Bariatric Surgery Outcomes in Patients with Kidney Transplantation
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Subjects’ Characteristics
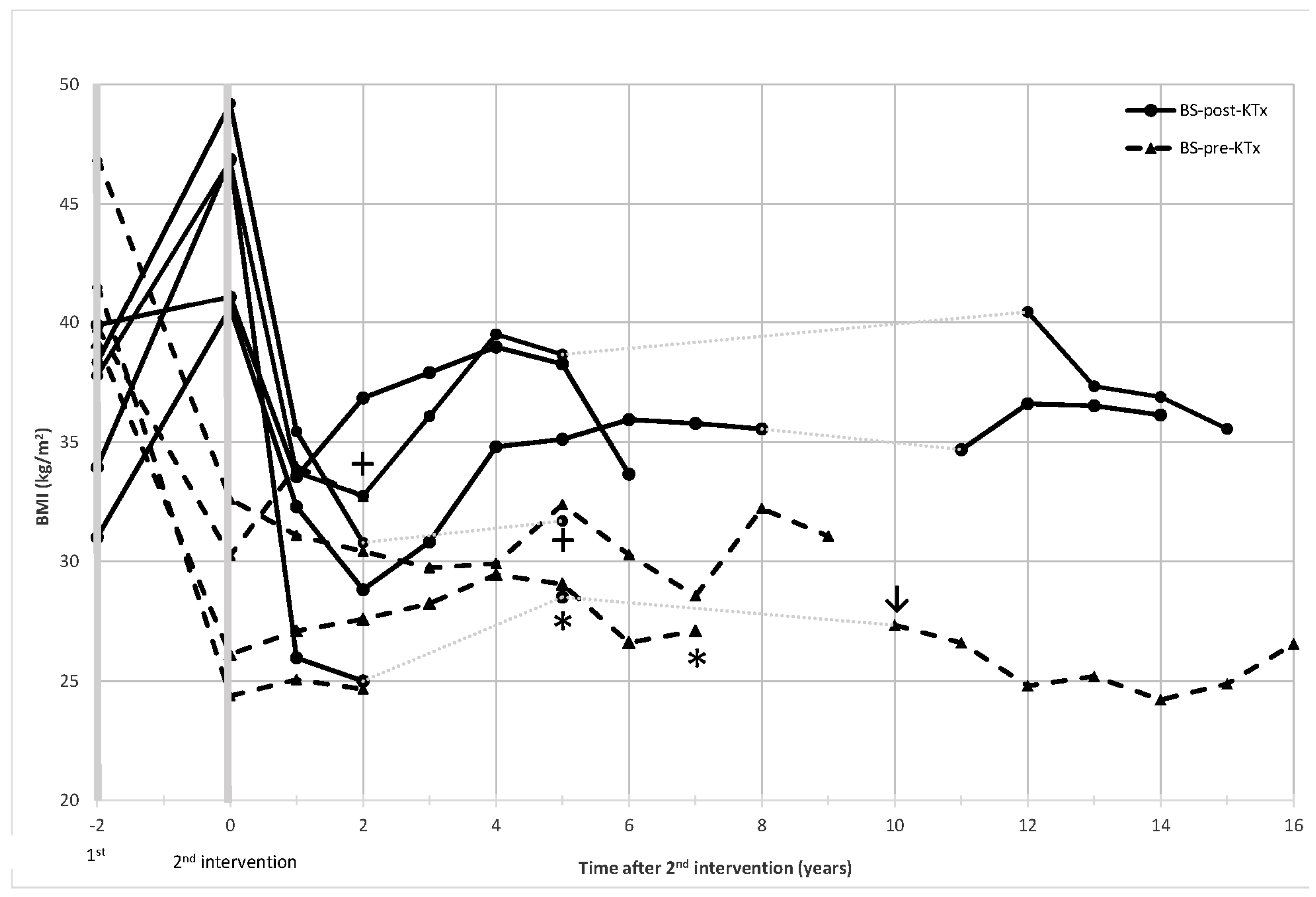
3.2. Metabolic Outcomes following Bariatric Surgery and Kidney Transplantation
3.3. Renal Outcomes following Bariatric Surgery and Kidney Transplantation
3.4. Immunosuppression after Kidney Transplantation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Praga, M.; Morales, E. The Fatty Kidney: Obesity and Renal Disease. Nephron 2017, 136, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Navarro Díaz, M. Consequences of Morbid Obesity on the Kidney. Where Are We Going? Clin. Kidney J. 2016, 9, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Bayés, B.; Granada, M.L.; Pastor, M.C.; Lauzurica, R.; Salinas, I.; Sanmartí, A.; Espinal, A.; Serra, A.; Navarro, M.; Bonal, J.; et al. Obesity, Adiponectin and Inflammation as Predictors of New-Onset Diabetes Mellitus After Kidney Transplantation. Am. J. Transplant. 2007, 7, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, S11–S103. [Google Scholar] [CrossRef]
- Al-Bahri, S.; Fakhry, T.K.; Gonzalvo, J.P.; Murr, M.M. Bariatric Surgery as a Bridge to Renal Transplantation in Patients with End-Stage Renal Disease. Obes. Surg. 2017, 13, 336–2955. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Weil, E.J.; Pham, P.T.; Pomeroy, J.; Knowler, W.C. Can New-Onset Diabetes after Kidney Transplant Be Prevented? Diabetes Care 2013, 36, 1406–1412. [Google Scholar] [CrossRef]
- Montgomery, J.R.; Cohen, J.A.; Brown, C.S.; Sheetz, K.H.; Chao, G.F.; Waits, S.A.; Telem, D.A. Perioperative Risks of Bariatric Surgery among Patients with and without History of Solid Organ Transplant. Am. J. Transplant. 2020, 20, 2530–2539. [Google Scholar] [CrossRef]
- Fagenson, A.M.; Mazzei, M.M.; Zhao, H.; Lu, X.; Edwards, M.A. Bariatric Surgery Outcomes in Patients with Prior Solid Organ Transplantation: An MBSAQIP Analysis. Obes. Surg. 2020, 30, 2313–2324. [Google Scholar] [CrossRef]
- Schindel, H.; Winkler, J.; Yemini, R.; Carmeli, I.; Nesher, E.; Keidar, A. Survival Benefit in Bariatric Surgery Kidney Recipients May Be Mediated through Effects on Kidney Graft Function and Improvement of Co-Morbidities: A Case-Control Study. Surg. Obes. Relat. Dis. 2019, 15, 621–627. [Google Scholar] [CrossRef]
