Low Dehydroepiandrosterone (DHEA) Level Is Associated with Poor Immunologic Response among People Living with HIV/AIDS
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
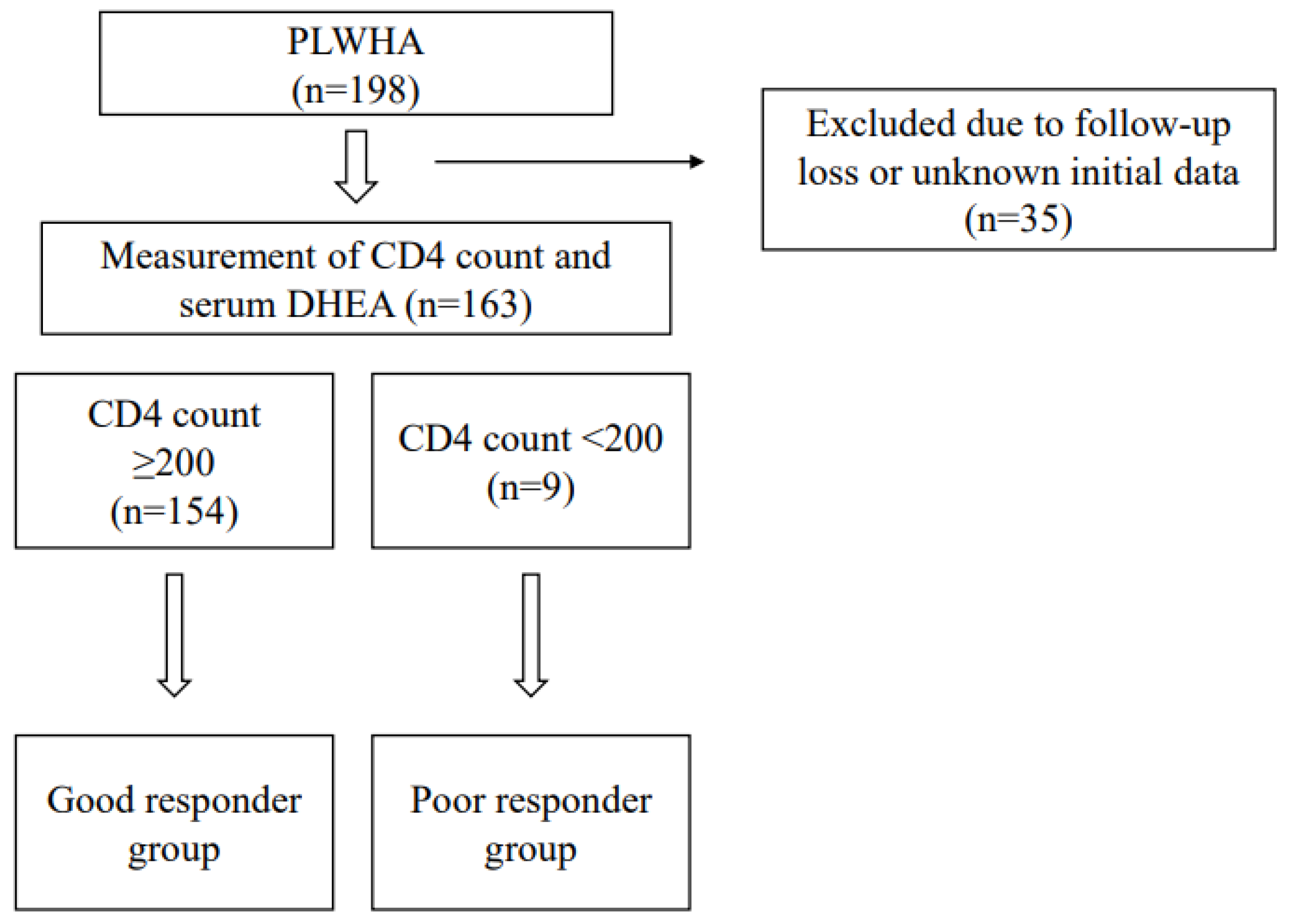
2.2. Study Design and Definitions
2.3. Measurement of Plasma DHEA
2.4. Data Collection
2.5. Statistical Analysis
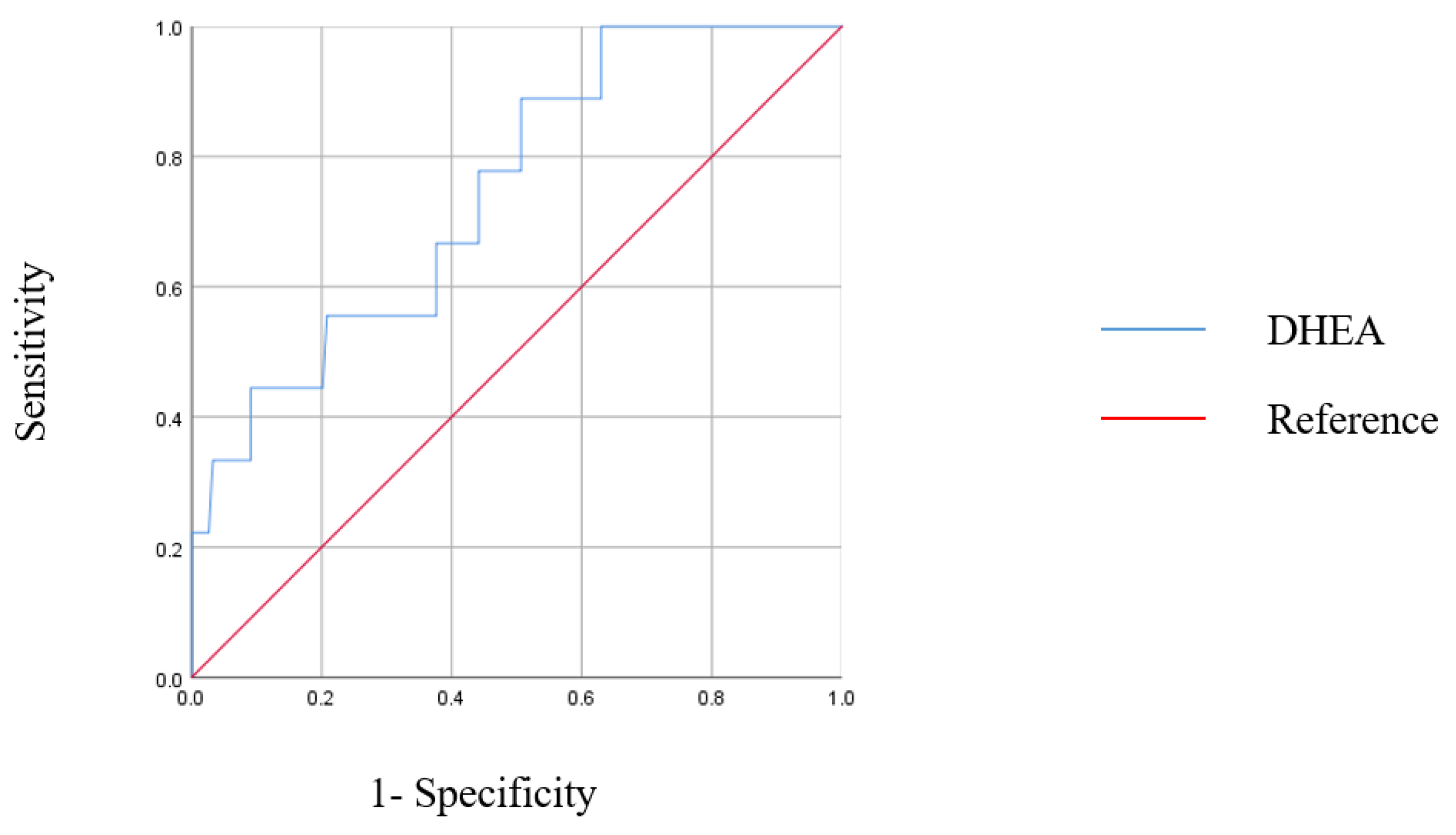
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younes, S.A.; Talla, A.; Pereira Ribeiro, S.; Saidakova, E.V.; Korolevskaya, L.B.; Shmagel, K.V.; Shive, C.L.; Freeman, M.L.; Panigrahi, S.; Zweig, S.; et al. Cycling CD4+ T Cells in HIV-Infected Immune Nonresponders Have Mitochondrial Dysfunction. J. Clin. Investig. 2018, 128, 5083–5094. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Cozzi-Lepri, A.; Reekie, J.; Kirk, O.; Lundgren, J.; Reiss, P.; Jevtovic, D.; Machala, L.; Zangerle, R.; Mocroft, A.; et al. Immuno-Virological Discordance and the Risk of Non-AIDS and AIDS Events in a Large Observational Cohort of HIV-Patients in Europe. PLoS ONE 2014, 9, e87160. [Google Scholar] [CrossRef] [PubMed]
- Engsig, F.N.; Zangerle, R.; Katsarou, O.; Dabis, F.; Reiss, P.; Gill, J.; Porter, K.; Sabin, C.; Riordan, A.; Fätkenheuer, G.; et al. Long-Term Mortality in HIV-Positive Individuals Virally Suppressed for >3 Years With Incomplete CD4 Recovery. Clin. Infect. Dis. 2014, 58, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.F.; Kitchen, C.M.R.; Hunt, P.W.; Rodriguez, B.; Hecht, F.M.; Kitahata, M.; Crane, H.M.; Willig, J.; Mugavero, M.; Saag, M.; et al. Incomplete Peripheral CD4+ Cell Count Restoration in HIV-Infected Patients Receiving Long-Term Antiretroviral Treatment. Clin. Infect. Dis. 2009, 48, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Robbins, G.K.; Spritzler, J.G.; Chan, E.S.; Asmuth, D.M.; Gandhi, R.T.; Rodriguez, B.A.; Skowron, G.; Skolnik, P.R.; Shafer, R.W.; Pollard, R.B.; et al. Incomplete Reconstitution of T Cell Subsets on Combination Antiretroviral Therapy in the AIDS Clinical Trials Group Protocol 384. Clin. Infect. Dis. 2009, 48, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Montes, C.G.; Canals, A.; Mackenna, M.J.; Wolff, M. Role and Effects of Zinc Supplementation in HIV-Infected Patients with Immunovirological Discordance: A Randomized, Double Blind, Case Control Study. PLoS ONE 2021, 16, e0244823. