Intraoperative Assessment of Surgical Stress Response Using Nociception Monitor under General Anesthesia and Postoperative Complications: A Narrative Review
Abstract
1. Introduction
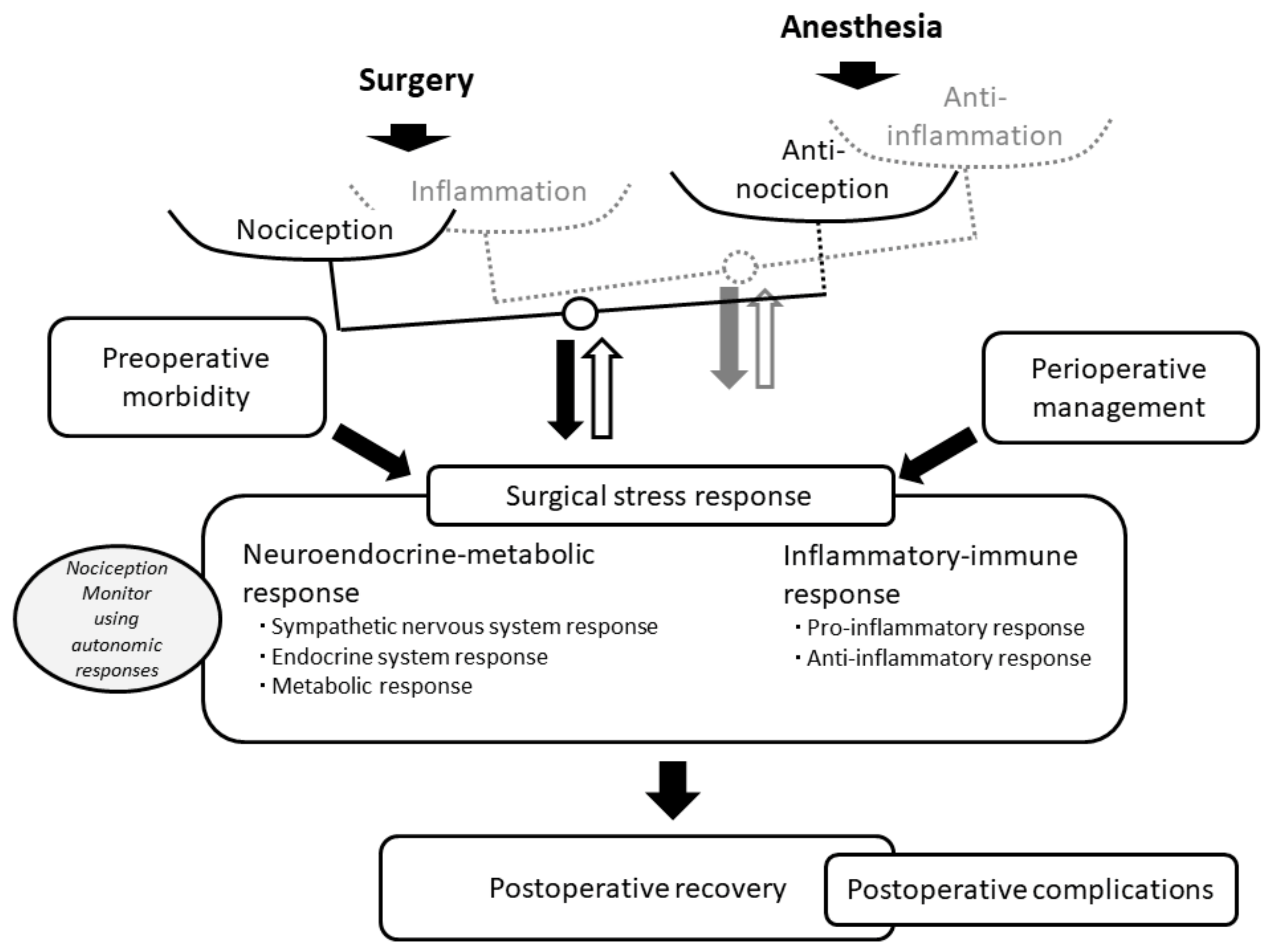
2. Surgical Trauma, Nociception and Anti-Nociception
3. Nociception Monitors
4. Disadvantages of Nociception Monitors for Assessing the Balance between Nociception and Anti-Nociception
5. Advantages of Nociception Monitors for Assessing Surgical Stress Responses
6. Postoperative Complications and Surgical Stress Responses
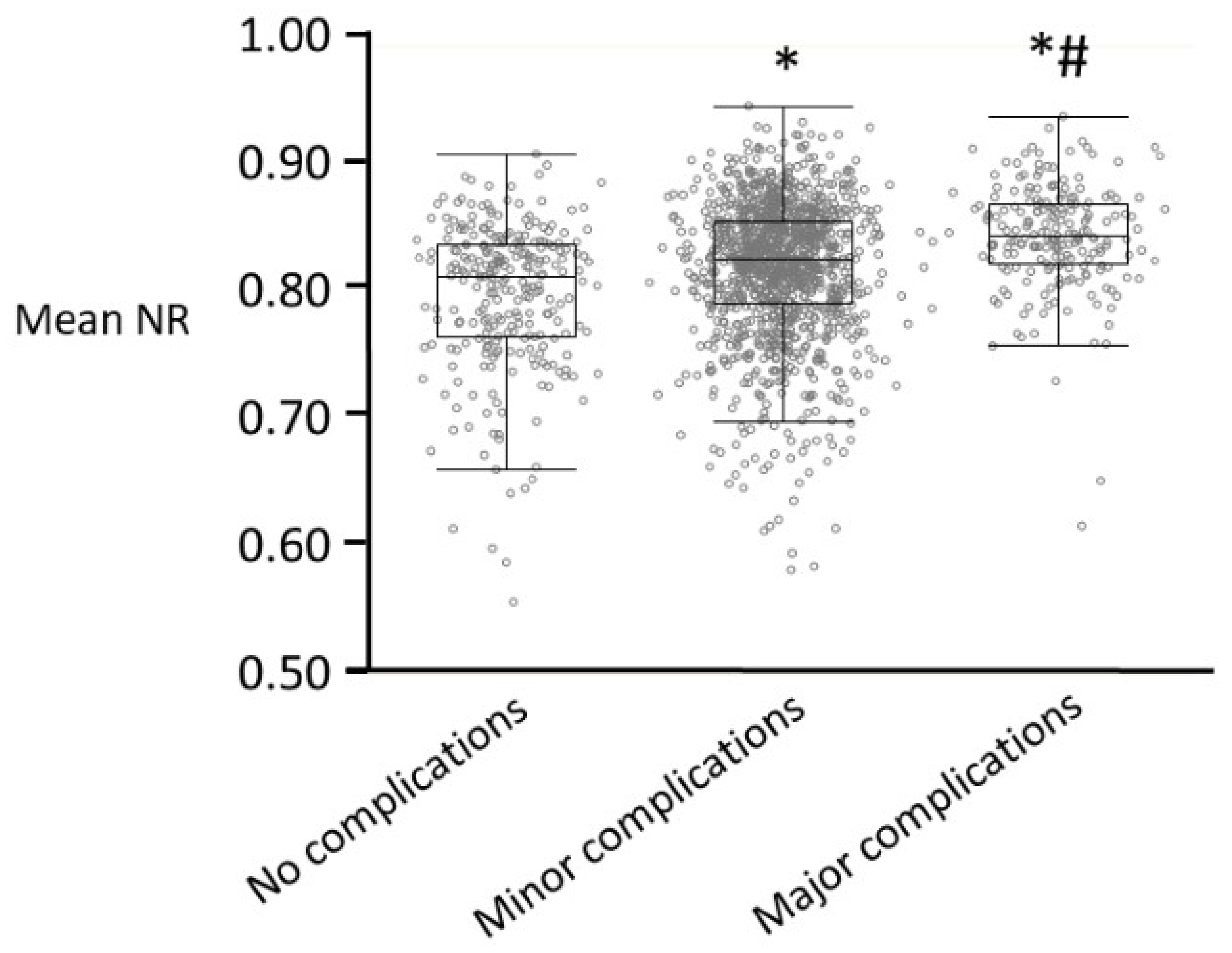
7. Nociception Monitors and Postoperative Complications
8. Nociception Monitor-Guided Anesthesia for Suppressing Surgical Stress Responses
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dobson, G.P. Addressing the Global Burden of Trauma in Major Surgery. Front. Surg. 2015, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Cusack, B.; Buggy, D.J. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Eappen, S.; Lane, B.H.; Rosenberg, B.; Lipsitz, S.A.; Sadoff, D.; Matheson, D.; Berry, W.R.; Lester, M.; Gawande, A.A. Relationship between occurrence of surgical complications and hospital finances. JAMA 2013, 309, 1599–1606. [Google Scholar] [CrossRef]
- Dobson, G.P. Trauma of major surgery: A global problem that is not going away. Int. J. Surg. 2020, 81, 47–54. [Google Scholar] [CrossRef]
- Ludbrook, G.L. The Hidden Pandemic: The Cost of Postoperative Complications. Curr. Anesthesiol. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Copeland, G.P. The POSSUM system of surgical audit. Arch. Surg. 2002, 137, 15–19. [Google Scholar] [CrossRef]
- Cohen, M.E.; Bilimoria, K.Y.; Ko, C.Y.; Hall, B.L. Development of an American College of Surgeons National Surgery Quality Improvement Program: Morbidity and mortality risk calculator for colorectal surgery. J. Am. Coll. Surg. 2009, 208, 1009–1016. [Google Scholar] [CrossRef]
- Glance, L.G.; Lustik, S.J.; Hannan, E.L.; Osler, T.M.; Mukamel, D.B.; Qian, F.; Dick, A.W. The Surgical Mortality Probability Model: Derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann. Surg. 2012, 255, 696–702. [Google Scholar] [CrossRef]
- Protopapa, K.L.; Simpson, J.C.; Smith, N.C.; Moonesinghe, S.R. Development and validation of the Surgical Outcome Risk Tool (SORT). Br. J. Surg. 2014, 101, 1774–1783. [Google Scholar] [CrossRef]
- Fritz, B.A.; Cui, Z.; Zhang, M.; He, Y.; Chen, Y.; Kronzer, A.; Ben Abdallah, A.; King, C.R.; Avidan, M.S. Deep-learning model for predicting 30-day postoperative mortality. Br. J. Anaesth. 2019, 123, 688–695. [Google Scholar] [CrossRef]
- Hofer, I.S.; Lee, C.; Gabel, E.; Baldi, P.; Cannesson, M. Development and validation of a deep neural network model to predict postoperative mortality, acute kidney injury, and reintubation using a single feature set. NPJ Digit. Med. 2020, 3, 58. [Google Scholar] [CrossRef]
