Oral Anticoagulant Discontinuation and Its Predictors in Patients with Atrial Fibrillation
Abstract
1. Introduction
2. Methods
2.1. Data Source
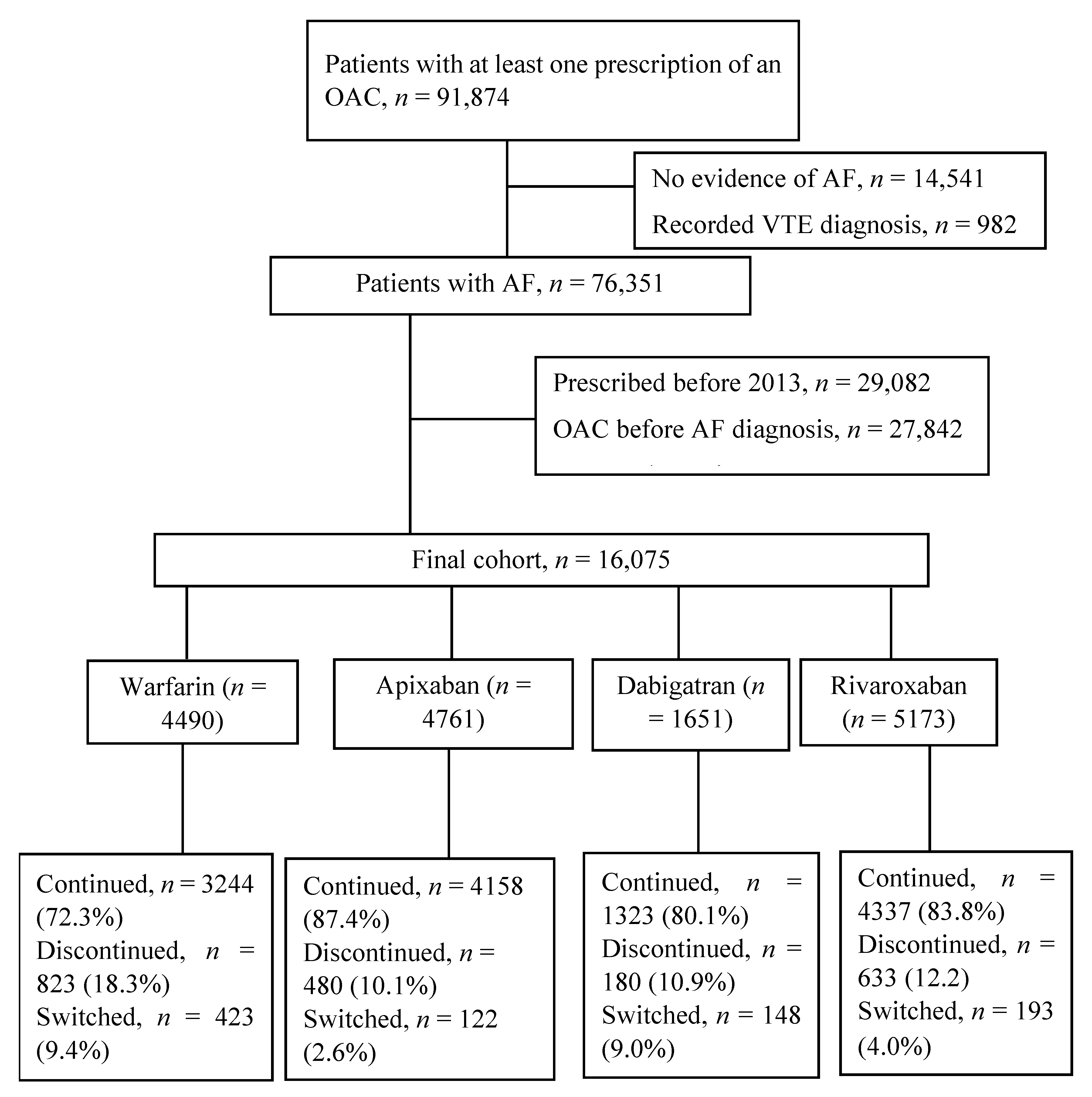
2.2. Study Population and Follow-Up
2.3. Study Outcomes
2.4. Study Covariates
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Discontinuation of OAC Therapy
3.3. Predictors of OAC Discontinuation
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pistoia, F.; Sacco, S.; Tiseo, C.; Degan, D.; Ornello, R.; Carolei, A. The Epidemiology of Atrial Fibrillation and Stroke. Cardiol. Clin. 2016, 34, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Wodchis, W.P.; Bhatia, R.S.; Leblanc, K.; Meshkat, N.; Morra, D. A Review of the Cost of Atrial Fibrillation. Value Health 2012, 15, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study 2019 (GBD 2019); Institute for Health Metrics and Evaluation (IHME), University of Washington: Seattle, WA, USA, 2019; Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 23 October 2020).
