Physicians’ Considerations and Practice Recommendations Regarding the Use of Sodium-Glucose Cotransporter-2 Inhibitors
Abstract
1. Introduction
2. SGLT-2is
3. Overcoming Physician-Related Barriers to SGLT-2i Use
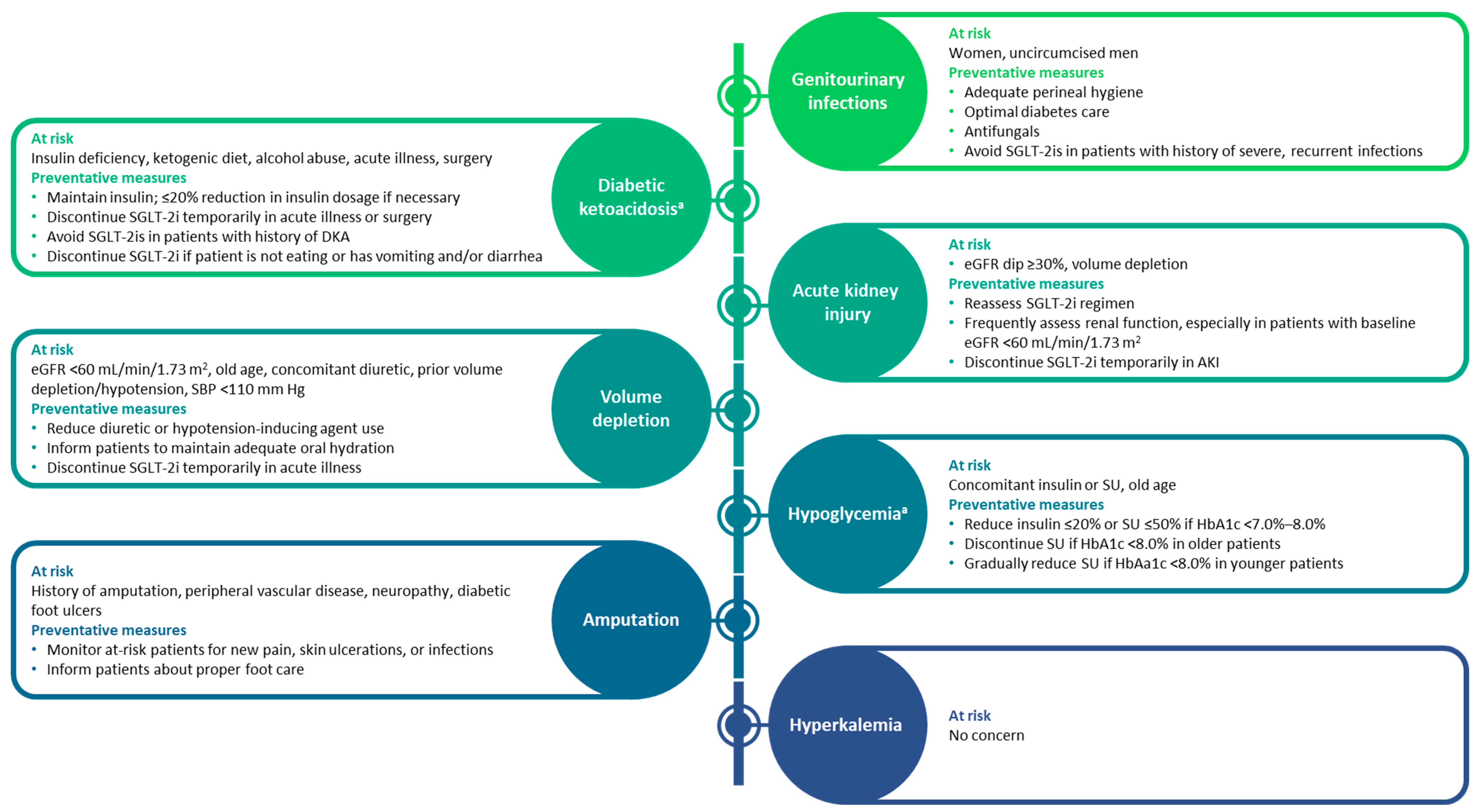
3.1. Acute Effects on Renal Function upon SGLT-2i Initiation
3.1.1. eGFR Acute Dip
3.1.2. AKI
3.2. Volume Depletion
3.3. DKA
3.4. Genital Mycotic Infections, Urinary Tract Infections, and Fournier’s Gangrene
3.5. Hyperkalemia
3.6. Hypoglycemia
3.7. Amputation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johansen, M.E.; Argyropoulos, C. The cardiovascular outcomes, heart failure and kidney disease trials tell that the time to use sodium glucose cotransporter 2 inhibitors is now. Clin. Cardiol. 2020, 43, 1376–1387. [Google Scholar] [CrossRef]
- Neuen, B.L.; Cherney, D.Z.; Jardine, M.J.; Perkovic, V. Sodium-glucose cotransporter inhibitors in type 2 diabetes: Thinking beyond glucose lowering. CMAJ 2019, 191, E1128–E1135. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Sathiyakumar, V.; Singh, A.; McCarthy, C.P.; Qamar, A.; Januzzi, J.L., Jr.; Scirica, B.M.; Butler, J.; Cannon, C.P.; Bhatt, D.L. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J. Am. Coll. Cardiol. 2018, 72, 3370–3372. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef]
- Seufert, J. SGLT2 inhibitors—An insulin-independent therapeutic approach for treatment of type 2 diabetes: Focus on canagliflozin. Diabetes Metab. Syndr. Obes. 2015, 8, 543–554. [Google Scholar] [CrossRef]
- Piperidou, A.; Loutradis, C.; Sarafidis, P. SGLT-2 inhibitors and nephroprotection: Current evidence and future perspectives. J. Hum. Hypertens. 2021, 35, 12–25. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Guidance for Industry: Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. Available online: https://www.fda.gov/media/71297/download (accessed on 4 November 2021).
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Vardeny, O.; Vaduganathan, M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 2019, 7, 169–172. [Google Scholar] [CrossRef]