- Xue, B.; Li, D.; Lu, C.; King, C.R.; Wildes, T.; Avidan, M.S.; Kannampallil, T.; Abraham, J. Use of Machine Learning to Develop and Evaluate Models Using Preoperative and Intraoperative Data to Identify Risks of Postoperative Complications. JAMA Netw. Open. 2021, 4, e212240. [Google Scholar] [CrossRef]
- Buis, N.; Esfandiari, H.; Hoch, A.; Fürnstahl, P. Overview of Methods to Quantify Invasiveness of Surgical Approaches in Orthopedic Surgery-A Scoping Review. Front. Surg. 2022, 8, 771275. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Brown, E.N.; Pavone, K.J.; Naranjo, M. Multimodal General Anesthesia: Theory and Practice. Anesth. Analg. 2018, 127, 1246–1258. [Google Scholar] [CrossRef]
- Lichtner, G.; Auksztulewicz, R.; Kirilina, E.; Velten, H.; Mavrodis, D.; Scheel, M.; Blankenburg, F.; von Dincklage, F. Effects of propofol anesthesia on the processing of noxious stimuli in the spinal cord and the brain. Neuroimage 2018, 172, 642–653. [Google Scholar] [CrossRef]
- Lichtner, G.; Auksztulewicz, R.; Velten, H.; Mavrodis, D.; Scheel, M.; Blankenburg, F.; von Dincklage, F. Nociceptive activation in spinal cord and brain persists during deep general anaesthesia. Br. J. Anaesth. 2018, 121, 291–302. [Google Scholar] [CrossRef]
- Cividjian, A.; Petitjeans, F.; Liu, N.; Ghignone, M.; de Kock, M.; Quintin, L. Do we feel pain during anesthesia? A critical review on surgery-evoked circulatory changes and pain perception. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 445–467. [Google Scholar] [CrossRef]
- Bruehl, S.; Chung, O.Y. Interactions between the cardiovascular and pain regulatory systems: An updated review of mechanisms and possible alterations in chronic pain. Neurosci. Biobehav. Rev. 2004, 28, 395–414. [Google Scholar] [CrossRef]
- Dampney, R.A. Central neural control of the cardiovascular system: Current perspectives. Adv. Physiol. Educ. 2016, 40, 283–296. [Google Scholar] [CrossRef]
- Suarez-Roca, H.; Klinger, R.Y.; Podgoreanu, M.V.; Ji, R.R.; Sigurdsson, M.I.; Waldron, N.; Mathew, J.P.; Maixner, W. Contribution of Baroreceptor Function to Pain Perception and Perioperative Outcomes. Anesthesiology 2019, 130, 634–650. [Google Scholar] [CrossRef]
- Brown, E.N.; Lydic, R.; Schiff, N.D. General anesthesia, sleep, and coma. N. Engl. J. Med. 2010, 363, 2638–2650. [Google Scholar] [CrossRef]
- Heier, T.; Steen, P.A. Assessment of anaesthesia depth. Acta Anaesthesiol. Scand. 1996, 40, 1087–1100. [Google Scholar] [CrossRef]
- Buhre, W.; Rossaint, R. Perioperative management and monitoring in anaesthesia. Lancet 2003, 362, 1839–1846. [Google Scholar] [CrossRef]
- Guignard, B. Monitoring analgesia. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 161–180. [Google Scholar] [CrossRef]