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Aguilar-Jimenez, W.; Rugeles, M.T. The Potential Protective Role of Vitamin D Supplementation on HIV-1 Infection. Front. Immunol. 2019, 10, 2291. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenhoven, R.F. Dehydroepiandrosterone: Uses and Abuses. In Textbook of Rheumatology; Kelley, W.N., Harris, J., Ruddy, S., Sledge, C.B., Eds.; W. B. Saunders Co.: Philadelphia, PA, USA, 1997; pp. 1–25. [Google Scholar]
- Prall, S.P.; Muehlenbein, M.P. DHEA Modulates Immune Function: A Review of Evidence. Vitam. Horm. 2018, 108, 125–144. [Google Scholar]
- Abrams, D.I.; Shade, S.B.; Couey, P.; McCune, J.M.; Lo, J.; Bacchetti, P.; Chang, B.; Epling, L.; Liegler, T.; Grant, R.M. Dehydroepiandrosterone (DHEA) Effects on HIV Replication and Host Immunity: A Randomized Placebo-Controlled Study. AIDS Res. Hum. Retrovir. 2007, 23, 77–85. [Google Scholar] [CrossRef]
- Damtie, D.; Yismaw, G.; Woldeyohannes, D.; Anagaw, B. Common Opportunistic Infections and Their CD4 Cell Correlates Among HIV-Infected Patients Attending at Antiretroviral Therapy Clinic of Gondar University Hospital, Northwest Ethiopia. BMC. Res. Notes 2013, 6, 534. [Google Scholar] [CrossRef]
- Rb-Silva, R.; Goios, A.; Kelly, C.; Teixeira, P.; João, C.; Horta, A.; Correia-Neves, M. Definition of Immunological Nonresponse to Antireviral Therapy: A systemic Review. J. Acquir. Immune Defic. Syndr. 2019, 82, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Wolbers, M.; Bucher, H.C.; Furrer, H.; Rickenbach, M.; Cavassini, M.; Weber, R.; Schmid, P.; Bernasconi, E.; Hirschel, B.; Battegay, M.; et al. Delayed Diagnosis of HIV Infection and Late Initiation of Antiretroviral Therapy in the Swiss HIV Cohort Study. HIV Med. 2008, 9, 397–405. [Google Scholar] [CrossRef]