- Lupsa, B.C.; Inzucchi, S.E. Use of SGLT2 inhibitors in type 2 diabetes: Weighing the risks and benefits. Diabetologia 2018, 61, 2118–2125. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- O’Meara, E.; McDonald, M.; Chan, M.; Ducharme, A.; Ezekowitz, J.A.; Giannetti, N.; Grzeslo, A.; Heckman, G.A.; Howlett, J.G.; Koshman, S.L.; et al. CCS/CHFS heart failure guidelines: Clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can. J. Cardiol. 2020, 36, 159–169. [Google Scholar] [CrossRef]
- Staels, B. Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: Potential mechanisms. Am. J. Med. 2017, 130, S30–S39. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium glucose cotransporter-2 inhibition in heart failure: Potential mechanisms, clinical applications, and summary of clinical trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef]
- Joshi, S.S.; Singh, T.; Newby, D.E.; Singh, J. Sodium-glucose co-transporter 2 inhibitor therapy: Mechanisms of action in heart failure. Heart 2021, 107, 1032–1038. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. The serendipitous story of SGLT2 inhibitors in heart failure. Circulation 2019, 139, 2537–2541. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Boehringer Ingelheim. Jardiance® (Empagliflozin Tablets), for Oral Use [Prescribing Information]. Available online: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf (accessed on 25 February 2022).
- Janssen Pharmaceuticals. Invokana® (Canagliflozin) Tablets, for Oral Use [Prescribing Information]. Available online: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVOKANA-pi.pdf (accessed on 2 November 2021).
- AstraZeneca. Farxiga® (Empagliflozin) Tablets, for Oral Use [Prescribing Information]. Available online: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/0be9cb1b-3b33-41c7-bfc2-04c9f718e442/0be9cb1b-3b33-41c7-bfc2-04c9f718e442_viewable_rendition__v.pdf (accessed on 2 November 2021).
- American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S175–S184. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S144–S174. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- De Boer, I.H.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]
- Maddox, T.M.; Januzzi, J.L.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Merck & Co. Steglatro® (Ertugliflozin) Tablets, for Oral Use [Prescribing Information]. Available online: https://www.merck.com/product/usa/pi_circulars/s/steglatro/steglatro_pi.pdf (accessed on 25 February 2022).