- Rampil, I.J.; Matteo, R.S. Changes in EEG spectral edge frequency correlate with the hemodynamic response to laryngoscopy and intubation. Anesthesiology 1987, 67, 139–142. [Google Scholar] [CrossRef]
- Kochs, E.; Bischoff, P.; Pichlmeier, U.; Schulte am Esch, J. Surgical stimulation induces changes in brain electrical activity during isoflurane/nitrous oxide anesthesia. A topographic electroencephalographic analysis. Anesthesiology 1994, 80, 1026–1034. [Google Scholar] [CrossRef]
- Vakkuri, A.; Yli-Hankala, A.; Talja, P.; Mustola, S.; Tolvanen-Laakso, H.; Sampson, T.; Viertiö-Oja, H. Time-frequency balanced spectral entropy as a measure of anesthetic drug effect in central nervous system during sevoflurane, propofol, and thiopental anesthesia. Acta Anaesthesiol. Scand. 2004, 48, 145–153. [Google Scholar] [CrossRef]
- García, P.S.; Kreuzer, M.; Hight, D.; Sleigh, J.W. Effects of noxious stimulation on the electroencephalogram during general anaesthesia: A narrative review and approach to analgesic titration. Br. J. Anaesth. 2021, 126, 445–457. [Google Scholar] [CrossRef]
- Raeder, L. EEG-based monitor on anti-nociception during general anaesthesia: Mission impossible? Acta Anaesthesiol. Scand. 2014, 58, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T. Objective monitoring of nociception: A review of current commercial solutions. Br. J. Anaesth. 2019, 123, e312–e321. [Google Scholar] [CrossRef] [PubMed]
- Seitsonen, E.R.; Korhonen, I.K.; van Gils, M.J.; Huiku, M.; Lötjönen, J.M.; Korttila, K.T.; Yli-Hankala, A.M. EEG spectral entropy, heart rate, photoplethysmography and motor responses to skin incision during sevoflurane anaesthesia. Acta Anaesthesiol. Scand. 2005, 49, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Huiku, M.; Uutela, K.; van Gils, M.; Korhonen, I.; Kymäläinen, M.; Meriläinen, P.; Paloheimo, M.; Rantanen, M.; Takala, P.; Viertiö-Oja, H.; et al. Assessment of surgical stress during general anaesthesia. Br. J. Anaesth. 2007, 98, 447–455. [Google Scholar] [CrossRef]
- Wennervirta, J.; Hynynen, M.; Koivusalo, A.M.; Uutela, K.; Huiku, M.; Vakkuri, A. Surgical stress index as a measure of nociception/antinociception balance during general anesthesia. Acta Anaesthesiol. Scand. 2008, 52, 1038–1045. [Google Scholar] [CrossRef]
- Bonhomme, V.; Uutela, K.; Hans, G.; Maquoi, I.; Born, J.D.; Brichant, J.F.; Lamy, M.; Hans, P. Comparison of the surgical Pleth Index™ with haemodynamic variables to assess nociception-anti-nociception balance during general anaesthesia. Br. J. Anaesth. 2011, 106, 101–111. [Google Scholar] [CrossRef]
- Hirose, M. Nociception during surgery. In Features and Assessments of Pain, Anesthetics and Analgesics; Rajendram, R., Preedy, V.R., Patel, V.B., Martin, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–245. [Google Scholar]
