Mitral Valve Systolic Anterior Motion in Robotic Thoracic Surgery as the Cause of Unexplained Hemodynamic Shock: From a Case Report to Recommendations
Abstract
1. Introduction
2. Case Report
3. Discussion
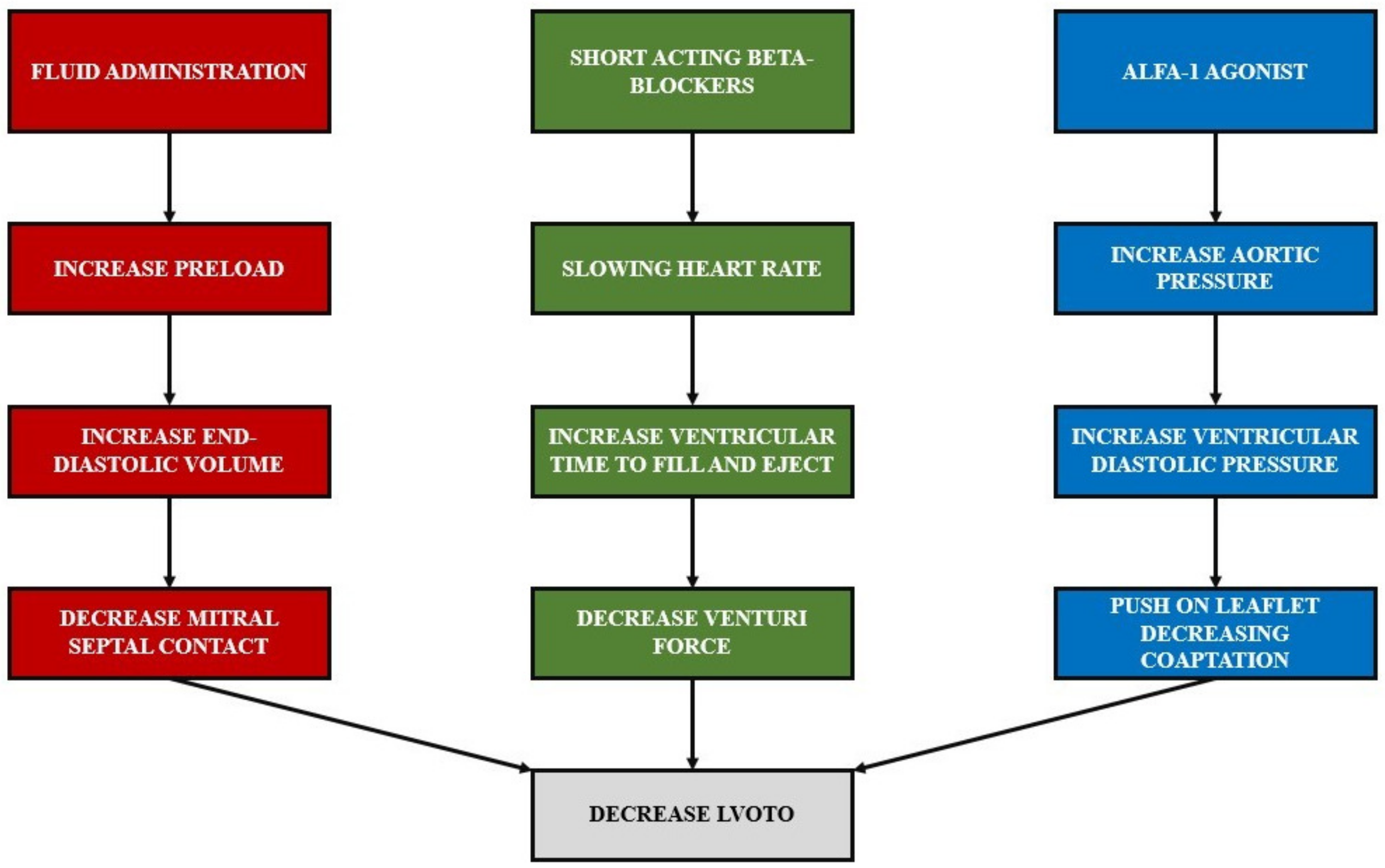
3.1. Hemodynamic Management Considerations
3.2. Respiratory Management Considerations
3.3. Pain Management Consideration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Parato, V.M.; Antoncecchi, V.; Sozzi, F.; Marazia, S.; Zito, A.; Maiello, M.; Palmiero, P. Echocardiographic diagnosis of the different phenotypes of hypertrophic car-diomyopathy. Cardiovasc. Ultrasound 2015, 14, 30. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rao, C.; Ashrafian, H.; Chaudhry, U.; Darzi, A.; Athanasiou, T. Modern management of systolic anterior motion of the mitral valve. Eur. J. Cardio-Thorac. Surg. 2012, 41, 260–1270. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Pa-tients Undergoing Noncardiac Surgery. J. Am. Coll. Cardiol. 2014, 64, e77–e137. [Google Scholar] [CrossRef]
- Caselli, S.; Martino, A.; Genuini, I.; Santini, D.; Carbone, I.; Agati, L.; Fedele, F. Pathophysiology of Dynamic Left Ventricular Outflow Tract Obstruction in a Critically Ill Patient. Echocardiography 2010, 27, E122–E124. [Google Scholar] [CrossRef]
- Cavallaro, F.; Marano, C.; Sandroni, C.; Dell’Anna, A.M. Systolic anterior motion causing hemodynamic instability and pulmonary edema during bleeding. Minerva Anestesiol. 2010, 76, 653–656. [Google Scholar]
- Zhang, Y.; Wang, S.; Sun, Y. Anesthesia of robotic thoracic surgery. Ann. Transl. Med. 2015, 3, 71. [Google Scholar] [CrossRef]
- Campos, J.H.; Keinich, U.; Falabella, A. Anesthesia for robotic thoracic surgery. In Atlas of Robotic Thoracic Surgery; Springer: Cham, Switzerland, 2018; pp. 15–25. [Google Scholar]
- Kernstine, K.H.; Anderson, C.A.; Falabella, A. Robotic lobectomy. Oper. Tech. Thorac. Cardiovasc. Surg. 2008, 13, 204-e1. [Google Scholar] [CrossRef]
- McCall, P.; Steven, M.; Shelley, B. Anaesthesia for video-assisted and robotic thoracic surgery. BJA Educ. 2019, 19, 405–411. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Shah, P.M.; Yipintsoi, T.; Amarasingham, R.; Oakley, C.M. Effects of respiration on the hemodynamics of hypertrophic obstructive cardiomyopathy. Am. J. Cardiol. 1965, 15, 793–800. [Google Scholar] [CrossRef]
- Maron, B.J.; McKenna, W.J. ACC/ESC clinical expert consensus document on hypertrophic cardiomyopathy: A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines (Committee to Develop an Expert Consensus Document on Hypertrophic Cardiomyo-pathy). J. Am. Coll. Cardiol. 2003, 42, 1687–1713. [Google Scholar] [PubMed]
- Vaughn-Whitley, K.; Mooss, A. Dynamic Left Ventricular Outflow Obstruction Related to Atrial Fibrillation. Chest 1992, 102, 1618–1619. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Jaramillo, R.; Monaco, F.; Zangrillo, A.; Landoni, G. Ultra-Short–Acting β-Blockers (Esmolol and Landiolol) in the Perioperative Period and in Critically Ill Patients. J. Cardiothorac. Vasc. Anesthesia 2018, 32, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Fifer, M.A.; Vlahakes, G.J. Management of Symptoms in Hypertrophic Cardiomyopathy. Circulation 2008, 117, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Oldham, H.N., Jr. The circulatory response of patients with idiopathic hypertrophic subaortic stenosis to nitro-glycerin and to the Valsalva maneuver. Circulation 1964, 29, 422–431. [Google Scholar] [CrossRef]