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.; Aguilar, M.I. Meta-analysis: Antithrombotic Therapy to Prevent Stroke in Patients Who Have Nonvalvular Atrial Fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Miller, C.S.; Grandi, S.M.; Shimony, A.; Filion, K.B.; Eisenberg, M.J. Meta-Analysis of Efficacy and Safety of New Oral Anticoagulants (Dabigatran, Rivaroxaban, Apixaban) Versus Warfarin in Patients With Atrial Fibrillation. Am. J. Cardiol. 2012, 110, 453–460. [Google Scholar] [CrossRef]
- Loffredo, L.; Perri, L.; Violi, F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: A meta-analysis of interventional trials. Dig. Liver Dis. 2015, 47, 429–431. [Google Scholar] [CrossRef]
- Bezabhe, W.M.; Bereznicki, L.R.; Radford, J.; Wimmer, B.C.; Curtain, C.; Salahudeen, M.S.; Peterson, G.M. Ten-Year Trends in the Use of Oral Anticoagulants in Australian General Practice Patients With Atrial Fibrillation. Front. Pharmacol. 2021, 12, 586370. [Google Scholar] [CrossRef]
- Bezabhe, W.M.; Bereznicki, L.R.; Radford, J.; Wimmer, B.C.; Curtain, C.; Salahudeen, M.S.; Peterson, G.M. Factors influencing oral anticoagulant use in patients newly diagnosed with atrial fibrillation. Eur. J. Clin. Investig. 2020, 51, e13457. [Google Scholar] [CrossRef]
- Komen, J.J.; Heerdink, E.R.; Klungel, O.H.; Mantel-Teeuwisse, A.K.; Forslund, T.; Wettermark, B.; Hjemdahl, P. Long-term persistence and adherence with non-vitamin K oral anticoagulants in patients with atrial fibrillation and their associations with stroke risk. Eur. Hearth J. Cardiovasc. Pharmacother. 2021, 7, f72–f80. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.A.; Ortiz, M.; Freedman, B.; Waterhouse, B.J.; Colquhoun, D. Medium- to long-term persistence with non-vitamin-K oral anticoagulants in patients with atrial fibrillation: Australian experience. Curr. Med. Res. Opin. 2017, 33, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.A.; Ortiz, M.; Freedman, B.; Waterhouse, B.J.; Colquhoun, D.; Thomas, G. Improved persistence with non-vitamin-K oral anticoagulants compared with warfarin in patients with atrial fibrillation: Recent Australian experience. Curr. Med. Res. Opin. 2016, 32, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- MedicineInsight Data Book Version 4; NPS MedicineWise: Sydney, Australia, December 2021.
- Busingye, D.; Gianacas, C.; Pollack, A.; Chidwick, K.; Merrifield, A.; Norman, S.; Mullin, B.; Hayhurst, R.; Blogg, S.; Havard, A.; et al. Data Resource Profile: MedicineInsight, an Australian national primary health care database. Int. J. Epidemiology 2019, 48, 1741–1741h. [Google Scholar] [CrossRef] [PubMed]
- Youens, D.; Moorin, R.; Harrison, A.; Varhol, R.; Robinson, S.; Brooks, C.; Boyd, J. Using general practice clinical information system data for research: The case in Australia. Int. J. Popul. Data Sci. 2020, 5, 1099. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Basic, E.; Nabauer, M. Changes in Oral Anticoagulation Therapy over One Year in 51,000 Atrial Fibrillation Patients at Risk for Stroke: A Practice-Derived Study. Thromb. Haemost. 2019, 119, 882–893. [Google Scholar] [CrossRef]
- The Royal Australian College of General Practitioners. Standards for General Practices; RACGP: East Melbourne, Australia, 2020. [Google Scholar]
