Rethinking the Body in the Brain after Spinal Cord Injury
Abstract
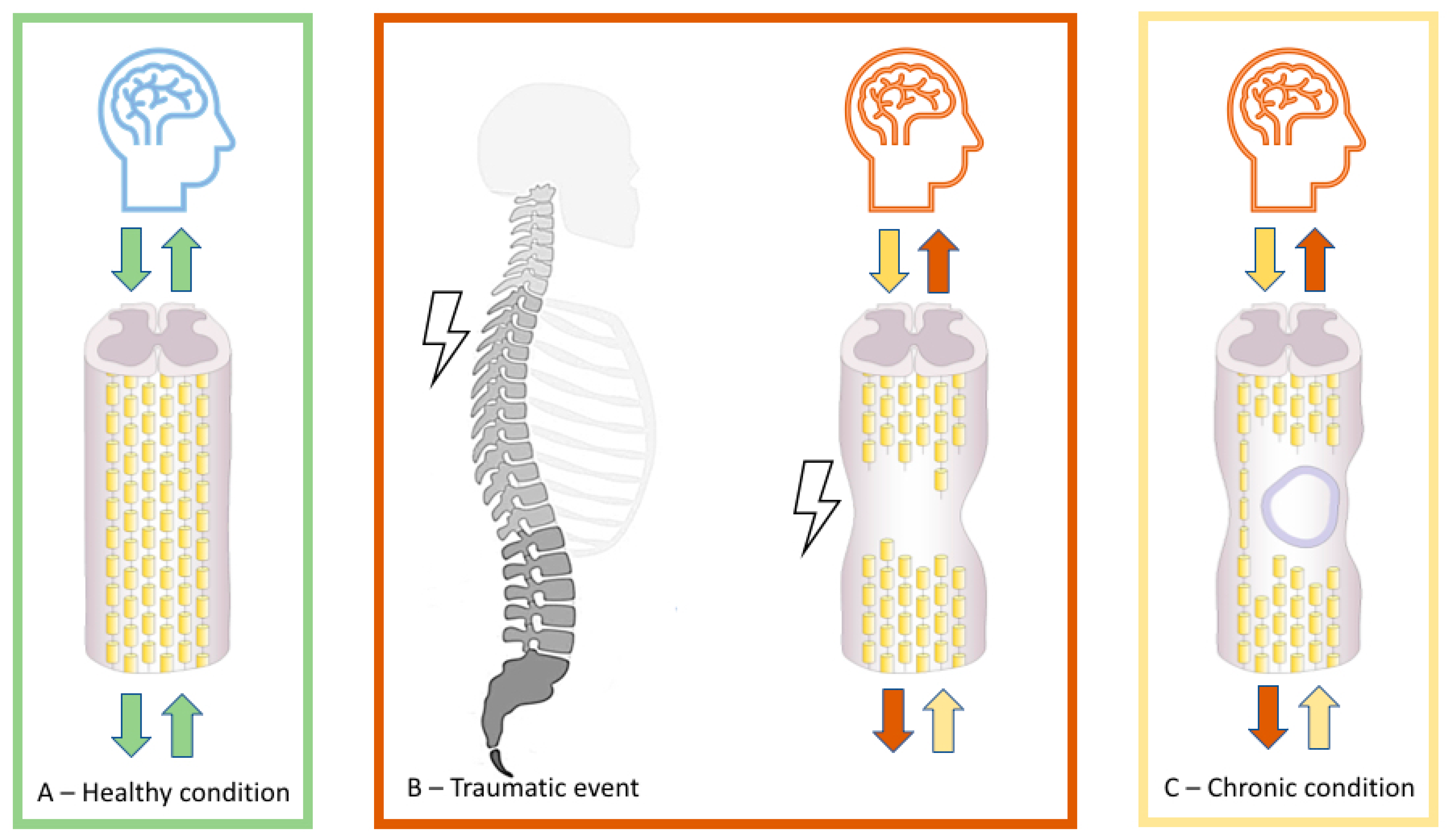
1. Introduction
2. Neural Plasticity following SCI
3. Body Reorganization and Body Stability of Somatosensory-Motor Topographies
3.1. Reorganization and Stability of the Lower Limb Representation
3.2. Reorganization and Stability of Upper Limb Representation
3.3. Reorganization and Stability of Face Representation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Tykocki, T.; Poniatowski, Ł.A.; Czyz, M.; Wynne-Jones, G. Oblique corpectomy in the cervical spine. Spinal Cord 2017, 56, 426–435. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Turczyn, P.; Frasuńska, J.; Paradowska-Gorycka, A.; Tarnacka, B. Significance of Omega-3 Fatty Acids in the Prophylaxis and Treatment after Spinal Cord Injury in Rodent Models. Mediat. Inflamm. 2020, 2020, 3164260. [Google Scholar] [CrossRef]
- Silva, N.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef]
- Nas, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.R.; Azañón, E.; Haggard, P. More than skin deep: Body representation beyond primary somatosensory cortex. Neuropsychologia 2010, 48, 655–668. [Google Scholar] [CrossRef]
- Cadotte, D.W.; Bosma, R.; Mikulis, D.; Nugaeva, N.; Smith, K.; Pokrupa, R.; Islam, O.; Stroman, P.W.; Fehlings, M.G. Plasticity of the Injured Human Spinal Cord: Insights Revealed by Spinal Cord Functional MRI. PLoS ONE 2012, 7, e45560. [Google Scholar] [CrossRef]
- Matsubayashi, K.; Nagoshi, N.; Komaki, Y.; Kojima, K.; Shinozaki, M.; Tsuji, O.; Iwanami, A.; Ishihara, R.; Takata, N.; Matsumoto, M.; et al. Assessing cortical plasticity after spinal cord injury by using resting-state functional magnetic resonance imaging in awake adult mice. Sci. Rep. 2018, 8, 14406. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.-S.; Park, J.W.; Jin, S.U.; Jang, K.E.; Nam, H.U.; Lee, Y.-S.; Jung, T.-D.; Chang, Y. Alteration of Resting-State Brain Sensorimotor Connectivity following Spinal Cord Injury: A Resting-State Functional Magnetic Resonance Imaging Study. J. Neurotrauma 2015, 32, 1422–1427. [Google Scholar] [CrossRef]
- Freund, P.; Weiskopf, N.; Ashburner, J.; Wolf, K.; Sutter, R.; Altmann, D.R.; Friston, K.; Thompson, A.; Curt, A. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: A prospective longitudinal study. Lancet Neurol. 2013, 12, 873–881. [Google Scholar] [CrossRef]
- Hou, J.; Xiang, Z.; Yan, R.; Zhao, M.; Wu, Y.; Zhong, J.; Guo, L.; Li, H.; Wang, J.; Wu, J.; et al. Motor recovery at 6 months after admission is related to structural and functional reorganization of the spine and brain in patients with spinal cord injury. Hum. Brain Mapp. 2016, 37, 2195–2209. [Google Scholar] [CrossRef]
- Oni-Orisan, A.; Kaushal, M.; Li, W.; Leschke, J.; Ward, B.D.; Vedantam, A.; Kalinosky, B.; Budde, M.D.; Schmit, B.D.; Li, S.-J.; et al. Alterations in Cortical Sensorimotor Connectivity following Complete Cervical Spinal Cord Injury: A Prospective Resting-State fMRI Study. PLoS ONE 2016, 11, e0150351. [Google Scholar] [CrossRef]