- Fitchett, D. A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes. Metab. 2019, 21, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Januzzi, J.L., Jr.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.; Morris, P.B.; Neumiller, J.J.; et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 1117–1145. [Google Scholar] [CrossRef] [PubMed]
- Van Bommel, E.J.M.; Muskiet, M.H.A.; van Baar, M.J.B.; Tonneijck, L.; Smits, M.M.; Emanuel, A.L.; Bozovic, A.; Danser, A.H.J.; Geurts, F.; Hoorn, E.J.; et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020, 97, 202–212. [Google Scholar] [CrossRef]
- Kraus, B.J.; Weir, M.R.; Bakris, G.L.; Mattheus, M.; Cherney, D.Z.I.; Sattar, N.; Heerspink, H.J.L.; Ritter, I.; von Eynatten, M.; Zinman, B.; et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021, 99, 750–762. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Cherney, D.Z.I. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin. J. Am. Soc. Nephrol. 2021, 16, 1278–1280. [Google Scholar] [CrossRef]
- Meraz-Muñoz, A.Y.; Weinstein, J.; Wald, R. eGFR decline after SGLT2 inhibitor initiation: The tortoise and the hare reimagined. Kidney360 2021, 2, 1042–1047. [Google Scholar] [CrossRef]
- Chertow, G.M.; Vart, P.; Jongs, N.; Toto, R.D.; Gorriz, J.L.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; et al. Effects of dapagliflozin in stage 4 chronic kidney disease. J. Am. Soc. Nephrol. 2021, 32, 2352–2361. [Google Scholar] [CrossRef]
- Iskander, C.; Cherney, D.Z.; Clemens, K.K.; Dixon, S.N.; Harel, Z.; Jeyakumar, N.; McArthur, E.; Muanda, F.T.; Parikh, C.R.; Paterson, J.M.; et al. Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: A population-based cohort study. CMAJ 2020, 192, E351–E360. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, G.N.; Ferrandino, R.; Chang, A.; Surapaneni, A.; Chauhan, K.; Poojary, P.; Saha, A.; Ferket, B.; Grams, M.E.; Coca, S.G. Acute kidney injury in patients on SGLT2 inhibitors: A propensity-matched analysis. Diabetes Care 2017, 40, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, C.; Kraut, E.; Whitlock, R.H.; Komenda, P.; Woo, V.; Rigatto, C.; Tangri, N. Acute kidney injury events in patients with type 2 diabetes using SGLT2 inhibitors versus other glucose-lowering drugs: A retrospective cohort study. Am. J. Kidney Dis. 2020, 76, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Gibson, A.K.; McGill, J.B.; Maddukuri, G.; Al-Aly, Z. Clinical implications of estimated glomerular filtration rate dip following sodium-glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 2021, 10, e020237. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, S.; Huang, Z.; Wang, T.; Tang, H. Network meta-analysis of novel glucose-lowering drugs on risk of acute kidney injury. Clin. J. Am. Soc. Nephrol. 2020, 16, 70–78. [Google Scholar] [CrossRef]
- Wilcox, C.S. Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors. Hypertension 2020, 75, 894–901. [Google Scholar] [CrossRef]
- Serenelli, M.; Böhm, M.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Solomon, S.D.; DeMets, D.L.; et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur. Heart J. 2020, 41, 3402–3418. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- Rong, X.; Li, X.; Gou, Q.; Liu, K.; Chen, X. Risk of orthostatic hypotension associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials. Diabetes Vasc. Dis. Res. 2020, 17, 1479164120953625. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Udell, J.A. Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists. Circulation 2016, 134, 1915–1917. [Google Scholar] [CrossRef]
- Mistry, S.; Eschler, D.C. Euglycemic diabetic ketoacidosis caused by SGLT2 inhibitors and a ketogenic diet: A case series and review of literature. AACE Clin. Case Rep. 2021, 7, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.M.; Berard, L.D.; Cheng, A.Y.Y.; Gilbert, J.D.; Verma, S.; Woo, V.C.; Yale, J.-F. SGLT2 inhibitor–associated diabetic ketoacidosis: Clinical review and recommendations for prevention and diagnosis. Clin. Ther. 2016, 38, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Neuen, B.L.; Jun, M.; Ohkuma, T.; Neal, B.; Jardine, M.J.; Heerspink, H.L.; Wong, M.G.; Ninomiya, T.; Wada, T.; et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J.; Taylor, S.I.; Weir, M.R. Diabetic ketoacidosis, sodium glucose transporter-2 inhibitors and the kidney. J. Diabetes Complicat. 2016, 30, 1162–1166. [Google Scholar] [CrossRef]
- Peters, A.L.; Buschur, E.O.; Buse, J.B.; Cohan, P.; Diner, J.C.; Hirsch, I.B. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015, 38, 1687–1693. [Google Scholar] [CrossRef]
- Nyirjesy, P.; Sobel, J.D. Genital mycotic infections in patients with diabetes. Postgrad. Med. 2013, 125, 33–46. [Google Scholar] [CrossRef]
- McGovern, A.P.; Hogg, M.; Shields, B.M.; Sattar, N.A.; Holman, R.R.; Pearson, E.R.; Hattersley, A.T.; Jones, A.G.; Dennis, J.M. Risk factors for genital infections in people initiating SGLT2 inhibitors and their impact on discontinuation. BMJ Open Diabetes Res. Care 2020, 8, e001238. [Google Scholar] [CrossRef]
- Engelhardt, K.; Ferguson, M.; Rosselli, J.L. Prevention and management of genital mycotic infections in the setting of sodium-glucose cotransporter 2 inhibitors. Ann. Pharmacother. 2021, 55, 543–548. [Google Scholar] [CrossRef]
- Williams, S.M.; Ahmed, S.H. Improving compliance with SGLT2 inhibitors by reducing the risk of genital mycotic infections: The outcomes of personal hygiene advice. Diabetes 2019, 68, 1224. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Vaginal Candidiasis. Available online: https://www.cdc.gov/fungal/diseases/candidiasis/genital/index.html (accessed on 15 November 2021).