- Oniscu, G.C.; Abramowicz, D.; Bolignano, D.; Gandolfini, I.; Hellemans, R.; Maggiore, U.; Nistor, I.; O’Neill, S.; Sever, M.S.; Koobasi, M.; et al. Management of Obesity in Kidney Transplant Candidates and Recipients: A Clinical Practice Guideline by the DESCARTES Working Group of ERA. Nephrol. Dial. Transplant 2021, 37, i1–i15. [Google Scholar] [CrossRef]
- Lee, Y.; Raveendran, L.; Lovrics, O.; Tian, C.; Khondker, A.; Koyle, M.A.; Farcas, M.; Doumouras, A.G.; Hong, D. The Role of Bariatric Surgery on Kidney Transplantation: A Systematic Review and Meta-Analysis. Can. Urol. Assoc. J. 2021, 15, E553–E562. [Google Scholar] [CrossRef] [PubMed]
- Veroux, M.; Mattone, E.; Cavallo, M.; Gioco, R.; Corona, D.; Volpicelli, A.; Veroux, P. Obesity and Bariatric Surgery in Kidney Transplantation: A Clinical Review. World J. Diabetes 2021, 12, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Marrahi, E.; Molina-Andújar, A.; Rovira, J.; Revuelta, I.; Ventura-Aguiar, P.; Piñeiro, G.; Ugalde-Altamirano, J.; Perna, F.; Torregrosa, J.-V.; Oppenheimer, F.; et al. The Impact of Functional Delayed Graft Function in the Modern Era of Kidney Transplantation - A Retrospective Study. Transpl. Int. 2021, 34, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical Practice Guidelines of the European Association for Endoscopic Surgery (EAES) on Bariatric Surgery: Update 2020 Endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, M.; Di Cocco, P.; Tulla, K.; Kaylan, K.B.; Masrur, M.A.; Hassan, C.; Alvarez, J.A.; Benedetti, E.; Tzvetanov, I. Simultaneous Robotic Kidney Transplantation and Bariatric Surgery for Morbidly Obese Patients with End-Stage Renal Failure. Am. J. Transplant 2021, 21, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Elli, E.F. Outcomes of Bariatric Surgery After Solid Organ Transplantation. Obes. Surg. 2020, 30, 4899–4904. [Google Scholar] [CrossRef]
- Outmani, L.; Kimenai, H.J.A.N.; Roodnat, J.I.; Leeman, M.; Biter, U.L.; Klaassen, R.A.; IJzermans, J.N.M.; Minnee, R.C. Clinical Outcome of Kidney Transplantation after Bariatric Surgery: A Single-Center, Retrospective Cohort Study. Clin. Transplant. 2021, 35, e14208. [Google Scholar] [CrossRef]
- Freeman, C.M.; Woodle, E.S.; Shi, J.; Alexander, J.W.; Leggett, P.L.; Shah, S.A.; Paterno, F.; Cuffy, M.C.; Govil, A.; Mogilishetty, G.; et al. Addressing Morbid Obesity as a Barrier to Renal Transplantation with Laparoscopic Sleeve Gastrectomy. Am. J. Transplant 2015, 15, 1360–1368. [Google Scholar] [CrossRef]