- Bunge, R.P.; Puckett, W.R.; Becerra, J.L.; Marcillo, A.; Quencer, R.M. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 1993, 59, 75–89. [Google Scholar] [PubMed]
- Bunge, R.P.; Puckett, W.R.; Hiester, E.D. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 1997, 72, 305–315. [Google Scholar] [PubMed]
- Guest, J.; Hiester, E.; Bunge, R. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 2005, 192, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Robert, S.; Huang, W.; Young, W.; Cotman, C.W. Activation of Complement Pathways after Contusion-Induced Spinal Cord Injury. J. Neurotrauma 2004, 21, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Grabher, P.; Blaiotta, C.; Ashburner, J.; Freund, P. Relationship between brainstem neurodegeneration and clinical impairment in traumatic spinal cord injury. NeuroImage Clin. 2017, 15, 494–501. [Google Scholar] [CrossRef]
- Grabher, P.; Callaghan, M.F.; Ashburner, J.; Weiskopf, N.; Thompson, A.; Curt, A.; Freund, P. Tracking sensory system atrophy and outcome prediction in spinal cord injury. Ann. Neurol. 2015, 78, 751–761. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, W.; Chen, X.; Li, X.; Wang, L.; Qin, W.; Li, K.; Chen, N. Reorganization of the somatosensory pathway after subacute incomplete cervical cord injury. NeuroImage Clin. 2019, 21, 101674. [Google Scholar] [CrossRef]
- Bruehlmeier, M.; Dietz, V.; Leenders, K.L.; Roelcke, U.; Missimer, J.; Curt, A. How does the human brain deal with a spinal cord injury? Eur. J. Neurosci. 1998, 10, 3918–3922. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Humanes-Valera, D.; Calviño, E.A.; Yague, J.G.; Moxon, K.A.; Oliviero, A.; Foffani, G. Spinal Cord Injury Immediately Changes the State of the Brain. J. Neurosci. 2010, 30, 7528–7537. [Google Scholar] [CrossRef] [PubMed]
- Jutzeler, C.R.; Huber, E.; Callaghan, M.F.; Luechinger, R.; Curt, A.; Kramer, J.L.K.; Freund, P. Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. 2016, 6, 18534. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Gustin, S.M.; Macey, P.M.; Wrigley, P.J.; Siddall, P.J. Functional Reorganization of the Brain in Humans Following Spinal Cord Injury: Evidence for Underlying Changes in Cortical Anatomy. J. Neurosci. 2011, 31, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Dou, W.-B.; Wang, Y.-H.; Luo, H.-W.; Ge, Y.-X.; Yan, S.-Y.; Xu, Q.; Tu, Y.-Y.; Xiao, Y.-Q.; Wu, Q.; et al. Non-concomitant cortical structural and functional alterations in sensorimotor areas following incomplete spinal cord injury. Neural Regen. Res. 2017, 12, 2059–2066. [Google Scholar] [CrossRef]
- Adams, M.M.; Hicks, A.L. Spasticity after spinal cord injury. Spinal Cord 2005, 43, 577–586. [Google Scholar] [CrossRef]
- Burchiel, K.J.; Hsu, F.P.K. Pain and Spasticity After Spinal Cord Injury. Spine 2001, 26, S146–S160. [Google Scholar] [CrossRef]