- Nitzschke, R.; Fischer, M.; Funcke, S. Nociception monitoring: Method for intraoperative opioid control? Anaesthesist 2021, 70, 735–752. [Google Scholar] [CrossRef]
- Ghanty, I.; Schraag, S. The quantification and monitoring of intraoperative nociception levels in Thoracic surgery: A review. J. Thorac. Dis. 2019, 11, 4059–4071. [Google Scholar] [CrossRef]
- Ledowski, T.; Pascoe, E.; Ang, B.; Schmarbeck, T.; Clarke, M.W.; Fuller, C.; Kapoor, V. Monitoring of intra-operative nociception: Skin conductance and surgical stress index versus stress hormone plasma levels. Anaesthesia 2010, 65, 1001–1006. [Google Scholar] [CrossRef]
- Chen, X.; Thee, C.; Gruenewald, M.; Ilies, C.; Höcker, J.; Hanss, R.; Steinfath, M.; Bein, B. Correlation of surgical pleth index with stress hormones during propofol-remifentanil anaesthesia. Sci. World J. 2012, 2012, 879158. [Google Scholar] [CrossRef]
- Colombo, R.; Raimondi, F.; Corona, A.; Rivetti, I.; Pagani, F.; Porta, V.D.; Guzzetti, S. Comparison of the Surgical Pleth Index with autonomic nervous system modulation on cardiac activity during general anaesthesia: A randomised cross-over study. Eur. J. Anaesthesiol. 2014, 31, 76–84. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Park, S.; Miyamoto, K.; Ueki, R.; Kariya, N.; Tatara, T.; Hirose, M. Modified model for predicting early C-reactive protein levels after gastrointestinal surgery: A prospective cohort study. PLoS ONE 2020, 15, e0239709. [Google Scholar] [CrossRef]
- Ogata, H.; Matsuki, Y.; Okamoto, T.; Ueki, R.; Kariya, N.; Tatara, T.; Shigemi, K.; Hirose, M. Intra-operative nociceptive responses and postoperative major complications after gastrointestinal surgery under general anaesthesia: A prospective cohort study. Eur. J. Anaesthesiol. 2021, 38, 1215–1222. [Google Scholar] [CrossRef]
- Ogata, H.; Nakamoto, S.; Miyawaki, H.; Ueki, R.; Kariya, N.; Tatara, T.; Hirose, M. Association between intraoperative nociception and postoperative complications in patients undergoing laparoscopic gastrointestinal surgery. J. Clin. Monit. Comput. 2020, 34, 575–581. [Google Scholar] [CrossRef]
- Okamoto, T.; Matsuki, Y.; Ogata, H.; Okutani, H.; Ueki, R.; Kariya, N.; Tatara, T.; Hirose, M. Association between averaged intraoperative nociceptive response index and postoperative complications after lung resection surgery. Interact. Cardiovasc. Thorac. Surg. 2022, ivac258, online ahead of print. [Google Scholar] [CrossRef]
- Ben-Israel, N.; Kliger, M.; Zuckerman, G.; Katz, Y.; Edry, R. Monitoring the nociception level: A multi-parameter approach. J. Clin. Monit. Comput. 2013, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Meijer, F.S.; Niesters, M.; van Velzen, M.; Martini, C.H.; Olofsen, E.; Edry, R.; Sessler, D.I.; van Dorp, E.L.A.; Dahan, A.; Boon, M. Does nociception monitor-guided anesthesia affect opioid consumption? A systematic review of randomized controlled trials. J. Clin. Monit. Comput. 2020, 34, 629–641. [Google Scholar] [CrossRef]