- Petruski-Ivleva, N.; Schneeweiss, S.; Eapen, S.; Rajan, A.; Jan, S. Fournier’s gangrene in patients with type 2 diabetes using second-line antidiabetic medications. Diabetes Obes. Metab. 2020, 22, 267–271. [Google Scholar] [CrossRef]
- Tran, B.A.; Updike, W.H.; Bullers, K.; Serag-Bolos, E. Sodium–glucose cotransporter 2 inhibitor use associated with Fournier’s gangrene: A review of case reports and spontaneous post-marketing cases. Clin. Diabetes 2022, 40, 78–86. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Warns about Rare Occurrences of a Serious Infection of the Genital Area with SGLT2 Inhibitors for Diabetes. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm617360.htm (accessed on 4 November 2021).
- Weir, M.R.; Kline, I.; Xie, J.; Edwards, R.; Usiskin, K. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr. Med. Res. Opin. 2014, 30, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Slee, A.; Sun, T.; Balis, D.; Oh, R.; de Zeeuw, D.; Perkovic, V. Effects of canagliflozin on serum potassium in the CANagliflozin cardioVascular Assessment Study (CANVAS) Program. Clin. Kidney J. 2021, 14, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Docherty, K.F.; Jhund, P.S.; Bengtsson, O.; Demets, D.L.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; et al. Dapagliflozin reduces the risk of hyperkalaemia in patients with heart failure and reduced ejection fraction: A secondary analysis DAPA-HF. Eur. Heart J. 2020, 41, ehaa946.0939. [Google Scholar] [CrossRef]
- Provenzano, M.; Jongs, N.; Vart, P.; Stefánsson, B.V.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; et al. The kidney protective effects of the sodium–glucose cotransporter-2 inhibitor, dapagliflozin, are present in patients with CKD treated with mineralocorticoid receptor antagonists. Kidney Int. Rep. 2021, 7, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Zannad, F.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Jamal, W.; Steubl, D.; Schueler, E.; et al. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: EMPEROR-Reduced. J. Am. Coll. Cardiol. 2021, 77, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Kohler, S.; Zeller, C.; Iliev, H.; Kaspers, S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: Pooled analysis of phase I-III clinical trials. Adv. Ther. 2017, 34, 1707–1726. [Google Scholar] [CrossRef]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020, 323, 1353–1368. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Invokana (Canagliflozin) Tablets, for Oral Use [Prescribing Information]. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204042s026lbl.pdf (accessed on 2 November 2021).
- Inzucchi, S.E.; Iliev, H.; Pfarr, E.; Zinman, B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care 2018, 41, e4–e5. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Removes Boxed Warning about Risk of Leg and Foot Amputations for the Diabetes Medicine Canagliflozin (Invokana, Invokamet, Invokamet XR). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-removes-boxed-warning-about-risk-leg-and-foot-amputations-diabetes-medicine-canagliflozin (accessed on 2 November 2021).