- Karlsson, A.-K. Overview: Autonomic dysfunction in spinal cord injury: Clinical presentation of symptoms and signs. Glial Cell Funct. 2006, 152, 1–8. [Google Scholar] [CrossRef]
- Yoon, E.J.; Kim, Y.K.; Shin, H.I.; Lee, Y.; Kim, S.E. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 2013, 1540, 64–73. [Google Scholar] [CrossRef]
- Gustin, S.M.; Wrigley, P.J.; Youssef, A.M.; McIndoe, L.; Wilcox, S.L.; Rae, C.D.; Edden, R.A.; Siddall, P.J.; Henderson, L.A. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain 2014, 155, 1027–1036. [Google Scholar] [CrossRef]
- Zhao, P.; Hill, M.; Liu, S.; Chen, L.; Bangalore, L.; Waxman, S.G.; Tan, A.M. Dendritic spine remodeling following early and late Rac1 inhibition after spinal cord injury: Evidence for a pain biomarker. J. Neurophysiol. 2016, 115, 2893–2910. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zheng, L.; Acosta, G.; Vega-Alvarez, S.; Chen, Z.; Muratori, B.; Cao, P.; Shi, R. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J. Neurochem. 2015, 135, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Meisner, J.G.; Marsh, A.D.; Marsh, D.R. Loss of GABAergic Interneurons in Laminae I–III of the Spinal Cord Dorsal Horn Contributes to Reduced GABAergic Tone and Neuropathic Pain after Spinal Cord Injury. J. Neurotrauma 2010, 27, 729–737. [Google Scholar] [CrossRef]
- Lucci, G.; Pazzaglia, M. Towards multiple interactions of inner and outer sensations in corporeal awareness. Front. Hum. Neurosci. 2015, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.L.; De Bartolo, M.; Leemhuis, E.; Pazzaglia, M. Rebuilding Body–Brain Interaction from the Vagal Network in Spinal Cord Injuries. Brain Sci. 2021, 11, 1084. [Google Scholar] [CrossRef]
- Curt, A.; Alkadhi, H.; Crelier, G.R.; Boendermaker, S.H.; Hepp-Reymond, M.-C.; Kollias, S.S. Changes of non-affected upper limb cortical representation in paraplegic patients as assessed by fMRI. Brain 2002, 125, 2567–2578. [Google Scholar] [CrossRef]
- Corbetta, M.; Burton, H.; Sinclair, R.J.; Conturo, T.E.; Akbudak, E.; McDonald, J.W. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 17066–17071. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Zheng, W.; Chen, X.; Wang, L.; Qin, W.; Li, K.; Lu, J.; Chen, N. Inconsistency between cortical reorganization and functional connectivity alteration in the sensorimotor cortex following incomplete cervical spinal cord injury. Brain Imaging Behav. 2019, 14, 2367–2377. [Google Scholar] [CrossRef]
- Huynh, V.; Rosner, J.; Curt, A.; Kollias, S.; Hubli, M.; Michels, L. Disentangling the Effects of Spinal Cord Injury and Related Neuropathic Pain on Supraspinal Neuroplasticity: A Systematic Review on Neuroimaging. Front. Neurol. 2020, 10. [Google Scholar] [CrossRef]