- Maura, G.; Billionnet, C.; Alla, F.; Gagne, J.J.; Pariente, A. Comparison of Treatment Persistence with Dabigatran or Rivaroxaban versus Vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients: A Competing Risk Analysis in the French National Health Care Databases. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 38, 6–18. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Australian Statistical Geography Standard; Australian Bureau of Statistics: Canberra, Australia, 2019. [Google Scholar]
- Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA); Australian Bureau of Statistics: Canberra, Australia, 2016. [Google Scholar]
- Bezabhe, W.M.; Bereznicki, L.R.; Radford, J.; Wimmer, B.C.; Salahudeen, M.S.; Garrahy, E.; Bindoff, I.; Peterson, G.M. Oral Anticoagulant Treatment and the Risk of Dementia in Patients With Atrial Fibrillation: A Population-Based Cohort Study. J. Am. Hearth Assoc. 2022, 11, e023098. [Google Scholar] [CrossRef]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- O’Brien, E.C.; Simon, D.N.; Thomas, L.E.; Hylek, E.M.; Gersh, B.J.; Ansell, J.E.; Kowey, P.R.; Mahaffey, K.W.; Chang, P.; Fonarow, G.C.; et al. The ORBIT bleeding score: A simple bedside score to assess bleeding risk in atrial fibrillation. Eur. Hearth J. 2015, 36, 3258–3264. [Google Scholar] [CrossRef]
- Banerjee, A.; Benedetto, V.; Gichuru, P.; Burnell, J.; Antoniou, S.; Schilling, R.J.; Strain, W.D.; Ryan, R.; Watkins, C.; Marshall, T.; et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: A population-based study. Heart 2020, 106, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Collings, S.-L.; Lefèvre, C.; Johnson, M.E.; Evans, D.; Hack, G.; Stynes, G.; Maguire, A. Oral anticoagulant persistence in patients with non-valvular atrial fibrillation: A cohort study using primary care data in Germany. PLoS ONE 2017, 12, e0185642. [Google Scholar] [CrossRef] [PubMed]
- Collings, S.-L.; Vannier-Moreau, V.; Johnson, M.E.; Stynes, G.; Lefèvre, C.; Maguire, A.; Asmar, J.; Bizouard, G.; Duhot, D.; Mouquet, F.; et al. Initiation and continuation of oral anticoagulant prescriptions for stroke prevention in non-valvular atrial fibrillation: A cohort study in primary care in France. Arch. Cardiovasc. Dis. 2018, 111, 370–379. [Google Scholar] [CrossRef]
- Ozaki, A.F.; Choi, A.S.; Le, Q.T.; Ko, D.T.; Han, J.K.; Park, S.S.; Jackevicius, C.A. Real-World Adherence and Persistence to Direct Oral Anticoagulants in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual Outcomes 2020, 13, e005969. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.R., II; Kim, S.; Blanco, R.; Thomas, L.; Ansell, J.; Fonarow, G.C.; Gersh, B.J.; Go, A.S.; Kowey, P.R.; Mahaffey, K.W. Discontinuation rates of warfarin versus direct acting oral anticoagulants in US clinical practice: Results from Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II). Am. Heart J. 2020, 226, 85–93. [Google Scholar] [CrossRef]
- Buck, J.; Fromings Hill, J.; Martin, A.; Springate, C.; Ghosh, B.; Ashton, R.; Lee, G.; Orlowski, A. Reasons for discontinuing oral anticoagulation therapy for atrial fibrillation: A systematic review. Age Ageing 2021, 50, 1108–1117. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Baker, C.L.; Dhamane, A.D.; Mardekian, J.; Dina, O.; Russ, C.; Rosenblatt, L.; Lingohr-Smith, M.; Menges, B.; Lin, J.; Nadkarni, A. Comparison of Drug Switching and Discontinuation Rates in Patients with Nonvalvular Atrial Fibrillation Treated with Direct Oral Anticoagulants in the United States. Adv. Ther. 2019, 36, 162–174. [Google Scholar] [CrossRef]
- Ruigómez, A.; Vora, P.; Balabanova, Y.; Brobert, G.; Roberts, L.; Fatoba, S.; Fernandez, O.; Rodríguez, L.A.G. Discontinuation of non-Vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation: A population-based cohort study using primary care data from The Health Improvement Network in the UK. BMJ Open 2019, 9, e031342. [Google Scholar] [CrossRef]