- Egger, M.; May, M.; Chêne, G.; Phillips, A.N.; Ledergerber, B.; Dabis, F.; Costagliola, D.; D’Arminio Monforte, A.; de Wolf, F.; Reiss, P.; et al. Prognosis of HIV-1-Infected Patients Starting Highly Active Antiretroviral Therapy: A Collaborative Analysis of Prospective Studies. Lancet 2002, 360, 119–129. [Google Scholar] [CrossRef]
- Hoffman, J.; van Griensven, J.; Colebunders, R.; McKellar, M. Role of the CD4 Count in HIV Management. HIV Ther. 2010, 4, 27–39. [Google Scholar] [CrossRef]
- Massanella, M.; Negredo, E.; Pérez-Alvarez, N.; Puig, J.; Ruiz-Hernández, R.; Bofill, M.; Clotet, B.; Blanco, J. CD4 T-Cell Hyperactivation and Susceptibility to Cell Death Determine Poor CD4 T-Cell Recovery During Suppressive HAART. AIDS 2010, 24, 959–968. [Google Scholar] [CrossRef]
- Von Roenn, J.H.; Armstrong, D.; Kotler, D.P.; Cohn, D.L.; Klimas, N.G.; Tchekmedyian, N.S.; Cone, L.; Brennan, P.J.; Weitzman, S.A. Megestrol Acetate in Patients With AIDS-Related Cachexia. Ann. Intern. Med. 1994, 121, 393–399. [Google Scholar] [CrossRef]
- Von Roenn, J.H.; Roth, E.L.; Craig, R. HIV-Related Cachexia: Potential Mechanisms and Treatment. Oncology 1992, 49 (Suppl. 2), 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Westfall, A.O.; Willig, J.H.; Mugavero, M.J.; Saag, M.S.; Kaslow, R.A.; Kempf, M.C. Clinical Outcome of HIV-Infected Antiretroviral-Naive Patients With Discordant Immunologic and Virologic Responses to Highly Active Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2008, 47, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Migeon, C.J.; Plager, J.E. Identification and Isolation of Dehydroisoandrosterone from Peripheral Human Plasma. J. Biol. Chem. 1954, 209, 767–772. [Google Scholar] [CrossRef]
- Hall, P.F.; Sozer, C.C.; Eik-Nes, K.B. Formation of Dehydroepiandrosterone during In Vivo and In Vitro Biosynthesis of Testosterone by Testicular Tissue. Endocrinology 1964, 74, 35–43. [Google Scholar] [CrossRef]