- Hill, C.J.; Courtney, A.E.; Cardwell, C.R.; Maxwell, A.P.; Lucarelli, G.; Veroux, M.; Furriel, F.; Cannon, R.M.; Hoogeveen, E.K.; Doshi, M.; et al. Recipient Obesity and Outcomes after Kidney Transplantation: A Systematic Review and Meta-Analysis. Nephrol. Dial. Transplant 2015, 30, 1403–1411. [Google Scholar] [CrossRef]
- Cohen, J.B.; Lim, M.A.; Tewksbury, C.M.; Torres-Landa, S.; Trofe-Clark, J.; Abt, P.L.; Williams, N.N.; Dumon, K.R.; Goral, S. Bariatric Surgery before and after Kidney Transplantation: Long-Term Weight Loss and Allograft Outcomes. Surg. Obes. Relat. Dis. 2019, 15, 935–941. [Google Scholar] [CrossRef]
- Kim, Y.; Bailey, A.J.; Morris, M.C.; Kassam, A.F.; Shah, S.A.; Diwan, T.S. Kidney Transplantation after Sleeve Gastrectomy in the Morbidly Obese Candidate: Results of a 2-Year Experience. Surg. Obes. Relat. Dis. 2020, 16, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Gheith, O.; Al-Otaibi, T.; Halim, M.A.; Mahmoud, T.; Mosaad, A.; Yagan, J.; Zakaria, Z.; Rida, S.; Nair, P.; Hassan, R. Bariatric Surgery in Renal Transplant Patients. Exp. Clin. Transplant. 2017, 15, 164–169. [Google Scholar] [CrossRef]
- Alexander, J.W.; Goodman, H.R.; Gersin, K.; Cardi, M.; Austin, J.; Goel, S.; Safdar, S.; Huang, S.; Woodle, E.S. Gastric Bypass in Morbidly Obese Patients with Chronic Renal Failure and Kidney Transplant. Transplantation 2004, 78, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Modanlou, K.A.; Muthyala, U.; Xiao, H.; Schnitzler, M.A.; Salvalaggio, P.R.; Brennan, D.C.; Abbott, K.C.; Graff, R.J.; Lentine, K.L. Bariatric Surgery among Kidney Transplant Candidates and Recipients: Analysis of the United States Renal Data System and Literature Review. Transplantation 2009, 87, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Castelli, C.; Foucher, Y.; Boucquemont, J.; Prezelin-Reydit, M.; Giral, M.; Savoye, E.; Hazzan, M.; Lenain, R. Impact of Kidney Transplantation in Obese Candidates: A Time-Dependent Propensity Score Matching Study. Nephrol. Dial. Transplant. 2022, 37, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Calabia, J.; Arcos, E.; Carrero, J.J.; Comas, J.; Vallés, M. Does the Obesity Survival Paradox of Dialysis Patients Differ with Age? Blood Purif. 2015, 39, 193–199. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, H.L.; Campbell, K.L. Obesity as a Barrier to Kidney Transplantation: Time to Eliminate the Body Weight Bias? Semin. Dial. 2019, 32, 219–222. [Google Scholar] [CrossRef]
