Accuracy of Six Intraocular Lens Power Calculations in Eyes with Axial Lengths Greater than 28.0 mm
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Procedures
2.2. Surgery and Intraocular Lenses
2.3. Retrospective and Statistical Analysis
- (1)
- Barrett Universal II (available at https://calc.apacrs.org/barrett_universal2105/, hereafter referred to as BU-II, accessed 12 September 2021);
- (2)
- Emmetropia Verifying Optical (available at https://www.evoiolcalculator.com/, referred to as EVO, accessed 12 September 2021);
- (3)
- Hill–Radial Bias Function 3.0 Calculator (available at https://rbfcalculator.com/, hereafter referred to as Hill-RBF, accessed 12 September 2021);
- (4)
- Holladay 1 [20];
- (5)
- Kane (available at https://www.iolformula.com/, accessed 12 September 2021);
- (6)
- SRK/T [21].
3. Results
3.1. Population Demographics
3.2. Accuracy of the Six Formulas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melles, R.B.; Holladay, J.T.; Chang, W.J. Accuracy of Intraocular Lens Calculation Formulas. Ophthalmology 2018, 125, 169–178. [Google Scholar] [CrossRef]
- Davis, G. The Evolution of Cataract Surgery. Mo. Med. 2016, 113, 58. [Google Scholar] [CrossRef]
- Doshi, D.; Limdi, P.; Parekh, N.; Gohil, N. A Comparative Study to Assess the Predictability of Different IOL Power Calculation Formulas in Eyes of Short and Long Axial Length. J. Clin. Diagn. Res. 2017, 11, NC01–NC04. [Google Scholar] [CrossRef] [PubMed]
- Amro, M.; Chanbour, W.; Arej, N.; Jarade, E. Third- and fourth-generation formulas for intraocular lens power calculation before and after phakic intraocular lens insertion in high myopia. J. Cataract Refract. Surg. 2018, 44, 1321–1325. [Google Scholar] [CrossRef]
- Kuthirummal, N.; Vanathi, M.; Mukhija, R.; Gupta, N.; Meel, R.; Saxena, R.; Tandon, R. Evaluation of Barrett universal II formula for intraocular lens power calculation in Asian Indian population. Indian J. Ophthalmol. 2020, 68, 59–64. [Google Scholar] [CrossRef]
- Aristodemou, P.; Cartwright, N.E.K.; Sparrow, J.M.; Johnston, R.L. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J. Cataract Refract. Surg. 2011, 37, 63–71. [Google Scholar] [CrossRef]
- Xia, T.; Martinez, C.E.; Tsai, L.M. Update on Intraocular Lens Formulas and Calculations. Asia-Pacific J. Ophthalmol. 2020, 9, 186–193. [Google Scholar] [CrossRef]
- Kane, J.X.; Van Heerden, A.; Atik, A.; Petsoglou, C. Accuracy of 3 new methods for intraocular lens power selection. J. Cataract Refract. Surg. 2017, 43, 333–339. [Google Scholar] [CrossRef]
- Savini, G.; Hoffer, K.J.; Balducci, N.; Barboni, P.; Schiano-Lomoriello, D. Comparison of formula accuracy for intraocular lens power calculation based on measurements by a swept-source optical coherence tomography optical biometer. J. Cataract. Refract. Surg. 2020, 46, 27–33. [Google Scholar] [PubMed]