- Funcke, S.; Pinnschmidt, H.O.; Brinkmann, C.; Wesseler, S.; Beyer, B.; Fischer, M.; Nitzschke, R. Nociception level-guided opioid administration in radical retropubic prostatectomy: A randomised controlled trial. Br. J. Anaesth. 2021, 126, 516–524. [Google Scholar] [CrossRef]
- Gruenewald, M.; Dempfle, A. Analgesia/nociception monitoring for opioid guidance: Meta-analysis of randomized clinical trials. Minerva Anestesiol. 2017, 83, 200–213. [Google Scholar] [CrossRef]
- Jiao, Y.; He, B.; Tong, X.; Xia, R.; Zhang, C.; Shi, X. Intraoperative monitoring of nociception for opioid administration: A meta-analysis of randomized controlled trials. Minerva Anestesiol. 2019, 85, 522–530. [Google Scholar] [CrossRef]
- Helander, E.M.; Webb, M.P.; Menard, B.; Prabhakar, A.; Helmstetter, J.; Cornett, E.M.; Urman, R.D.; Nguyen, V.H.; Kaye, A.D. Metabolic and the Surgical Stress Response Considerations to Improve Postoperative Recovery. Curr. Pain Headache Rep. 2019, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Ljungqvist, O.; Carli, F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br. J. Anaesth. 2022, 128, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Alazawi, W.; Pirmadjid, N.; Lahiri, R.; Bhattacharya, S. Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Ann. Surg. 2016, 264, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Watt, D.G.; Horgan, P.G.; McMillan, D.C. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: A systematic review. Surgery 2015, 157, 362–380. [Google Scholar] [CrossRef]
- Webster, J.I.; Tonelli, L.; Sternberg, E.M. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002, 20, 125–163. [Google Scholar] [CrossRef]
- Whelton, S.P.; Narla, V.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Jenny, N.S.; Al-Mallah, M.H.; Michos, E.D. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2014, 113, 644–649. [Google Scholar] [CrossRef]
- Papaioannou, V.; Pnevmatikos, I. Heart Rate Variability: A Potential Tool for Monitoring Immunomodulatory Effects of Parenteral Fish Oil Feeding in Patients with Sepsis. Nutr. Metab. Insights 2019, 12, 1178638819847486. [Google Scholar] [CrossRef]
- Khuri, S.F.; Henderson, W.G.; DePalma, R.G.; Mosca, C.; Healey, N.A.; Kumbhani, D.J.; Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann. Surg. 2005, 242, 326–341. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Hu, D.M.; Gong, T.P.; Xu, R.; Gao, J. Impact of postoperative complications on long-term outcomes of patients following surgery for gastric cancer: A systematic review and meta-analysis of 64 follow-up studies. Asian J. Surg. 2020, 43, 719–729. [Google Scholar] [CrossRef]