- Braunwald, E.; Ebert, P.A. Hemodynamic alterations in idiopathic hypertrophic subaortic stenosis induced by sympathomimetic drugs. Am. J. Cardiol. 1962, 10, 489–495. [Google Scholar] [CrossRef]
- Ebert, T.J.; Harkin, C.P.; Muzi, M. Cardiovascular responses to sevoflurane: A review. Anesth. Analg. 1995, 81, 11S–22S. [Google Scholar] [CrossRef]
- Veronesi, G.; Galetta, D.; Maisonneuve, P.; Melfi, F.; Schmid, R.A.; Borri, A.; Vannucci, F.; Spaggiari, L. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 19–25. [Google Scholar] [CrossRef]
- Jones, D.R.; Graeber, G.M.; Tanguilig, G.G.; Hobbs, G.; Murray, G.F. Effects of insufflation during thoracoscopy. Ann. Thorac. Surg. 1993, 55, 1379–1382. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Nakajima, J.; Kotsuka, Y.; Takamoto, S. Hemodynamic responses to intrapleural insufflation with hemipulmonary collapse. Surg. Endosc. 2001, 15, 1327–1330. [Google Scholar] [CrossRef]
- Hill, R.C.; Jones, D.R.; Vance, R.A.; Kalantarian, B. Selective lung ventilation during thoracoscopy: Effects of insufflation on hemodynamics. Ann. Thorac. Surg. 1996, 61, 945–948. [Google Scholar] [CrossRef]
- Brock, H.; Rieger, R.; Gabriel, C.; Polz, W.; Moosbauer, W.; Necek, S. Haemodynamic changes during thoracoscopic surgery the effects of one-lung ventilation compared with carbon dioxide insufflation. Anaesthesia 2000, 55, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Muraji, T.; Asai, T.; Hamada, Y.; Hioki, K. Hemodynamic Effects of Carbon Dioxide Insufflation of the Thoracic Cavity During Thoracoscopic Surgery. Pediatr. Endosurgery Innov. Tech. 2002, 6, 185–189. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Imanaka, K.; Endoh, M.; Kohno, T.; Nakajima, J.; Kotsuka, Y.; Takamoto, S. Hemodynamic effects of carbon dioxide insufflation under single-lung ventilation during thoracoscopy. Ann. Thorac. Surg. 1999, 68, 29–32. [Google Scholar] [CrossRef]
- Shekerdemian, L.; Desmond, B. Cardiovascular effects of mechanical ventilation. Arch. Dis. Child. 1999, 80, 475–480. [Google Scholar] [CrossRef]
- Defosse, J.; Schieren, M. A Germany-wide survey on anaesthesia in thoracic surgery. Anaesthesist 2016, 65, 449–457. [Google Scholar] [CrossRef]
- Della Rocca, G.; Langiano, N. Survey of thoracic anesthetic practice in Italy. J. Cardiothorac. Vasc. Anesth. 2013, 27, 1321–1329. [Google Scholar] [CrossRef]
- Gayraud, G.; Bastien, O. A French survey on the practice of analgesia for thoracic surgery. Ann. Fr. Anesth. Reanim. 2013, 32, 684–690. [Google Scholar] [CrossRef]
- Eldawlatly, A.; Turkistani, A.; Shelley, B.; El-Tahan, M.; MacFie, A.; Kinsella, J. Anesthesia for thoracic surgery: A survey of middle eastern practice. Saudi J. Anaesth. 2012, 6, 192–196. [Google Scholar] [CrossRef]
