Sanguineti, F.; Garot, P.; Mana, M.; O’H-Ici, D.; Hovasse, T.; Unterseeh, T.; Louvard, Y.; Troussier, X.; Morice, M.-C.; Garot, J. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2015, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; Eitel, I.; Adam, J.; Steiner, J.; Grothoff, M.; Desch, S.; Fuernau, G.; de Waha, S.; Sareban, M.; Luecke, C.; et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. J. Am. Coll. Cardiol. Imaging 2012, 5, 513–524. [Google Scholar] [CrossRef] [PubMed]

| Whole Population (n = 61) | No CV Events (n = 54) | Composite CV Event (n = 7) | p Value | |
|---|---|---|---|---|
| Baseline features | ||||
| Age (years) | 39 ± 12 | 38 ± 12 | 44 ± 9 | 0.175 |
| Sex (male) | 49 (80) | 42 (78) | 7 (100) | 0.327 |
| Hypertension, n (%) | 15 (25) | 14 (26) | 1 (14) | 0.588 |
| Dyslipidemia, n (%) | 13 (21) | 11 (20) | 1 (14) | 0.670 |
| Diabetes, n (%) | 1 (2) | 0 (0) | 1 (14) | 0.130 |
| Smoking habit, n (%) | 19 (31) | 17 (31) | 2 (29) | 0.661 |
| Body mass index (kg/m2) | 25.2 ± 3.6 | 25.3 ± 3.8 | 24.6 ± 1.2 | 0.702 |
| Prior myocarditis, n (%) | 4 (7) | 4 (7) | 0 (0) | 0.506 |
| Laboratory findings | ||||
| C reactive protein (mg/dL) | 1.96 (0.62–5.42) | 1.96 (0.62–5.87) | 2.35 (0.80–3.40) | 0.798 |
| WBC (cells/mmc) | 8792 ± 2681 | 8824 ± 2766 | 8530 ± 2137 | 0.813 |
| Neutrophils (%) | 63 ± 11 | 61 ± 11 | 76 ± 7 | 0.014 |
| Hs-troponin (ng/L) | 648 (251–1555) | 710 (264–1583) | 473 (90–1843) | 0.677 |
| Serum creatinine (mg/dL) | 0.92 ± 0.12 | 0.91 ± 0.11 | 0.97 ± 0.15 | 0.452 |
| GOT (UI/L) | ||||
| Baseline echocardiographic findings | ||||
| Baseline LVEF (%) | 57 ± 3 | 57 ± 3 | 56 ± 2 | 0.459 |
| IVS (mm) | 9.9 ± 1.3 | 9.9 ± 1.3 | 10.0 ± 2.0 | 0.882 |
| TAPSE (mm) | 23 ± 4 | 23 ± 3 | 26 ± 7 | 0.049 |
| sPAP (mmHg) | 26 ± 5 | 25 ± 5 | 25 ± 5 | 0.798 |
| Cardiac magnetic resonance findings | ||||
| Indexed LVEDV (mL/m2) | 80 ± 14 | 80 ± 14 | 81 ± 15 | 0.970 |
| Indexed LVM (g/m2) | 75 ± 13 | 75 ± 13 | 74 ± 16 | 0.974 |
| LVEF (%) | 62 ± 5 | 62 ± 5 | 61 ± 8 | 0.666 |
| Indexed RVEDV (mL/m2) | 63 (58–75) | 63 (58–75) | 59 (53–68) | 0.367 |
| RVEF (%) | 57 ± 8 | 57 ± 8 | 58 ± 8 | 0.876 |
| Presence of myocardial edema, n (%) | 30 (49) | 26 (48) | 4 (57) | 0.707 |
| IVS edema, n (%) | 14 (23) | 12 (22) | 2 (29) | 0.655 |
| Lateral/inferolateral edema, n (%) | 15 (25) | 13 (24) | 2 (29) | 0.795 |
| Inferior edema, n (%) | 7 (12) | 6 (11) | 1 (14) | 0.804 |
| Anterior edema, n (%) | 9 (15) | 8 (15) | 1 (14) | 0.970 |
| DE, n (%) | 57 (93) | 52 (96) | 5 (71) | 0.012 |
| IVS DE, n (%) | 16 (26) | 14 (26) | 2 (29) | 0.881 |