- O’Brien, W.J.; Gupta, K.; Itani, K.M.F. Association of Postoperative Infection with Risk of Long-term Infection and Mortality. JAMA Surg. 2020, 155, 61–68. [Google Scholar] [CrossRef]
- Portuondo, J.I.; Itani, K.M.F.; Massarweh, N.N. Association Between Postoperative Complications and Long-term Survival after Non-cardiac Surgery among Veterans. Ann. Surg. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Saxena, S.; Maze, M. Impact on the brain of the inflammatory response to surgery. Presse Med. 2018, 47, e73–e81. [Google Scholar] [CrossRef]
- Yang, T.; Velagapudi, R.; Terrando, N. Neuroinflammation after surgery: From mechanisms to therapeutic targets. Nat. Immunol. 2020, 21, 1319–1326. [Google Scholar] [CrossRef]
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef]
- Patil, S.; Cornett, E.M.; Jesunathadas, J.; Belani, K.; Fox, C.J.; Kaye, A.D.; Lambert, L.A.; Urman, R.D. Implementing enhanced recovery pathways to improve surgical outcomes. J. Anaesthesiol. Clin. Pharmacol. 2019, 35 (Suppl. S1), S24–S28. [Google Scholar]
- Cabellos Olivares, M.; Labalde Martinez, M.; Torralba, M.; Rodriguez Fraile, J.R.; Atance Martínez, J.C. C-reactive protein as a marker of the surgical stress reduction within an ERAS protocol (Enhanced Recovery After Surgery) in colorectal surgery: A prospective cohort study. J. Surg. Oncol. 2018, 117, 717–724. [Google Scholar] [CrossRef]
- Stowers, M.D.; Lemanu, D.P.; Hill, A.G. Health economics in Enhanced Recovery After Surgery programs. Can. J. Anaesth. 2015, 62, 219–230. [Google Scholar] [CrossRef]
- Brooks, N.A.; Kokorovic, A.; McGrath, J.S.; Kassouf, W.; Collins, J.W.; Black, P.C.; Douglas, J.; Djaladat, H.; Daneshmand, S.; Catto, J.W.F.; et al. Critical analysis of quality of life and cost-effectiveness of enhanced recovery after surgery (ERAS) for patient’s undergoing urologic oncology surgery: A systematic review. World J. Urol. 2022, 40, 1325–1342. [Google Scholar] [CrossRef]
- Noba, L.; Rodgers, S.; Chandler, C.; Balfour, A.; Hariharan, D.; Yip, V.S. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2020, 24, 918–932. [Google Scholar] [CrossRef]
- Tong, Y.; Fernandez, L.; Bendo, J.A.; Spivak, J.M. Enhanced Recovery After Surgery Trends in Adult Spine Surgery: A Systematic Review. Int. J. Spine Surg. 2020, 14, 623–640. [Google Scholar] [CrossRef]
- Elsherbini, N.; Carli, F. Advocating for prehabilitation for patients undergoing gynecology-oncology surgery. Eur. J. Surg. Oncol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Futier, E.; Lefrant, J.Y.; Guinot, P.G.; Godet, T.; Lorne, E.; Cuvillon, P.; Bertran, S.; Leone, M.; Pastene, B.; Piriou, V.; et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA 2017, 318, 1346–1357. [Google Scholar] [CrossRef]