- American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S185–S194. [Google Scholar] [CrossRef]
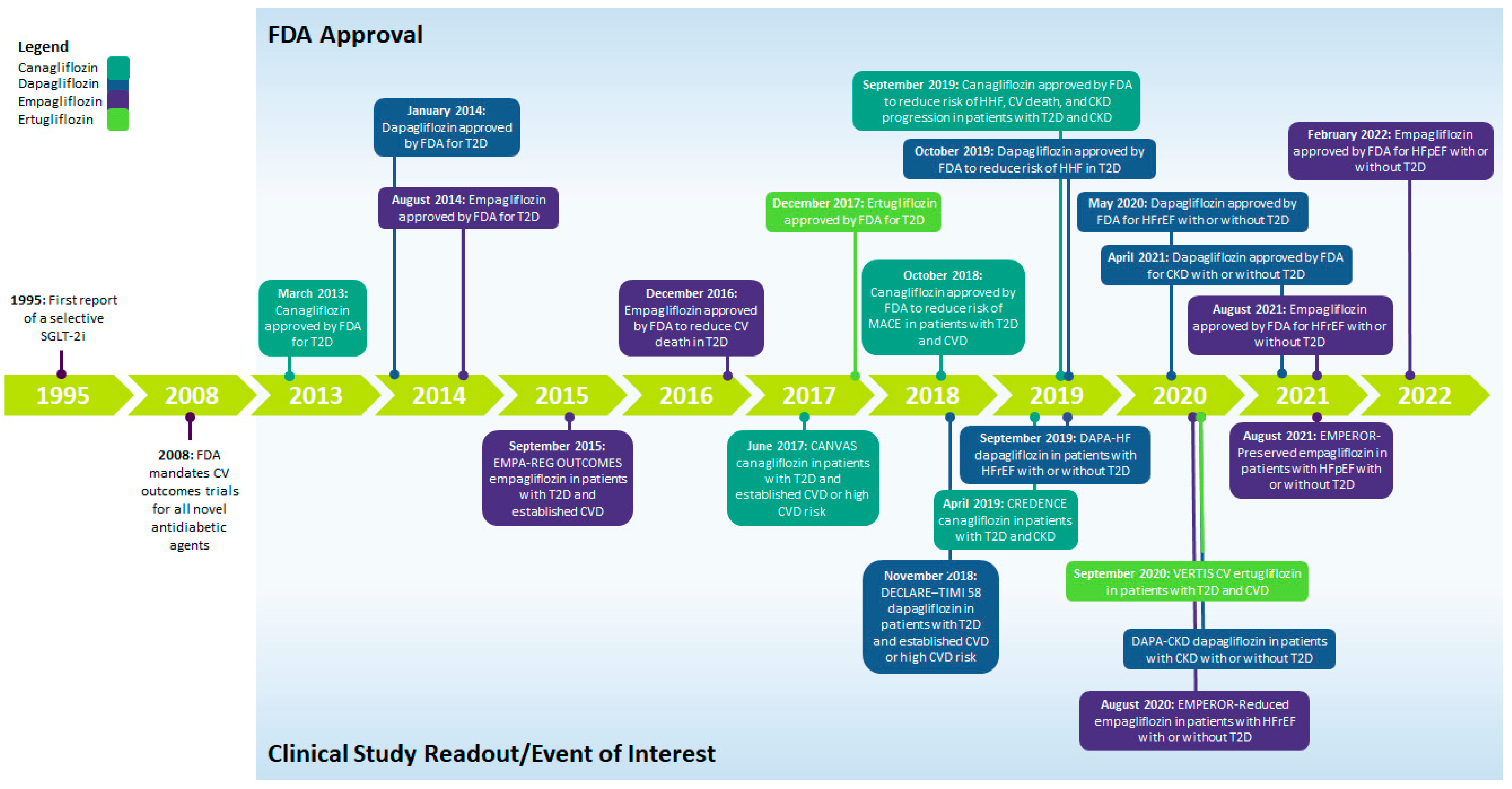
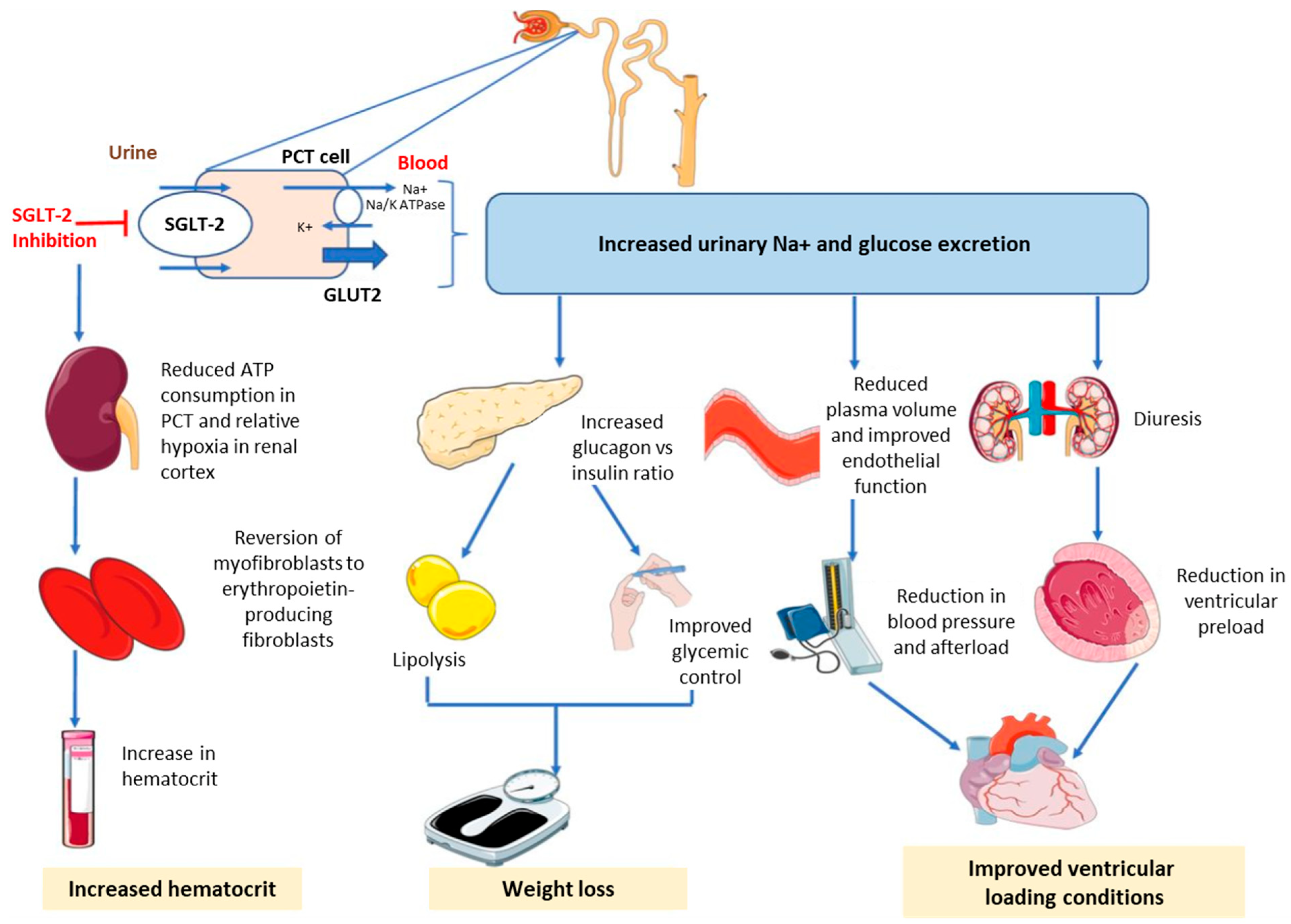
| Scheme 1 | Population | Median Follow-Up | HbA1c Difference vs. Placebo, Mean (95% CI) | CV Outcomes, HR (95% CI) | Renal Outcomes, HR (95% CI) |
|---|---|---|---|---|---|
| Canagliflozin | |||||
| CANVAS [9] | Age ≥ 30 y with T2D and established CVD OR Age ≥ 50 y with T2D and ≥2 CVD risk factors (n = 10,142) | ~126 wk | −0.58% (−0.61%, −0.56%) | MACE a: 0.86 (0.75–0.97); p < 0.001 for noninferiority and p = 0.02 for superiority CV death or HHF: 0.78 (0.67–0.91) HHF: 0.67 (0.52–0.87) CV death: 0.87 (0.72–1.06) | Progression of albuminuria: 0.73 (0.67–0.79) 40% reduction in eGFR, RRT initiation, or death from renal causes: 0.60 (0.47–0.77) |
| CREDENCE [22] | Age ≥30 y with T2D and CKD b (n = 4401) | ~2.6 y | −0.25% (−0.31%, −0.20%) | MACE a: 0.80 (0.67–0.95); p = 0.01 CV death or HHF: 0.69 (0.57–0.83) p < 0.001 HHF: 0.61 (0.47–0.80) p < 0.001 CV death: 0.78 (0.61–1.00); p = 0.05 | ESKD, doubling of sCr, or death from renal causes: 0.66 (0.53–0.81); p < 0.001 Doubling of sCr: 0.60 (0.48–0.76); p < 0.001 ESKD: 0.68 (0.54–0.86); p = 0.002 |
| Dapagliflozin | |||||
| DECLARE–TIMI 58 [10] | Age ≥ 40 y with T2D and established CVD OR Age ≥ 55 y (men) or ≥60 y (women) with T2D and ≥1 CVD risk factor (n = 17,160) | 4.2 y | −0.42% (−0.45%, −0.40%) | MACE a: 0.93 (0.84–1.03); p < 0.001 for noninferiority and p = 0.17 for superiority CV death or HHF: 0.83 (0.84–0.95); p = 0.005 HHF: 0.73 (0.61–0.88) CV death: 0.98 (0.82–1.17) | ≥40% reduction in eGFR to <60 mL/min/1.73 m2, ESKD, or death from CV or renal causes: 0.76 (0.67–0.87) ≥40% reduction in eGFR to <60 mL/min/1.73 m2, ESKD, or death from renal causes: 0.53 (0.43–0.66) |