- Widerström-Noga, E.; Cruz-Almeida, Y.; Felix, E.R.; Pattany, P.M. Somatosensory phenotype is associated with thalamic metabolites and pain intensity after spinal cord injury. Pain 2015, 156, 166–174. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Chen, Q.; Hu, Y.; Du, J.; Chen, X.; Zheng, W.; Lu, J.; Chen, N. The Reorganization of Insular Subregions in Individuals with Below-Level Neuropathic Pain following Incomplete Spinal Cord Injury. Neural Plast. 2020, 2020, 2796571. [Google Scholar] [CrossRef]
- Bors, E. Phantom limbs of patients with spinal cord injury. AMA Arch. Neurol. Psychiatry 1951, 66, 610–631. [Google Scholar] [CrossRef]
- Pazzaglia, M.; Leemhuis, E.; Giannini, A.M.; Haggard, P. The Homuncular Jigsaw: Investigations of Phantom Limb and Body Awareness Following Brachial Plexus Block or Avulsion. J. Clin. Med. 2019, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Scivoletto, G.; Giannini, A.M.; Leemhuis, E. My hand in my ear: A phantom limb re-induced by the illusion of body ownership in a patient with a brachial plexus lesion. Psychol. Res. 2018, 83, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Galli, G.; Lucci, G.; Scivoletto, G.; Molinari, M.; Haggard, P. Phantom limb sensations in the ear of a patient with a brachial plexus lesion. Cortex 2019, 117, 385–395. [Google Scholar] [CrossRef]
- Diaz-Segarra, N.; McKay, O.; Kirshblum, S.; Yonclas, P. Management of nonpainful supernumerary phantom limbs after incomplete spinal cord injury with visual–tactile feedback therapy: A case report. Spinal Cord Ser. Cases 2020, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Muret, D.; Makin, T.R. The homeostatic homunculus: Rethinking deprivation-triggered reorganisation. Curr. Opin. Neurobiol. 2021, 67, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, W.; Zhang, Q.; Liu, L.; Liu, J.; Zhou, S.; Shi, J.; Chen, J.; Ning, B. Reorganization of the brain in spinal cord injury: A meta-analysis of functional MRI studies. Neuroradiology 2019, 61, 1309–1318. [Google Scholar] [CrossRef]
- Dimitrijević, M.R. Residual motor functions in spinal cord injury. Adv. Neurol. 1988, 47, 138–155. [Google Scholar]
- Wrigley, P.J.; Siddall, P.J.; Gustin, S. New evidence for preserved somatosensory pathways in complete spinal cord injury: A fMRI study. Hum. Brain Mapp. 2018, 39, 588–598. [Google Scholar] [CrossRef]
- Awad, A.; Levi, R.; Waller, M.; Westling, G.; Lindgren, L.; Eriksson, J. Preserved somatosensory conduction in complete spinal cord injury: Discomplete SCI. Clin. Neurophysiol. 2020, 131, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.C.; Macedo, D.R.; Soares, A.B. Divergent Findings in Brain Reorganization After Spinal Cord Injury: A Review. J. Neuroimaging 2020, 30, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Sharp, K.G.; Gramer, R.; Page, S.J.; Cramer, S.C. Increased Brain Sensorimotor Network Activation after Incomplete Spinal Cord Injury. J. Neurotrauma 2017, 34, 623–631. [Google Scholar] [CrossRef]
- Gao, F.; Guo, Y.; Chu, H.; Yu, W.; Chen, Z.; Chen, L.; Li, J.; Yang, D.; Yang, M.; Du, L.; et al. Lower-Limb Sensorimotor Deprivation-Related Brain Activation in Patients with Chronic Complete Spinal Cord Injury. Front. Neurol. 2020, 11, 555733. [Google Scholar] [CrossRef] [PubMed]