- Aakvaag, A.; Hagen, A.A.; Eik-Nes, K.B. Biosynthesis In Vivo of Testosterone and Δ4-Androstenedione From Dehydroepiandrosterone-Sodium Sulfate by the Canine Testis and Ovary. Biochim. Biophys. Acta (BBA) Gen. Subj. 1964, 86, 622–627. [Google Scholar] [CrossRef]
- Christeff, N.; Gherbi, N.; Mammes, O.; Dalle, M.T.; Gharakhanian, S.; Lortholary, O.; Melchior, J.C.; Nunez, E.A. Serum Cortisol and DHEA Concentrations During HIV Infection. Psychoneuroendocrinology 1997, 22 (Suppl. 1), S11–S18. [Google Scholar] [CrossRef]
- Simpson, E.R.; Davis, S.R. Minireview: Aromatase and the Regulation of Estrogen Biosynthesis—Some New Perspectives. Endocrinology 2001, 142, 4589–4594. [Google Scholar] [CrossRef]
- Rabkin, J.G.; McElhiney, M.C.; Rabkin, R.; McGrath, P.J.; Ferrando, S.J. Placebo-Controlled Trial of Dehydroepiandrosterone (DHEA) for Treatment of Nonmajor Depression in Patients with HIV/AIDS. Am. J. Psychiatry 2006, 163, 59–66. [Google Scholar] [CrossRef]
- Piketty, C.; Jayle, D.; Leplege, A.; Castiel, P.; Ecosse, E.; Gonzalez-Canali, G.; Sabatier, B.; Boulle, N.; Debuire, B.; Le Bouc, Y.; et al. Double-Blind Placebo-Controlled Trial of Oral Dehydroepiandrosterone in Patients With Advanced HIV Disease. Clin. Endocrinol. (Oxf.) 2001, 55, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Hasheeve, D.; Salvato, P.; Thompson, C. DHEA: A Potential Treatment for HIV Disease. In Proceedings of the International Conference on AIDS, Yokohama, Japan, 7–12 August 1994; Volume 10, p. 223. [Google Scholar]
- Wang, F.; He, Y.; Santos, H.O.; Sathian, B.; Price, J.C.; Diao, J. The effects of dehydroepiandrosterone (DHEA) supplementation on body composition and blood pressure: A meta-analysis of randomized clinical trials. Steroids 2020, 163, 108710. [Google Scholar] [CrossRef]
| All Patients | |
|---|---|
| Baseline characteristics | |
| Number of Patients | 163 |
| Age, years | 44.1 ± 12.1 |
| Gender, male | 155 (95.1) |
| BMI (kg/m2) | 23.1 ± 4.1 |
| Weight (kg) | 68.5 ± 12.7 |
| Waist (inch) | 31.9 ± 5.9 |
| Underlying conditions | |
| DM | 16 (9.8) |
| HTN | 31 (19.0) |