- Ageno, W.; Beyer-Westendorf, J.; Rubboli, A. Once- versus twice-daily direct oral anticoagulants in non-valvular atrial fibrillation. Expert Opin. Pharmacother. 2017, 18, 1325–1332. [Google Scholar] [CrossRef]
- Fralick, M.; Colacci, M.; Schneeweiss, S.; Huybrechts, K.F.; Lin, K.J.; Gagne, J.J. Effectiveness and Safety of Apixaban Compared With Rivaroxaban for Patients With Atrial Fibrillation in Routine Practice: A Cohort Study. Ann. Intern. Med. 2020, 172, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Kefale, A.T.; Peterson, G.M.; Bezabhe, W.M.; Bereznicki, L.R. Switching of oral anticoagulants in patients with non-valvular atrial fibrillation: A narrative review. Br. J. Clin. Pharmacol. 2022, 88, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Marchetti, G.; Bernardini, F.; Urbinati, S. Switching between direct oral anticoagulants: A systematic review and me-ta-analysis. J. Thromb. Thrombolysis 2021, 52, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Kefale, A.T.; Peterson, G.M.; Bezabhe, W.M.; Bereznicki, L.R. Switching of oral anticoagulants in atrial fibrillation: A cohort study using Aus-tralian general practice data. Expert Rev. Clin. Pharmacol. 2022, 15, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lowres, N.; Giskes, K.; Hespe, C.; Freedman, B. Reducing Stroke Risk in Atrial Fibrillation: Adherence to Guidelines Has Improved, but Patient Persistence with Anticoagulant Therapy Remains Suboptimal. Korean Circ. J. 2019, 49, 883–907. [Google Scholar] [CrossRef]
- Hellfritzsch, M.; Husted, S.E.; Grove, E.L.; Rasmussen, L.; Poulsen, M.H.; Johnsen, S.P.; Hallas, J.; Pottegård, A. Treatment Changes among Users of Non-Vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation. Basic Clin. Pharmacol. Toxicol. 2017, 120, 187–194. [Google Scholar] [CrossRef]
- Dhamane, A.D.; Hernandez, I.; Di Fusco, M.; Gutierrez, C.; Ferri, M.; Russ, C.; Tsai, W.-L.; Emir, B.; Yuce, H.; Keshishian, A. Non-persistence to Oral Anticoagulation Treatment in Patients with Non-valvular Atrial Fibrillation in the USA. Am. J. Cardiovasc. Drugs 2022, 22, 333–343. [Google Scholar] [CrossRef]
| Variables | All Patients (%) (n = 16,075) | Persisted (%) (n = 13,959) | Discontinued (%) (n = 2116) | p-Value |
|---|---|---|---|---|
| Follow up in days, Mean (SD) | 329.7 (79.1) | 348.9 (64.3) | 202.9 (42.0) | <0.001 |
| Sex, female | 7602 (47.3) | 6642 (47.6) | 960 (45.4) | 0.057 |
| Age, mean (SD), years | 74.6 (10.2) | 74.8 (9.8) | 73.4 (12.4) | <0.001 |
| Age group | <0.001 | |||
| <65 years | 2439 (15.2) | 1981 (14.2) | 458 (21.6) | |
| 65–74 years | 5002 (31.1) | 4421 (31.7) | 581 (27.5) | |
| ≥75 years | 8634 (53.7) | 7557 (54.1) | 1077 (50.9) | |
| Index year | <0.001 | |||
| 2013 | 2629 (16.4) | 2175 (15.6) | 454 (21.5) | |
| 2014 | 2986 (18.6) | 2590 (18.6) | 396 (18.7) | |
| 2015 | 3159 (19.6) | 2759 (19.8) | 400 (18.9) | |
| 2016 | 3431 (21.3) | 3027 (21.7) | 404 (19.1) | |
| 2017 | 3870 (24.1) | 3408 (24.4) | 462 (21.8) | |
| ATSI | n = 13,542 | n = 11,828 | n = 1714 | 0.72 |
| Yes | 177 (1.3) | 153 (1.3) | 24 (1.4) | |
| No | 13,365 (98.7) | 11,675 (98.7) | 1690 (98.6) | |
| Rurality | n = 15,994 | n = 13,897 | n = 2097 | <0.001 |
| Major cities | 9452 (59.1) | 8225 (59.2) | 1227 (58.5) | |
| Inner regional | 4863 (30.4) | 4270 (30.7) | 593 (28.3) | |
| Outer regional | 1444 (9.0) | 1204 (8.7) | 240 (11.4) | |