- Shelley, B.; Macfie, A.; Kinsella, J. Anesthesia for Thoracic Surgery: A Survey of UK Practice. J. Cardiothorac. Vasc. Anesth. 2011, 25, 1014–1017. [Google Scholar] [CrossRef]
- Minzter, B.H.; Johnson, R.F. The practice of thoracic epidural analgesia: A survey of academic medical centers in the United States. Anesth. Analg. 2002, 95, 472–475. [Google Scholar] [CrossRef] [PubMed]
- El-Tahan, M.R. Role of Thoracic Epidural Analgesia for Thoracic Surgery and Its Perioperative Effects. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Gates, S.; Naidu, B.V.; Wilson, M.J.; Smith, F.G. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst. Rev. 2016, 2, CD009121. [Google Scholar] [CrossRef] [PubMed]
- Baidya, D.K.; Khanna, P.; Maitra, S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-J.; Lai, Y.-C.; Ou, S.-Y.; Chen, C.-H. Unexpected systolic anterior motion of the mitral valve-related hypoxemia during transurethral resection of the prostate under spinal anesthesia: A case report. BMC Anesthesiol. 2022, 22, 207. [Google Scholar] [CrossRef]
- D’Souza, S. Optimum Anesthesia Technique for a Patient with Hypertrophic Obstructive Cardiomyopathy (HOCM) and Systolic Anterior Motion of the Mitral Valve (SAM). In Proceedings of the American Society of Anesthesiologists (ASA) Conference 2019, Orlando, FL, USA, 19–23 October 2019. [Google Scholar]
- Lasala, J.D.; Tsai, J.; Rodriguez-Restrepo, A.; Atay, S.M.; Sepesi, B. Systolic anterior motion of the mitral valve—The mechanism of postural hypotension following left intrapericardial pneumonectomy. J. Thorac. Dis. 2017, 9, E354–E357. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, F.; D’Amico, F.; Barucco, G.; Licheri, M.; Novellis, P.; Ciriaco, P.; Veronesi, G. Mitral Valve Systolic Anterior Motion in Robotic Thoracic Surgery as the Cause of Unexplained Hemodynamic Shock: From a Case Report to Recommendations. J. Clin. Med. 2022, 11, 6044. https://doi.org/10.3390/jcm11206044
Monaco F, D’Amico F, Barucco G, Licheri M, Novellis P, Ciriaco P, Veronesi G. Mitral Valve Systolic Anterior Motion in Robotic Thoracic Surgery as the Cause of Unexplained Hemodynamic Shock: From a Case Report to Recommendations. Journal of Clinical Medicine. 2022; 11(20):6044. https://doi.org/10.3390/jcm11206044
Chicago/Turabian StyleMonaco, Fabrizio, Filippo D’Amico, Gaia Barucco, Margherita Licheri, Pierluigi Novellis, Paola Ciriaco, and Giulia Veronesi. 2022. "Mitral Valve Systolic Anterior Motion in Robotic Thoracic Surgery as the Cause of Unexplained Hemodynamic Shock: From a Case Report to Recommendations" Journal of Clinical Medicine 11, no. 20: 6044. https://doi.org/10.3390/jcm11206044
APA StyleMonaco, F., D’Amico, F., Barucco, G., Licheri, M., Novellis, P., Ciriaco, P., & Veronesi, G. (2022). Mitral Valve Systolic Anterior Motion in Robotic Thoracic Surgery as the Cause of Unexplained Hemodynamic Shock: From a Case Report to Recommendations. Journal of Clinical Medicine, 11(20), 6044. https://doi.org/10.3390/jcm11206044