| Chronic kidney disease | 3 (1.8) |
| Dyslipidemia | 28 (17.2) |
| Fatty liver | 9 (5.5) |
| HIV status | |
| Known duration of HIV (days) | 3168.7 ± 2223.1 |
| Treatment duration with cART (days) | 2774.2 ± 2036.9 |
| Time from diagnosis to treatment initiation (days) | 28.0 (11.3, 308.0) |
| Initial CD4+ T-cell count (/µL) | 278.0 ± 191.7 |
| Initial CD8+ T-cell count (/µL) | 953.4 ± 930.6 |
| Initial log10HIV-RNA titre (copies/mm3) | 4.8 (4.3, 5.4) |
| Follow-up CD4+ T-cell count (/µL) | 694.9 ± 325.5 |
| Follow-up CD8+ T-cell count (/µL) | 780.7 ± 399.5 |
| Follow-up log10HIV-RNA titre (copies/mm3) | 0 (0, 0) |
| Dehydroepiandrosterone (ng/mL) | 4.8 ± 3.4 |
| All Patients | Good Responders | Poor Responders | p-Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Number of Patients | 163 | 154 | 9 | |
| Age, years | 44.1 ± 12.1 | 44.0 ± 12.3 | 44.9 ± 8.7 | 0.711 |
| Gender, male | 155 (95.1) | 147 (95.5) | 7 (87.5) | 0.372 |
| BMI (kg/m2) | 23.1 ± 4.1 | 23.7 ± 3.6 | 20.6 ± 2.7 | 0.013 |
| Weight (kg) | 68.5 ± 12.7 | 70.3 ± 12.6 | 59.6 ± 10.7 | 0.013 |
| Waist (inch) | 31.9 ± 5.9 | 32.1 ± 6.0 | 29.1 ± 2.4 | 0.007 |
| Underlying conditions | ||||
| DM | 16 (9.8) | 16 (11.0) | 0 (0) | 0.599 |
| HTN | 31 (19.0) | 31 (21.2) | 0 (0) | 0.206 |
| Chronic kidney disease | 3 (1.8) | 3 (2.1) | 0 (0) | >0.999 |
| Dyslipidemia | 28 (17.2) | 28 (19.2) | 0 (0) | 0.365 |
| Fatty liver | 9 (5.5) | 9 (6.2) | 0 (0) | >0.999 |
| HIV status | ||||
| Known duration of HIV (days) | 3168.7 ± 2223.1 | 3352.1 ± 2222.3 | 3276.6 ± 2710.9 | 0.931 |
| Treatment duration with cART (days) | 2774.2 ± 2036.9 | 2898.3 ± 2001.9 | 3139.3 ± 2587.8 | 0.808 |
| Time from diagnosis to treatment initiation (days) | 28.0 (11.3, 308.0) | 488.6 ± 1030.3 | 83.2 ± 225.5 | 0.006 |
| Initial CD4+ T-cell count (/µL) | 278.0 ± 191.7 | 282.7 ± 187.9 | 92.4 ± 97.1 | 0.001 |
| Initial CD8+ T-cell count (/µL) | 953.4 ± 930.6 | 966.7 ± 863.0 | 497.3 ± 325.0 | 0.025 |
| Initial log10HIV-RNA titre (copies/mm3) | 4.8 (4.3, 5.4) | 4.7 (4.2, 5.3) | 5.6 (5.1, 6.1) | 0.001 |