| DAPA-HF [23] | Age ≥18 y with NYHA class II–IV HFrEF (EF ≤40%) with or without T2D (n = 4744) | 18.2 mo | −0.24% (−0.34%, −0.13%); p < 0.001 c | Worsening HF d or CV death: 0.74 (0.65–0.85); p < 0.001 CV death or HHF: 0.75 (0.65–0.85); p < 0.001 Worsening HF d: 0.70 (0.59–0.83) HHF: 0.70 (0.59–0.83) Urgent HF visit: 0.43 (0.20–0.90) CV death: 0.82 (0.69–0.98) | Worsening renal function e: 0.71 (0.44–1.16) |
| DAPA-CKD [21] | Age ≥18 y with CKD f with or without T2D (n = 4094) | 2.4 y | NR | CV death or HHF: 0.71 (0.55–0.92); p = 0.009 CV death: 0.81 (0.58–1.12) | Sustained ≥50% reduction in eGFR, ESKD, or death from CV or renal causes: 0.61 (0.51–0.72); p < 0.001 Sustained ≥50% reduction in eGFR, ESKD, or death from renal causes: 0.56 (0.45–0.68); p < 0.001 ≥ 50% reduction in eGFR: 0.53 (0.42–0.67) ESKD: 0.64 (0.50–0.82) |
| Empagliflozin | |||||
| EMPA-REG OUTCOME [11,24] | Age ≥18 y with T2D and established CVD (n = 7020) | 3.1 y | Adjusted mean difference, 10 mg dose: −0.24% (−0.40%, −0.08%); 25 mg dose: −0.36% (−0.51%, −0.20%) | MACE a: 0.86 (0.74–0.99); p < 0.001 for noninferiority and p = 0.04 for superiority MACE a or hospitalization for UA: 0.89 (0.78–1.01); p < 0.001 for noninferiority and p = 0.08 for superiority CV death or HHF: 0.66 (0.55–0.79); p < 0.001 HHF: 0.65 (0.50–0.85); p = 0.002 CV death: 0.62 (0.49–0.77); p < 0.001 | Incident or worsening nephropathy g: 0.61 (0.53–0.70); p < 0.001 Doubling of sCr with eGFR ≤45 mL/min/1.73 m2, RRT initiation, or death from renal causes: 0.54 (0.40–0.75); p < 0.001 |
| EMPEROR-Reduced [25] | Age ≥18 y with NYHA class II–IV HFrEF (EF ≤40%) with or without T2D (n = 3730) | 16 mo | Absolute difference: −0.16 (−0.25, −0.08) c | CV death or HHF: 0.75 (0.65–0.86); p < 0.001 HHF: 0.69 (0.59–0.81) CV death: 0.92 (0.75–1.12) | Composite renal outcome h: 0.50 (0.32–0.77) |
| EMPEROR-Preserved [26] | Age ≥18 y with NYHA class II–IV HFpEF (EF >40%) with or without T2D (n = 5988) | 26.2 mo | Adjusted mean difference: −0.19% (−0.25%, −0.14%)c | CV death or HHF: 0.79 (0.69–0.90); p < 0.001 HHF: 0.71 (0.60–0.83) CV death: 0.91 (0.76–1.09) | Mean difference (95% CI) in eGFR slope change per year vs. placebo: 1.36 (1.06–1.66) mL/min/1.73 m2; p < 0.001 Composite renal outcome h: 0.95 (0.73–1.24) |