- Krings, T.; Naujokat, C.; Keyserlingk, D.G.V. Representation of cortical motor function as revealed by stereotactic transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1998, 109, 85–93. [Google Scholar] [CrossRef]
- Fassett, H.J.; Turco, C.V.; El-Sayes, J.; Nelson, A.J. Alterations in Motor Cortical Representation of Muscles Following Incomplete Spinal Cord Injury in Humans. Brain Sci. 2018, 8, 225. [Google Scholar] [CrossRef]
- Björkman, A.; Weibull, A.; Rosén, B.; Svensson, J.; Lundborg, G. Rapid cortical reorganisation and improved sensitivity of the hand following cutaneous anaesthesia of the forearm. Eur. J. Neurosci. 2009, 29, 837–844. [Google Scholar] [CrossRef]
- Waters, R.; Moore, K.R.; Graboff, S.R.; Paris, K. Brachioradialis to flexor pollicis longus tendon transfer for active lateral pinch in the tetraplegic. J. Hand Surg. 1985, 10, 385–391. [Google Scholar] [CrossRef]
- Wester, K.; Hove, L.M.; Barndon, R.; Craven, A.R.; Hugdahl, K. Cortical Plasticity After Surgical Tendon Transfer in Tetraplegics. Front. Hum. Neurosci. 2018, 12, 234. [Google Scholar] [CrossRef]
- Käll, L.B.; Fridén, J.; Björnsdotter, M. Regional estimates of cortical thickness in brain areas involved in control of surgically restored limb movement in patients with tetraplegia. J. Spinal Cord Med. 2018, 43, 462–469. [Google Scholar] [CrossRef]
- Jain, N.; Catania, K.C.; Kaas, J.H. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nat. Cell Biol. 1997, 386, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Florence, S.L.; Qi, H.-X.; Kaas, J.H. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc. Natl. Acad. Sci. USA 2000, 97, 5546–5550. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Qi, H.-X.; Collins, C.E.; Kaas, J.H. Large-Scale Reorganization in the Somatosensory Cortex and Thalamus after Sensory Loss in Macaque Monkeys. J. Neurosci. 2008, 28, 11042–11060. [Google Scholar] [CrossRef]
- Tandon, S.; Kambi, N.; Lazar, L.; Mohammed, H.; Jain, N. Large-Scale Expansion of the Face Representation in Somatosensory Areas of the Lateral Sulcus after Spinal Cord Injuries in Monkeys. J. Neurosci. 2009, 29, 12009–12019. [Google Scholar] [CrossRef]
- Pons, T.P.; Garraghty, P.E.; Ommaya, A.K.; Kaas, J.H.; Taub, E.; Mishkin, M. Massive Cortical Reorganization After Sensory Deafferentation in Adult Macaques. Science 1991, 252, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Pollin, B.; Albe-Fessard, D. Organization of somatic thalamus in monkeys with and without section of dorsal spinal tracts. Brain Res. 1979, 173, 431–449. [Google Scholar] [CrossRef]
- Garraghty, P.; Florence, S.; Kaas, J. Ablations of areas 3a and 3b of monkey somatosensory cortex abolish cutaneous responsivity in area 1. Brain Res. 1990, 528, 165–169. [Google Scholar] [CrossRef]