| Follow-up CD4+ T-cell count (/µL) * | 694.9 ± 325.5 | 726.6 ± 303.3 | 187.4 ± 76.9 | <0.0001 |
| Follow-up CD8+ T-cell count (/µL) * | 780.7 ± 399.5 | 821.4 ± 396.5 | 569.0 ± 368.1 | 0.034 |
| Follow-up log10HIV-RNA titre (copies/mm3) * | 0 (0, 0) | 0 (0, 0) | 1.7 (0, 4.1) | <0.0001 |
| Dehydroepiandrosterone (ng/mL) | 4.8 ± 3.4 | 4.3 ± 3.4 | 2.9 ± 1.1 | 0.013 |
| All Patients | Good Responders | Poor Responders | p-Value | |
|---|---|---|---|---|
| Antiretroviral therapy | 163 | 154 | 9 | |
| Total number of antiretroviral agents | 3.1 ± 0.35 | 3.0 ± 0.02 | 3.3 ± 0.16 | <0.001 |
| Integrase inhibitor | 125 (81.2) | 117 (80.7) | 8 (88.9) | >0.999 |
| Nucleoside/nucleotide reverse transcriptase inhibitor | 153 (99.4) | 144 (99.3) | 9 (100.0) | >0.999 |
| Non-nucleoside/nucleotide reverse transcriptase inhibitor | 3 (1.9) | 3 (2.1) | 0 (0) | >0.999 |
| Protease inhibitor | 36 (23.4) | 31 (21.4) | 5 (55.6) | 0.033 |
| Age (at HIV Diagnosis) | Sex | CD4+ Count (Baseline, /mm3) | CD4+ Count (at the End of the Study, /mm3) | Antiretroviral Regimen | Duration of HIV Infection(Days) | Duration of HIV Treatment (Days) | |
|---|---|---|---|---|---|---|---|
| Poor responder 1 | 40 | Male | 9 | 290 | INI + PI | 391 | 386 |
| Poor responder 2 | 54 | Female | 4 | 137 | INI-based | 184 | 183 |
| Poor responder 3 | 42 | Male | 116 | 76 | INI + PI | 4655 | 4630 |
| Poor responder 4 | 29 | Male | 14 | 519 | INI-based | 1 | 1 |
| Poor responder 5 | 42 | Male | 56 | 201 | INI + PI | 2854 | 2844 |
| Poor responder 6 | 54 | Male | 229 | 282 | INI-based | 7322 | 6638 |
| Poor responder 7 | 54 | Male | 246 | 133 | INI + PI | 4712 | 4690 |
| Poor responder 8 | 46 | Male | 8 | 122 | INI based | 6372 | 6372 |
| Poor responder 9 | 56 | Male | 150 | 185 | INI + PI | 2999 | 2997 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Baseline characteristics | ||||
| Age, years | 1.006 (0.952–1.063) | 0.836 | ||
| Gender, male | 2.625 (0.287–23.996) | 0.393 | ||
| BMI (kg/m2) | 0.863 (0.746–0.999) | 0.048 | 0.763 (0.603–0.966) | 0.025 |