| Ertugliflozin | |||||
| VERTIS CV [8] | Age ≥40 y with T2D and established CVD (n = 8246) | 3.0 y | LSM difference at wk 18 vs. baseline, 5 mg: −0.70% (−0.73%, −0.67%); 15 mg: −0.72% (−0.75%, −0.69%); placebo: −0.22% (−0.25%, −0.19%) | MACE: 0.97 (0.85–1.11); p < 0.001 for noninferiority CV death or HHF: 0.88 (0.75–1.03); p = 0.11 HHF: 0.70 (0.54–0.90) CV death: 0.92 (0.77–1.11) | Doubling of sCr, RRT initiation, or death from renal causes: 0.81 (0.63–1.04) |
| Year | Guidelines | SGLT-2is | Indications |
|---|---|---|---|
| 2019 | American College of Cardiology (ACC)/American Heart Association (AHA) [33] | Canagliflozin, dapagliflozin, and empagliflozin | T2D and ASCVD |
| 2019 | European Society of Cardiology (ESC) [34] | Canagliflozin, dapagliflozin, and empagliflozin | T2D and CVD |
| 2020 | Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease [35] | Canagliflozin, dapagliflozin, and empagliflozin | T2D and CKD |
| 2021 | ESC/Heart Failure Association (HFA) of the ESC [37] | Canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and sotagliflozin | T2D and CVD |
| Dapagliflozin, empagliflozin, and sotagliflozin | T2D and HFrEF | ||
| 2021 | ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment [36] | Dapagliflozin and a empagliflozin a | HFrEF with or without T2D |
| 2022 | American Diabetes Association [31,32] | SGLT-2i drug class recommended An SGLT-2i with proven benefit for the individual patient’s comorbidities is recommended (CVD: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin; DKD: canagliflozin, dapagliflozin, and empagliflozin) | T2D, ASCVD, HF, and DKD |
| Study Name | Population | Risk of AEs |
|---|---|---|
| Canagliflozin | ||
| CANVAS [9] | Age ≥30 y with T2D and established CVD OR Age ≥50 y with T2D and ≥2 CVD risk factors (n = 10,142) |