| Lateral/inferolateral DE, n (%) | 44 (72) | 40 (74) | 4 (57) | 0.386 |
| Inferior DE, n (%) | 14 (23) | 11 (20) | 3 (43) | 0.335 |
| Anterior DE, n (%) | 7 (12) | 7 (13) | 0 (0) | 0.586 |
| Number of segments with DE, n | 2.0 ± 1.7 | 2.0 ± 1.4 | 1.9 ± 1.5 | 0.813 |
| DE-LVM (g) | 12 (8–17) | 12 (8–16) | 18 (14–29.5) | 0.028 |
| DE-LVM/LVM, % | 7.9 (6.4–11.7) | 7.7 (6.2–11.6) | 11.3 (9.9–19.9) | 0.047 |
| Follow-up | ||||
| Echocardiographic LVEF (%) | 57 ± 4 | 57 ± 5 | 55 ± 2 | 0.261 |
| B | HR | CI (95%) | p Value | |
|---|---|---|---|---|
| Age | 0.049 | 1.050 | 0.985–1.120 | 0.131 |
| C reactive protein | −0.138 | 0.872 | 0.639–1.189 | 0.386 |
| Neutrophils | 0.010 | 1.010 | 0.962–1.061 | 0.677 |
| Hs-troponin | 0.045 | 1.045 | 0.979–1.028 | 0.860 |
| sPAP | 0.006 | 1.006 | 0.811–1.248 | 0.957 |
| LVEF | −0.017 | 0.983 | 0.850–1.136 | 0.814 |
| RVEF | 0.010 | 1.010 | 0.909–1.122 | 0.856 |
| IVS DE | −0.123 | 0.884 | 0.171–4.583 | 0.884 |
| DE-LVM | 0.122 | 1.130 | 1.017–1.256 | 0.022 |
| DE-LVM/LVM | 4.573 | 2.132 | 1.272–3.573 | 0.018 |
| Whole Population (n = 61) | DE-LVM below Median Value (n = 31) | DE-LVM above Median Value (n = 30) | p Value | |
|---|---|---|---|---|
| Baseline features | ||||
| Age (years) | 39 ± 12 | 38 ± 12 | 38 ± 10 | 0.175 |
| Sex (male) | 49 (80) | 21 (68) | 28 (93) | 0.327 |
| Hypertension, n (%) | 15 (25) | 9 (29) | 6 (20) | 0.592 |
| Dyslipidemia, n (%) | 13 (21) | 7 (23) | 6 (20) | 0.856 |
| Diabetes, n (%) | 1 (2) | 0 | 1 (3) | 0.130 |
| Smoking habit, n (%) | 19 (31) | 11 (35) | 8 (27) | 0.948 |
| Body mass index (kg/m2) | 25.2 ± 3.6 | 25.0 ± 3.8 | 25.5 ± 3.5 | 0.418 |
| Prior myocarditis, n (%) | 4 (7) | 2 (6) | 2 (7) | 0.506 |
| Laboratory findings | ||||
| C reactive protein (mg/dL) | 1.96 (0.62–5.42) | 1.82 (0.45–4.35) | 2.70 (0.67–7.00) | 0.775 |
| WBC (cells/mmc) | 8792 ± 2681 | 8794 ± 2823 | 8738 ± 2753 | 0.813 |
| Neutrophils (%) | 63 ± 11 | 59 ± 11 | 64 ± 11 | 0.014 |
| Hs-troponin (ng/L) | 648 (251–1555) | 396 (195–1055) | 720 (355–1915) | 0.669 |
| Serum creatinine (mg/dL) | 0.92 ± 0.12 | 0.91 ± 0.11 | 0.93 ± 0.13 | 0.452 |
| GOT (UI/L) | 27 (18–54) | 20 (16–29) | 40 (23–71) | 0.296 |
| Baseline echocardiographic findings | ||||
| Baseline LVEF (%) | 57 ± 3 | 57 ± 3 | 57 ± 3 | 0.459 |
| IVS (mm) | 9.9 ± 1.3 | 9.4 ± 1.1 | 10.3 ± 1.4 | 0.882 |
| TAPSE (mm) | 23 ± 4 | 24 ± 4 | 23 ± 4 | 0.049 |
| sPAP (mmHg) | 26 ± 5 | 25 ± 3 | 26 ± 6 | 0.798 |
| Cardiac magnetic resonance findings | ||||
| Indexed LVEDV (mL/m2) | 80 ± 14 | 78 ± 11 | 83 ± 15 | 0.970 |
| Indexed LVM (g/m2) | 75 ± 13 | 69 ± 9 | 81 ± 14 | 0.974 |
| LVEF (%) | 62 ± 5 | 63 ± 5 | 61 ± 5 | 0.666 |