- Turnbull, A.M.; Hill, W.E.; Barrett, G.D. Accuracy of intraocular lens power calculation methods when targeting low myopia in monovision. J. Cataract Refract. Surg. 2020, 46, 862–866. [Google Scholar] [CrossRef]
- Wang, L.; Shirayama, M.; Ma, X.J.; Kohnen, T.; Koch, D.D. Optimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mm. J. Cataract Refract. Surg. 2011, 37, 2018–2027. [Google Scholar] [CrossRef]
- Fernández, J.; Rodríguez-Vallejo, M.; Martínez, J.; Tauste, A.; Piñero, D.P. New Approach for the Calculation of the Intraocular Lens Power Based on the Fictitious Corneal Refractive Index Estimation. J. Ophthalmol. 2019, 2019, 279612. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.L.; Cooke, T.L. Approximating sum-of-segments axial length from a traditional optical low-coherence reflectometry measurement. J. Cataract Refract. Surg. 2019, 45, 351–354. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, X.; Wang, W.; Yang, G.; Xu, J.; Ruan, X.; Gu, X.; Luo, L. Effect of Axial Length Adjustment Methods on Intraocular Lens Power Calculation in Highly Myopic Eyes. Am. J. Ophthalmol. 2020, 214, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tsessler, M.; Cohen, S.; Wang, L.; Koch, D.D.; Zadok, D.; Abulafia, A. Evaluating the prediction accuracy of the Hill-RBF 3.0 formula using a heteroscedastic statistical method. J. Cataract Refract. Surg. 2021, 48, 37–43. [Google Scholar] [CrossRef]
- Hoffer, K.J.; Savini, G. Effect of Gender and Race on Ocular Biometry. Int. Ophthalmol. Clin. 2017, 57, 137–142. [Google Scholar] [CrossRef]
- Ikuno, Y. Overview of the complications of high myopia. Retina 2017, 37, 2347–2351. [Google Scholar] [CrossRef]
- Lin, M.C.; Chen, Y.Q.; Polse, K.A. The Effects of Ocular and Lens Parameters on the Postlens Tear Thickness. Eye Contact Lens: Sci. Clin. Pract. 2003, 29 (Suppl. S1), S33–S36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hickson-Curran, S.; Young, G.; Brennan, N.; Hunt, C. Chinese and Caucasian ocular topography and soft contact lens fit. Clin. Exp. Optom. 2016, 99, 149–156. [Google Scholar] [CrossRef]
- Holladay, J.T.; Musgrove, K.H.; Prager, T.C.; Lewis, J.W.; Chandler, T.Y.; Ruiz, R.S. A three-part system for refining intraocular lens power calculations. J. Cataract Refract. Surg. 1988, 14, 17–24. [Google Scholar] [CrossRef]
- Retzlaff, J.A.; Sanders, D.R.; Kraff, M.C. Development of the SRK/T intraocular lens implant power calculation formula. J. Cataract Refract. Surg. 1990, 16, 333–340. [Google Scholar] [CrossRef]
- Hoffer, K.J.; Savini, G. Update on Intraocular Lens Power Calculation Study Protocols: The Better Way to Design and Report Clinical Trials. Ophthalmology 2020, 128, e115–e120. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.S.; Chong, G.S.; Yiu, E.P.; Ho, C.K. Intraocular lens power calculation formulas in Chinese eyes with high axial myopia. J. Cataract Refract. Surg. 2003, 29, 1358–1364. [Google Scholar] [CrossRef]