- Merzenich, M.M.; Nelson, R.J.; Stryker, M.P.; Cynader, M.S.; Schoppmann, A.; Zook, J.M. Somatosensory cortical map changes following digit amputation in adult monkeys. J. Comp. Neurol. 1984, 224, 591–605. [Google Scholar] [CrossRef]
- Florence, S.; Kaas, J. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J. Neurosci. 1995, 15, 8083–8095. [Google Scholar] [CrossRef] [PubMed]
- Garraghty, P.E.; Kaas, J.H. Functional reorganization in adult monkey thalamus after peripheral nerve injury. NeuroReport 1991, 2, 747–750. [Google Scholar] [CrossRef]
- Lenz, F.A.; Kwan, H.C.; Martin, R.; Tasker, R.; Richardson, R.T.; Dostrovsky, J.O. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J. Neurophysiol. 1994, 72, 1570–1587. [Google Scholar] [CrossRef]
- Florence, S.L.; Taub, H.B.; Kaas, J.H. Large-Scale Sprouting of Cortical Connections After Peripheral Injury in Adult Macaque Monkeys. Science 1998, 282, 1117–1121. [Google Scholar] [CrossRef]
- Mikulis, D.J.; Jurkiewicz, M.T.; McIlroy, W.E.; Staines, W.R.; Rickards, L.; Kalsi–Ryan, S.; Crawley, A.P.; Fehlings, M.G.; Verrier, M.C. Adaptation in the motor cortex following cervical spinal cord injury. Neurology 2002, 58, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Scandola, M.; Tidoni, E.; Avesani, R.; Brunelli, G.; Aglioti, S.M.; Moro, V. Rubber hand illusion induced by touching the face ipsilaterally to a deprived hand: Evidence for plastic “somatotopic” remapping in tetraplegics. Front. Hum. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef]
- Moore, C.I.; Stern, C.E.; Dunbar, C.; Kostyk, S.; Gehi, A.; Corkin, S. Referred phantom sensations and cortical reorganization after spinal cord injury in humans. Proc. Natl. Acad. Sci. USA 2000, 97, 14703–14708. [Google Scholar] [CrossRef] [PubMed]
- Makin, T.; Bensmaia, S. Stability of Sensory Topographies in Adult Cortex. Trends Cogn Sci. 2017, 21, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Lee, J.; Martinez, O.; Medlin, A.; Schandler, S.; Cohen, M. Somatotopy of the motor cortex after long-term spinal cord injury or amputation. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Laubis-Herrmann, U.; Topka, H. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor. Neurol. Neurosci. 2006, 24, 97–107. [Google Scholar]
- Vaso, A.; Adahan, H.-M.; Gjika, A.; Zahaj, S.; Zhurda, T.; Vyshka, G.; Devor, M. Peripheral nervous system origin of phantom limb pain. Pain 2014, 155, 1384–1391. [Google Scholar] [CrossRef]
- Cirstea, C.M.; Choi, I.-Y.; Lee, P.; Peng, H.; Kaufman, C.L.; Frey, S.H. Magnetic resonance spectroscopy of current hand amputees reveals evidence for neuronal-level changes in former sensorimotor cortex. J. Neurophysiol. 2017, 117, 1821–1830. [Google Scholar] [CrossRef]
- Kambi, N.; Halder, P.; Rajan, R.; Arora, V.; Chand, P.; Arora, M.; Jain, N. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat. Commun. 2014, 5, 3602. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, E.; De Gennaro, L.; Pazzaglia, A.M. Disconnected Body Representation: Neuroplasticity Following Spinal Cord Injury. J. Clin. Med. 2019, 8, 2144. [Google Scholar] [CrossRef]