| Weight (kg) | 0.908 (0.837–0.984) | 0.019 | ||
| Waist (inch) | 0.619 (0.419–0.914) | 0.016 | ||
| HIV status | ||||
| Known duration of HIV (days) | 1.000 (1.000–1.000) | 0.881 | ||
| Treatment duration with cART (days) | 1.000 (1.000–1.000) | 0.521 | ||
| Time from Diagnosis to treatment initiation (days) | 0.999 (0.995–1.002) | 0.342 | ||
| Initial CD4+ T-cell count (/µL) | 0.990 (0.983–0.997) | 0.005 | 0.992 (0.984–0.999) | 0.023 |
| Initial CD8+ T-cell count (/µL) | 0.998 (0.995–1.000) | 0.025 | ||
| Initial HIV-RNA titre (copies/mm3) | 1.000 (1.000–1.000) | 0.055 | ||
| Dehydroepiandrosterone (ng/mL) | 0.469 (0.230–0.959) | 0.038 | 0.311 (0.102–0.948) | 0.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.H.; Lee, K.H.; Lee, S.J.; Kim, J.; Kim, J.H.; Ahn, J.Y.; Ku, N.S.; Choi, J.Y.; Yeom, J.-S.; Jeong, S.J. Low Dehydroepiandrosterone (DHEA) Level Is Associated with Poor Immunologic Response among People Living with HIV/AIDS. J. Clin. Med. 2022, 11, 6077. https://doi.org/10.3390/jcm11206077
Lee EH, Lee KH, Lee SJ, Kim J, Kim JH, Ahn JY, Ku NS, Choi JY, Yeom J-S, Jeong SJ. Low Dehydroepiandrosterone (DHEA) Level Is Associated with Poor Immunologic Response among People Living with HIV/AIDS. Journal of Clinical Medicine. 2022; 11(20):6077. https://doi.org/10.3390/jcm11206077
Chicago/Turabian StyleLee, Eun Hwa, Ki Hyun Lee, Se Ju Lee, Jinnam Kim, Jung Ho Kim, Jin Young Ahn, Nam Su Ku, Jun Yong Choi, Joon-Sup Yeom, and Su Jin Jeong. 2022. "Low Dehydroepiandrosterone (DHEA) Level Is Associated with Poor Immunologic Response among People Living with HIV/AIDS" Journal of Clinical Medicine 11, no. 20: 6077. https://doi.org/10.3390/jcm11206077
APA StyleLee, E. H., Lee, K. H., Lee, S. J., Kim, J., Kim, J. H., Ahn, J. Y., Ku, N. S., Choi, J. Y., Yeom, J.-S., & Jeong, S. J. (2022). Low Dehydroepiandrosterone (DHEA) Level Is Associated with Poor Immunologic Response among People Living with HIV/AIDS. Journal of Clinical Medicine, 11(20), 6077. https://doi.org/10.3390/jcm11206077