| BS-Post-KTx n = 6 | Baseline | 1 Year after BS | p Value |
|---|---|---|---|
| Sex (female) n (%) | 3 (50.0) | - | |
| Age (years) | 48.1 (28.6–53.1) | - | |
| Weight (Kg) | 110.7 (104.0–120.0) | 79.3 (66.5–94.7) | 0.027 |
| BMI (Kg/m2) | 43.9 (40.6–46.9) | 32.93 (26.7–33.7) | 0.028 |
| TWL (%) | - | 26.3 (20.5–28.0) | - |
| Hypertension, n (%) | 5 (83.3) | 5 (83.3) | 0.999 |
| Diabetes, n (%) | 1 (16.7) | 1 (16.7) | 0.999 |
| Serum creatinine (mg/dL) | 1.40 (1.1–1.7) | 1.53 (1.1–1.6) | 0.500 |
| eGFR (mL/min/1.73 m2) | 45.1 (40.5–62.9) | 47.8 (45.6–58.6) | 0.500 |
| Glucose (mg/dL) | 94 (86–109) | 84 (77–90) | 0.459 |
| Haemoglobin A1c (%) | 5.4 (5.15–5.6) | 4.8 (4.8–5.1) | 0.090 |
| Total Cholesterol (mg/dL) | 195 (180–232) | 203 (169–210) | 0.917 |
| LDL-Cholesterol (mg/dL) | 86 (79–143) | 113 (103–116) | 0.686 |
| Triglycerides (mg/dL) | 205 (158–309) | 103 (84–118) | 0.046 |
| BS-pre-KTx n = 5 | Baseline | 1 year after BS | p value |
| Sex (female), n (%) | 1 (20.0) | - | |
| Age (years) | 38.6 (34.7–61.1) | - | |
| Weight (Kg) | 129.0 (120.0–135.0) | 81.0 (80.5–82.0) | 0.043 |
| BMI (Kg/m2) | 41.7 (39.8–46.9) | 26.8 (25.9–27.4) | 0.043 |
| TWL (%) | - | 40.4 (31.7–41.7) | - |
| Hypertension, n (%) | 4 (80.0) | 1 (20.0) | 0.250 |
| Diabetes, n (%) | 3 (60.0) | 2 (40.0) | 0.999 |
| Glucose (mg/dL) | 92 (77–97) | 86 (72–88) | 0.345 |
| Haemoglobin A1c (%) | 5.9 (5.8–7.2) | 5.5 (4.8–5.7) | 0.080 |
| Total Cholesterol (mg/dL) | 182 (133–208) | 194 (183–247) | 0.500 |
| LDL-Cholesterol (mg/dL) | 79 (63–161.5) | 120 (105.5–151) | 0.715 |
| Triglycerides (mg/dL) | 163 (122–356) | 132 (118–182) | 0.225 |
| BS-Post-KTx n = 6 | BS-Pre-KTx n = 5 | ||
|---|---|---|---|
| Bariatric surgery parameters | |||
| Length of stay (days) | 4.5 (3.9–5.9) | 6.5 (3.5–8.8) | |
| Surgical time (min) | 90 (85–90) | 110 (85–130) | |
| Post-operative complications and clinical intercurrences | |||
| Early complications (<30 days) | 1 (16.7) | 1 (20.0) | |
| Surgical site infection | 1 (16.7) | 0 (0) | |
| Nausea and vomiting requiring IV fluids | 0 (0) | 1 (16.7) | |
| Late complications (>30 days) | 0 (0) | 0 (0) | |
| Kidney transplantation parameters | |||
| Length of stay (days) | 12.0 (8.3–15.2) | 16.7 (9.7–20.3) | |