| Indexed RVEDV (mL/m2) | 63 (58–75) | 62 (57–68) | 66 (60–75) | 0.321 |
| RVEF (%) | 57 ± 8 | 57 ± 9 | 57 ± 7 | 0.876 |
| Presence of myocardial edema, n (%) | 30 (49) | 11 (35) | 19 (63) | 0.707 |
| IVS edema, n (%) | 14 (23) | 5 (16) | 9 (30) | 0.655 |
| Lateral/inferolateral edema, n (%) | 15 (25) | 4 (13) | 11 (37) | 0.795 |
| Inferior edema, n (%) | 7 (12) | 3 (10) | 4 (13) | 0.804 |
| Anterior edema, n (%) | 9 (15) | 5 (16) | 4 (13) | 0.971 |
| DE, n (%) | 57 (93) | 27 (87) | 30 (100) | 0.061 |
| IVS DE, n (%) | 16 (26) | 5 (16) | 11 (37) | 0.882 |
| Lateral/inferolateral DE, n (%) | 44 (72) | 22 (70) | 22 (73) | 0.386 |
| Inferior DE, n (%) | 14 (23) | 6 (19) | 8 (27) | 0.335 |
| Anterior DE, n (%) | 7 (12) | 3 (10) | 4 (13) | 0.586 |
| Number of segments with DE, n | 2.0 ± 1.7 | 1.4 ± 0.6 | 2.7 ± 1.4 | 0.813 |
| DE-LVM/LVM, % | 7.9 (6.4–11.7) | 6.3 (4.9–7.7) | 11.3 (8.1–12.8) | <0.001 |
| Follow-up | ||||
| Echocardiographic LVEF (%) | 57 ± 4 | 58 ± 3 | 57 ± 6 | 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghionzoli, N.; Gismondi, A.; Mandoli, G.E.; Spera, L.; Di Florio, A.; D’Ascenzi, F.; Cameli, M.; Cavigli, L.; Sciaccaluga, C.; Carbone, S.F.; et al. Left Ventricular Mass with Delayed Enhancement as a Predictor of Major Events in Patients with Myocarditis with Preserved Ejection Fraction. J. Clin. Med. 2022, 11, 6082. https://doi.org/10.3390/jcm11206082
Ghionzoli N, Gismondi A, Mandoli GE, Spera L, Di Florio A, D’Ascenzi F, Cameli M, Cavigli L, Sciaccaluga C, Carbone SF, et al. Left Ventricular Mass with Delayed Enhancement as a Predictor of Major Events in Patients with Myocarditis with Preserved Ejection Fraction. Journal of Clinical Medicine. 2022; 11(20):6082. https://doi.org/10.3390/jcm11206082
Chicago/Turabian StyleGhionzoli, Nicolò, Annalaura Gismondi, Giulia Elena Mandoli, Lucia Spera, Alex Di Florio, Flavio D’Ascenzi, Matteo Cameli, Luna Cavigli, Carlotta Sciaccaluga, Salvatore Francesco Carbone, and et al. 2022. "Left Ventricular Mass with Delayed Enhancement as a Predictor of Major Events in Patients with Myocarditis with Preserved Ejection Fraction" Journal of Clinical Medicine 11, no. 20: 6082. https://doi.org/10.3390/jcm11206082
APA StyleGhionzoli, N., Gismondi, A., Mandoli, G. E., Spera, L., Di Florio, A., D’Ascenzi, F., Cameli, M., Cavigli, L., Sciaccaluga, C., Carbone, S. F., Aquaro, G. D., Valente, S., & Focardi, M. (2022). Left Ventricular Mass with Delayed Enhancement as a Predictor of Major Events in Patients with Myocarditis with Preserved Ejection Fraction. Journal of Clinical Medicine, 11(20), 6082. https://doi.org/10.3390/jcm11206082