| Remote/very remote | 235 (1.5) | 198 (1.4) | 37 (1.8) | |
| SEIFA quintiles | n = 15,975 | n = 13,750 | n = 2225 | 0.18 |
| 1 | 2626 (16.4) | 2239 (16.3) | 387 (17.4) | |
| 2 | 3418 (21.4) | 2956 (21.5) | 462 (20.8) | |
| 3 | 4029 (25.2) | 3505 (25.5) | 524 (23.6) | |
| 4 | 2724 (17.0) | 2338 (17.0) | 386 (17.3) | |
| 5 | 3178 (19.9) | 2712 (19.5) | 466 (20.9) | |
| Comorbidities | ||||
| Hypertension | 11,397 (70.9) | 10,045 (72.0) | 1352 (63.9) | <0.001 |
| Heart failure | 4206 (26.2) | 3694 (26.5) | 512 (24.2) | 0.027 |
| Diabetes | 4080 (25.4) | 3609 (25.8) | 471 (22.3) | <0.001 |
| Stroke | 3135 (19.5) | 2760 (19.8) | 375 (17.7) | 0.027 |
| Vascular disease | 6187 (38.5) | 5391(38.6) | 796 (37.6) | 0.38 |
| Anxiety | 2802 (17.4) | 2426 (17.4) | 376 (17.8) | 0.66 |
| Arthritis | 9768 (60.8) | 8628 (61.8) | 1140 (53.9) | <0.001 |
| Asthma | 2959 (18.4) | 2594 (18.6) | 365 (17.2) | 0.14 |
| COPD | 2721 (16.9) | 2365 (16.9) | 356 (16.8) | 0.89 |
| Depression | 4015 (25.0) | 3510 (25.2) | 505 (23.9) | 0.20 |
| Dementia | 724 (4.5) | 621 (4.4) | 103 (4.9) | 0.39 |
| eGFR in mL/min | n = 12,736 | n = 11,135 | n = 1601 | 0.16 |
| ≥60 | 6752 (53.0) | 5886 (52.9) | 866 (54.1) | |
| 45–59 | 2992 (23.5) | 2635 (23.7) | 357 (22.3) | |
| 30–44 | 2027 (15.9) | 1787 (16.0) | 240 (15.0) | |
| <30 | 965 (7.6) | 827 (7.4) | 138 (8.6) | |
| CHA2DS2-VASc risk score | ||||
| Mean (SD) | 3.9 (1.8) | 3.9 (1.7) | 3.6 (2.0) | <0.001 |
| 0 | 558 (3.5) | 364 (2.6) | 194 (9.2) | <0.001 |
| 1 | 853 (5.3) | 695 (5.0) | 158 (7.5) | |
| ≥2 | 14,664 (91.2) | 12,900 (92.4) | 1764 (83.4) | |
| ORBIT risk | ||||
| Mean (SD) | 1.9 (1.4) | 1.9 (1.4) | 2.1 (1.5) | <0.001 |
| Low | 10,661 (66.3) | 9351 (67.0) | 1310 (61.9) | <0.001 |
| Medium | 2991 (18.6) | 2568 (18.4) | 423 (20.0) | |
| High | 2423 (15.1) | 2040 (14.6) | 383 (18.1) |
| AOR (95% CI) | p-Value | |
|---|---|---|
| Age category | ||
| <65 years | Ref | |
| 65–74 years | 0.60 (0.52–0.71) | <0.001 |
| ≥75 years | 0.68 (0.56–0.76) | <0.001 |
| Rurality | ||
| Major cities | 0.94 (0.60–1.52) | 0.78 |
| Inner regional | 0.85 (0.54–1.38) | 0.49 |
| Outer regional | 1.26 (0.80–2.04) | 0.33 |
| Remote/very remote | Ref | |
| Comorbidities | ||
| Hypertension | 0.73 (0.65–0.82) | <0.001 |
| Diabetes | 0.88 (0.77–0.99) | 0.043 |
| Stroke | 0.94 (0.88–1.01) | 0.081 |
| Arthritis | 0.81 (0.72–0.90) | 0.0001 |
| Anxiety | 1.13 (0.98–1.29) | 0.086 |
| OAC | ||
| Dabigatran | Ref | |
| Warfarin | 1.70 (1.39–2.08) | <0.001 |
| Rivaroxaban | 1.06 (0.87–1.30) | 0.54 |
| Apixaban | 0.90 (0.73–1.10) | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kefale, A.T.; Bezabhe, W.M.; Peterson, G.M. Oral Anticoagulant Discontinuation and Its Predictors in Patients with Atrial Fibrillation. J. Clin. Med. 2022, 11, 6022. https://doi.org/10.3390/jcm11206022
Kefale AT, Bezabhe WM, Peterson GM. Oral Anticoagulant Discontinuation and Its Predictors in Patients with Atrial Fibrillation. Journal of Clinical Medicine. 2022; 11(20):6022. https://doi.org/10.3390/jcm11206022
Chicago/Turabian StyleKefale, Adane Teshome, Woldesellassie M. Bezabhe, and Gregory M. Peterson. 2022. "Oral Anticoagulant Discontinuation and Its Predictors in Patients with Atrial Fibrillation" Journal of Clinical Medicine 11, no. 20: 6022. https://doi.org/10.3390/jcm11206022
APA StyleKefale, A. T., Bezabhe, W. M., & Peterson, G. M. (2022). Oral Anticoagulant Discontinuation and Its Predictors in Patients with Atrial Fibrillation. Journal of Clinical Medicine, 11(20), 6022. https://doi.org/10.3390/jcm11206022