| Post-operative complications and clinical intercurrences | |||
| Acute tubular necrosis | 0 (0) | 1 (20.0) | |
| Delayed graft function | 1 (16.7) | 0 (0) | |
| Renal graft function and survival | |||
| Rejection before BS | 2 (33.3) | - | |
| Time since transplantation (months) | 1.9 (0.5–3.3) | - | |
| Rejection after BS | 2 (33.3) | 1 (20.0) | |
| Time since transplantation (months) | 71.9 (65.4–78.5) | 7.1 | |
| Time since bariatric surgery (months) | 38.7 (33.9–43.4) | 18.6 | |
| Graft Dysfunction # | 4 (66.7) | 3 (60.0) | |
| Need for renal replacement therapy | 2 (33.3) | 1 (20.0) | |
| Time since transplantation (years) | 13.4 (8.00–17.1) | 7.1 (0.7–10.6) | |
| Time since bariatric surgery (years) | 6.1 (3.6–14.2) | 8.3 (1.6–11.2) | |
| Mortality | |||
| n/cause | 1 (16.7) COVID-19 | 1 (20.0) septic shock ¢ | |
| Time since bariatric surgery (years) | 6.2 | 3.1 | |
| Time since renal transplant (years) | 10.6 | 2.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pané, A.; Molina-Andujar, A.; Olbeyra, R.; Romano-Andrioni, B.; Boswell, L.; Montagud-Marrahi, E.; Jiménez, A.; Ibarzabal, A.; Viaplana, J.; Ventura-Aguiar, P.; et al. Bariatric Surgery Outcomes in Patients with Kidney Transplantation. J. Clin. Med. 2022, 11, 6030. https://doi.org/10.3390/jcm11206030
Pané A, Molina-Andujar A, Olbeyra R, Romano-Andrioni B, Boswell L, Montagud-Marrahi E, Jiménez A, Ibarzabal A, Viaplana J, Ventura-Aguiar P, et al. Bariatric Surgery Outcomes in Patients with Kidney Transplantation. Journal of Clinical Medicine. 2022; 11(20):6030. https://doi.org/10.3390/jcm11206030
Chicago/Turabian StylePané, Adriana, Alicia Molina-Andujar, Romina Olbeyra, Bárbara Romano-Andrioni, Laura Boswell, Enrique Montagud-Marrahi, Amanda Jiménez, Ainitze Ibarzabal, Judith Viaplana, Pedro Ventura-Aguiar, and et al. 2022. "Bariatric Surgery Outcomes in Patients with Kidney Transplantation" Journal of Clinical Medicine 11, no. 20: 6030. https://doi.org/10.3390/jcm11206030
APA StylePané, A., Molina-Andujar, A., Olbeyra, R., Romano-Andrioni, B., Boswell, L., Montagud-Marrahi, E., Jiménez, A., Ibarzabal, A., Viaplana, J., Ventura-Aguiar, P., Amor, A. J., Vidal, J., Flores, L., & de Hollanda, A. (2022). Bariatric Surgery Outcomes in Patients with Kidney Transplantation. Journal of Clinical Medicine, 11(20), 6030. https://doi.org/10.3390/jcm11206030