- Wang, J.-K.; Hu, C.-Y.; Chang, S.-W. Intraocular lens power calculation using the IOLMaster and various formulas in eyes with long axial length. J. Cataract Refract. Surg. 2008, 34, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, X.; Miao, Y.; Zheng, G.; Sun, Y.; Xu, X. Accuracy of Intraocular Lens Power Formulas Involving 148 Eyes with Long Axial Lengths: A Retrospective Chart-Review Study. J. Ophthalmol. 2015, 2015, 976847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.Y.; Liu, S.; Lee, J.W.Y.; Bhaskar, S.; Lam, D.S.C. Accuracy of Intraocular Lens Power Calculation Formulas for Highly Myopic Eyes. J. Ophthalmol. 2016, 2016, 1917268. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, Y.; Fang, X.; Luo, Q.; Wang, Y. Accuracy of the Haigis and SRK/T Formulas in Eyes Longer than 29.0 mm and the Influence of Central Corneal Keratometry Reading. Curr. Eye Res. 2018, 43, 1316–1321. [Google Scholar] [CrossRef]
- Rong, X.; He, W.; Zhu, Q.; Qian, D.; Lu, Y.; Zhu, X. Intraocular lens power calculation in eyes with extreme myopia: Comparison of Barrett Universal II, Haigis, and Olsen formulas. J. Cataract Refract. Surg. 2019, 45, 732–737. [Google Scholar] [CrossRef]
- Wan, K.H.; Lam, T.C.; Yu, M.C.; Chan, T.C. Accuracy and Precision of Intraocular Lens Calculations Using the New Hill-RBF Version 2.0 in Eyes With High Axial Myopia. Am. J. Ophthalmol. 2019, 205, 66–73. [Google Scholar] [CrossRef]
- Deng, G.; Zhou, D.; Sun, Z. Accuracy of the refractive prediction determined by intraocular lens power calculation formulas in high myopia. Indian J. Ophthalmol. 2019, 67, 484–489. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Zou, X.-Y.; Zheng, D.-Y.; Chen, W.-R.; Sun, A.; Luo, L.-X. Effect of lens constants optimization on the accuracy of intraocular lens power calculation formulas for highly myopic eyes. Int. J. Ophthalmol. 2019, 12, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, L.; Kane, J.X.; Liangping, L.; Liu, L.; Wu, M. Accuracy of Artificial Intelligence Formulas and Axial Length Adjustments for Highly Myopic Eyes. Am. J. Ophthalmol. 2020, 223, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S.; Tran, E.M.; Chen, A.J.; Rivera, D.R.; Rivera, J.J.; Greenberg, P.B. Accuracy of biometric formulae for intraocular lens power calculation in a teaching hospital. Int. J. Ophthalmol. 2020, 13, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Roessler, G.F.; Dietlein, T.S.; Plange, N.; Roepke, A.-K.; Dinslage, S.; Walter, P.; Mazinani, B.A. Accuracy of intraocular lens power calculation using partial coherence interferometry in patients with high myopia. Ophthalmic Physiol. Opt. 2012, 32, 228–233. [Google Scholar] [CrossRef]
- Fuest, M.; Plange, N.; Kuerten, D.; Schellhase, H.; Mazinani, B.A.E.; Walter, P.; Kohnen, S.; Widder, R.A.; Roessler, G. Intraocular lens power calculation for plus and minus lenses in high myopia using partial coherence interferometry. Int. Ophthalmol. 2021, 41, 1585–1592. [Google Scholar] [CrossRef]