|
| CREDENCE [22] | Age ≥30 y with T2D and CKD a (n = 4401) |
|
| Dapagliflozin | ||
| DECLARE–TIMI 58 [10] | Age ≥40 y with T2D and established CVD OR Age ≥55 y (men) or ≥60 y (women) with T2D and ≥1 CVD risk factor (n = 17,160) |
|
| DAPA-HF [23] | Age ≥18 y with NYHA class II–IV HFrEF (EF ≤ 40%) with or without T2D (n = 4744) |
|
| DAPA-CKD [21] | Age ≥18 y with CKD b with or without T2D (n = 4094) |
|
| Empagliflozin | ||
| EMPA-REG OUTCOME [11] | Age ≥18 y with T2D and established CVD (n = 7020) |
|
| EMPEROR-Reduced [25] | Age ≥18 y with NYHA class II–IV HFrEF (EF ≤40%) with or without T2D (n = 3730) |
|
| EMPEROR-Preserved [26] | Age ≥18 y with NYHA class II–IV HFpEF (EF >40%) with or without T2D (n = 5988) |
|
| Ertugliflozin | ||
| VERTIS CV [8] | Age ≥40 y with T2D and established CVD (n = 8246) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbour, S.A.; Ibrahim, N.E.; Argyropoulos, C.P. Physicians’ Considerations and Practice Recommendations Regarding the Use of Sodium-Glucose Cotransporter-2 Inhibitors. J. Clin. Med. 2022, 11, 6051. https://doi.org/10.3390/jcm11206051
Jabbour SA, Ibrahim NE, Argyropoulos CP. Physicians’ Considerations and Practice Recommendations Regarding the Use of Sodium-Glucose Cotransporter-2 Inhibitors. Journal of Clinical Medicine. 2022; 11(20):6051. https://doi.org/10.3390/jcm11206051
Chicago/Turabian StyleJabbour, Serge A., Nasrien E. Ibrahim, and Christos P. Argyropoulos. 2022. "Physicians’ Considerations and Practice Recommendations Regarding the Use of Sodium-Glucose Cotransporter-2 Inhibitors" Journal of Clinical Medicine 11, no. 20: 6051. https://doi.org/10.3390/jcm11206051
APA StyleJabbour, S. A., Ibrahim, N. E., & Argyropoulos, C. P. (2022). Physicians’ Considerations and Practice Recommendations Regarding the Use of Sodium-Glucose Cotransporter-2 Inhibitors. Journal of Clinical Medicine, 11(20), 6051. https://doi.org/10.3390/jcm11206051