- Salmasi, V.; Maheshwari, K.; Yang, D.; Mascha, E.J.; Singh, A.; Sessler, D.I.; Kurz, A. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology 2017, 126, 47–65. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, Z.; Ying, J.; Han, Y.; Chen, Z. Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients: A randomized study: Optimal blood pressure reduces acute kidney injury. J. Clin. Anesth. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Abbott, T.E.F.; Pearse, R.M.; Archbold, R.A.; Ahmad, T.; Niebrzegowska, E.; Wragg, A.; Rodseth, R.N.; Devereaux, P.J.; Ackland, G.L. A Prospective International Multicentre Cohort Study of Intraoperative Heart Rate and Systolic Blood Pressure and Myocardial Injury After Noncardiac Surgery: Results of the VISION Study. Anesth. Analg. 2018, 126, 1936–1945. [Google Scholar] [CrossRef]
- Wesselink, E.M.; Kappen, T.H.; Torn, H.M.; Slooter, A.J.C.; van Klei, W.A. Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br. J. Anaesth. 2018, 121, 706–721. [Google Scholar] [CrossRef]
- Agerskov, M.; Thusholdt, A.N.W.; Holm-Sørensen, H.; Wiberg, S.; Meyhoff, C.S.; Højlund, J.; Secher, N.H.; Foss, N.B. Association of the intraoperative peripheral perfusion index with postoperative morbidity and mortality in acute surgical patients: A retrospective observational multicentre cohort study. Br. J. Anaesth. 2021, 127, 396–404. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Gawande, A.A.; Kwaan, M.R.; Regenbogen, S.E.; Lipsitz, S.A.; Zinner, M.J. An Apgar score for surgery. J. Am. Coll. Surg. 2007, 204, 201–208. [Google Scholar] [CrossRef]
- Regenbogen, S.E.; Bordeianou, L.; Hutter, M.M.; Gawande, A.A. The intraoperative Surgical Apgar Score predicts postdischarge complications after colon and rectal resection. Surgery 2010, 148, 559–566. [Google Scholar] [CrossRef][Green Version]
- House, L.M.; Marolen, K.N.; St Jacques, P.J.; McEvoy, M.D.; Ehrenfeld, J.M. Surgical Apgar score is associated with myocardial injury after noncardiac surgery. J. Clin. Anesth. 2016, 34, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Guerrisi, M.; Dauri, M.; Coniglione, F.; Tisone, G.; De Carolis, E.; Cillis, A.; Canichella, A.; Toschi, N.; Heldt, T. Prediction of postoperative outcomes using intraoperative hemodynamic monitoring data. Sci. Rep. 2017, 7, 16376. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Matsuki, Y.; Ogata, H.; Ueki, R.; Kariya, N.; Tatara, T.; Shigemi, K.; Hirose, M. Quantitative evaluation of the effects of interscalene block on physiological responses to the balance between nociception and anti-nociception among inpatients undergoing total shoulder arthroplasty under general anesthesia. J. Clin. Monit. Comput. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Onoe, K.; Ogata, H.; Okamoto, T.; Okutani, H.; Ueki, R.; Kariya, N.; Tatara, T.; Hashimoto, M.; Hasegawa, S.; Matsuki, Y.; et al. Association between thoracic epidural block and major complications after pleurectomy/decortication for malignant pleural mesothelioma under general anesthesia. Reg. Anesth. Pain Med. 2022, 47, 494–499. [Google Scholar] [CrossRef]