- Zantedeschi, M.; Pazzaglia, M. Commentary: Non-invasive Brain Stimulation, a Tool to Revert Maladaptive Plasticity in Neuropathic Pain. Front. Hum. Neurosci. 2016, 10, 544. [Google Scholar] [CrossRef]
- Leemhuis, E.; Esposito, R.; Gennaro, L.; Pazzaglia, M. Go Virtual to Get Real: Virtual Reality as a Resource for Spinal Cord Treatment. Int. J. Environ. Res. Public Health 2021, 18, 1819. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, E.; Giuffrida, V.; Giannini, A.M.; Pazzaglia, M.A. Therapeutic Matrix: Virtual Reality as a Clinical Tool for Spinal Cord Injury-Induced Neuropathic Pain. Brain Sci. 2021, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Galli, G.; Lewis, J.W.; Scivoletto, G.; Giannini, A.M.; Molinari, M. Embodying functionally relevant action sounds in patients with spinal cord injury. Sci. Rep. 2018, 8, 15641. [Google Scholar] [CrossRef] [PubMed]
- Scivoletto, G.; Galli, G.; Torre, M.; Molinari, M.; Pazzaglia, M. The Overlooked Outcome Measure for Spinal Cord Injury: Use of Assistive Devices. Front. Neurol. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Molinari, M. The re-embodiment of bodies, tools, and worlds after spinal cord injury: An intricate picture. Phys. Life Rev. 2016, 16, 191–194. [Google Scholar] [CrossRef]
- Pazzaglia, M.; Galli, G. Loss of agency in apraxia. Front. Hum. Neurosci. 2014, 8, 751. [Google Scholar] [CrossRef]
- Pazzaglia, M.; Galli, G. Action Observation for Neurorehabilitation in Apraxia. Front. Neurol. 2019, 10, 309. [Google Scholar] [CrossRef]
- Pazzaglia, M.; Galli, G. Translating novel findings of perceptual-motor codes into the neuro-rehabilitation of movement disorders. Front. Behav. Neurosci. 2015, 9, 222. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leemhuis, E.; Giuffrida, V.; De Martino, M.L.; Forte, G.; Pecchinenda, A.; De Gennaro, L.; Giannini, A.M.; Pazzaglia, M. Rethinking the Body in the Brain after Spinal Cord Injury. J. Clin. Med. 2022, 11, 388. https://doi.org/10.3390/jcm11020388
Leemhuis E, Giuffrida V, De Martino ML, Forte G, Pecchinenda A, De Gennaro L, Giannini AM, Pazzaglia M. Rethinking the Body in the Brain after Spinal Cord Injury. Journal of Clinical Medicine. 2022; 11(2):388. https://doi.org/10.3390/jcm11020388
Chicago/Turabian StyleLeemhuis, Erik, Valentina Giuffrida, Maria Luisa De Martino, Giuseppe Forte, Anna Pecchinenda, Luigi De Gennaro, Anna Maria Giannini, and Mariella Pazzaglia. 2022. "Rethinking the Body in the Brain after Spinal Cord Injury" Journal of Clinical Medicine 11, no. 2: 388. https://doi.org/10.3390/jcm11020388
APA StyleLeemhuis, E., Giuffrida, V., De Martino, M. L., Forte, G., Pecchinenda, A., De Gennaro, L., Giannini, A. M., & Pazzaglia, M. (2022). Rethinking the Body in the Brain after Spinal Cord Injury. Journal of Clinical Medicine, 11(2), 388. https://doi.org/10.3390/jcm11020388