- Funcke, S.; Pinnschmidt, H.O.; Wesseler, S.; Brinkmann, C.; Beyer, B.; Jazbutyte, V.; Behem, C.R.; Trepte, C.; Nitzschke, R. Guiding opioid administration by 3 different analgesia nociception monitoring indices during general anesthesia alters intraoperative sufentanil consumption and stress hormone release: A randomized controlled pilot study. Anesth. Analg. 2020, 130, 1264–1273. [Google Scholar] [CrossRef]
- Kehlet, H.; Wilmore, D.W. Multimodal strategies to improve surgical outcome. Am. J. Surg. 2002, 183, 630–641. [Google Scholar] [CrossRef]
| Nociception Monitor | Sources of Measurement [32,37,38] | Surgical Stress Responses Correlating with Nociception Monitor Values [40,41,42,43] | Surgical Procedures for which Incidence of Postoperative Complications Correlates with Nociception Monitor Values [44,45,46] |
|---|---|---|---|
| ANI | Heart rate variability | Parasympathetic activity | - |
| NoL | Accelerometry Galvanic skin response Photoplethysmography Temperature | - | - |
| NR | Heart rate Perfusion index Systolic blood pressure | CRP | Gastrointestinal surgery Lung resection surgery |
| PPI | Pupillometry | Sympathetic activity | - |
| SPI | Heartbeat intervals Plethysmographic amplitude | Cortisol Epinephrine Norepinephrine Sympathetic activity | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirose, M.; Okutani, H.; Hashimoto, K.; Ueki, R.; Shimode, N.; Kariya, N.; Takao, Y.; Tatara, T. Intraoperative Assessment of Surgical Stress Response Using Nociception Monitor under General Anesthesia and Postoperative Complications: A Narrative Review. J. Clin. Med. 2022, 11, 6080. https://doi.org/10.3390/jcm11206080
Hirose M, Okutani H, Hashimoto K, Ueki R, Shimode N, Kariya N, Takao Y, Tatara T. Intraoperative Assessment of Surgical Stress Response Using Nociception Monitor under General Anesthesia and Postoperative Complications: A Narrative Review. Journal of Clinical Medicine. 2022; 11(20):6080. https://doi.org/10.3390/jcm11206080
Chicago/Turabian StyleHirose, Munetaka, Hiroai Okutani, Kazuma Hashimoto, Ryusuke Ueki, Noriko Shimode, Nobutaka Kariya, Yumiko Takao, and Tsuneo Tatara. 2022. "Intraoperative Assessment of Surgical Stress Response Using Nociception Monitor under General Anesthesia and Postoperative Complications: A Narrative Review" Journal of Clinical Medicine 11, no. 20: 6080. https://doi.org/10.3390/jcm11206080
APA StyleHirose, M., Okutani, H., Hashimoto, K., Ueki, R., Shimode, N., Kariya, N., Takao, Y., & Tatara, T. (2022). Intraoperative Assessment of Surgical Stress Response Using Nociception Monitor under General Anesthesia and Postoperative Complications: A Narrative Review. Journal of Clinical Medicine, 11(20), 6080. https://doi.org/10.3390/jcm11206080