- Ji, J.; Liu, Y.; Zhang, J.; Wu, X.; Shao, W.; Ma, B.; Luo, M. Comparison of six methods for the intraocular lens power calculation in high myopic eyes. Eur. J. Ophthalmol. 2019, 31, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Qian, S.; Wang, Y.; Li, S.; Yang, F.; Hu, Y.; Liu, Z.; Zhao, Y.-E. Accuracy of new-generation intraocular lens calculation formulas in eyes with variations in predicted refraction. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 1–9. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Qiu, K.; Zhang, M. Comparison of accuracy of intraocular lens power calculation for eyes with an axial length greater than 29.0 mm. Int. Ophthalmol. 2022, 42, 2029–2038. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Huang, T.-L.; Chang, P.-Y.; Ho, W.-T.; Hsu, Y.-R.; Chang, S.-W.; Wang, J.-K. Predictability of 6 Intraocular Lens Power Calculation Formulas in People With Very High Myopia. Front. Med. 2022, 9, 762761. [Google Scholar] [CrossRef]
- Lin, L.; Xu, M.; Mo, E.; Huang, S.; Qi, X.; Gu, S.; Sun, W.; Su, Q.; Li, J.; Zhao, Y.-E. Accuracy of Newer Generation IOL Power Calculation Formulas in Eyes With High Axial Myopia. J. Refract. Surg. 2021, 37, 754–758. [Google Scholar] [CrossRef]
- Tan, Q.; Lin, D.; Wang, L.; Chen, B.; Tang, Q.; Chen, X.; Chen, M.; Tan, J.; Zhang, J.; Wu, L.; et al. Comparison of IOL Power Calculation Formulas for a Trifocal IOL in Eyes With High Myopia. J. Refract. Surg. 2021, 37, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, J.; Raimundo, M.; Lobo, C.; Murta, J.N. A Comparison of Intraocular Lens Power Calculation Formulas in High Myopia. J. Refract. Surg. 2021, 37, 207–211. [Google Scholar] [CrossRef] [PubMed]
| n | (%) | |
| Gender (F/M) | 15/10 | (60.0%, 40.0%) |
| Eye (OD/OS) | 20/15 | (57.1%, 42.9%) |
| Mean ± SD | Range | |
| Age, y | 56.94 ± 9.56 | 37, 76 |
| Axial length (mm) | 28.71 ± 0.87 | 28.01, 31.1 |
| ACD (mm) | 3.66 ± 0.38 | 2.38, 4.24 |
| Lens thickness (mm) | 4.25 ± 0.52 | 2.96, 5.6 |
| Average keratometry (D) | 43.30 ± 1.61 | 41.59, 49.22 |
| n | (%) | |
| Keratometry subgroups | ||
| Flat (<42.0 D) | 10 | (28.6%) |
| Medium (42.0 D–46.0 D) | 23 | (65.7%) |
| Steep (>46.0 D) | 2 | (5.7%) |
| IOL Type | ||
| Alcon MA60MA | 2 | (5.7%) |
| AMO AR40e | 2 | (5.7%) |
| enVista MX60E | 1 | (2.9%) |
| Tecnis ZCB00 | 24 | (68.6%) |
| Tecnis ZCT225 | 2 | (5.7%) |
| Tecnis ZXR00 | 4 | (11.4%) |
| Mean ± SD | Range | |
| IOL power (D) | 7.76 ± 3.06 | –1.00, +12.00 |
| Preoperative | ||
| SE (D) | –11.28 ± 4.29 | –18.88, −3.63 |
| UDVA (LogMAR) | 1.69 ± 0.39 | 0.3, 1.90 |
| CDVA (LogMAR) | 0.22 ± 0.17 | 0, 1.00 |
| Postoperative | ||
| SE (D) | –0.58 ± 0.79 | –2.13, 0.75 |
| UDVA (LogMAR) | 0.20 ± 0.23 | 0, 0.80 |
| CDVA (LogMAR) | 0.01 ± 0.07 | –0.12, 0.30 |
| Postoperative refraction, days after surgery | 147.62 ± 179.90 | 21, 686 |
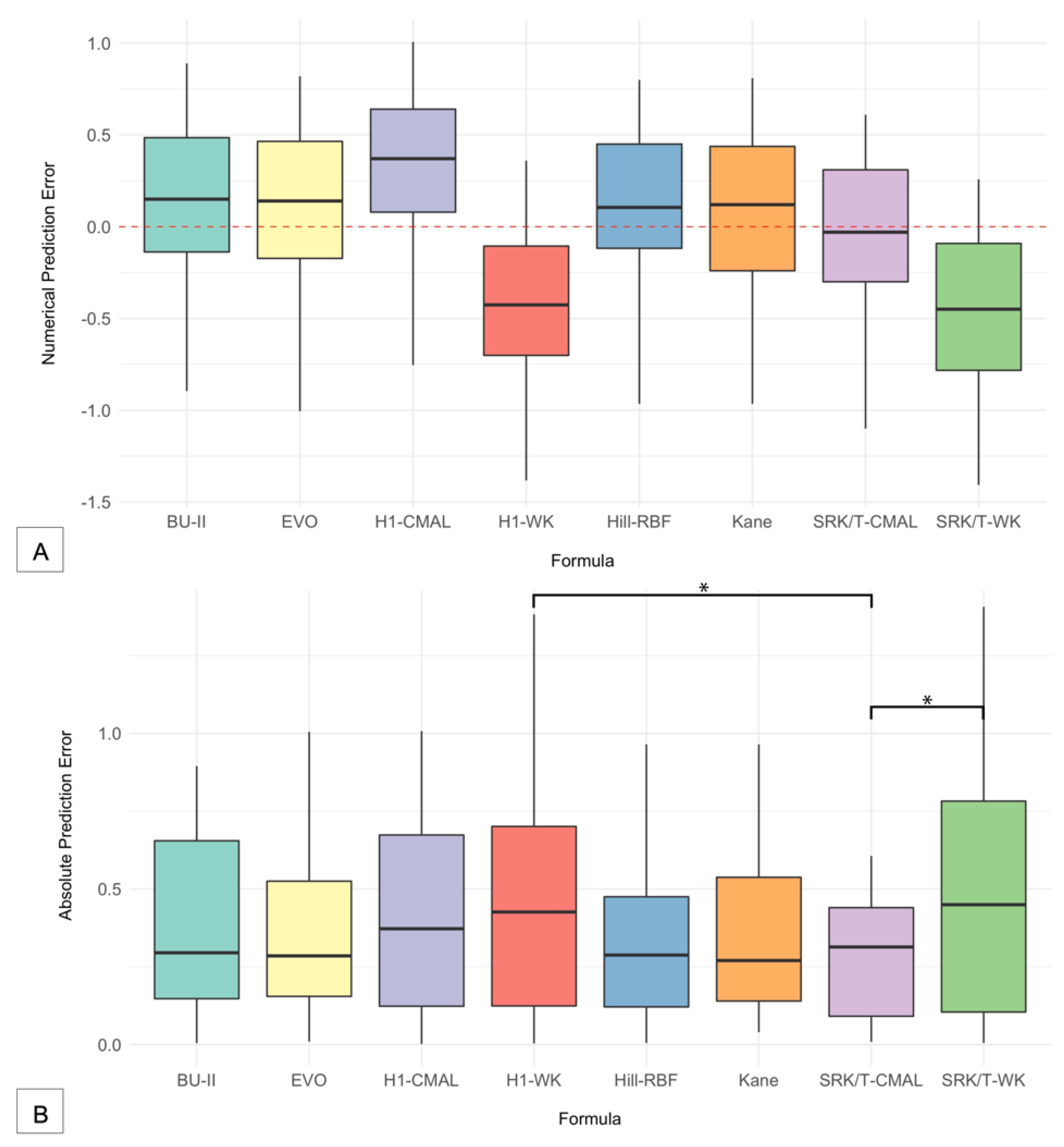
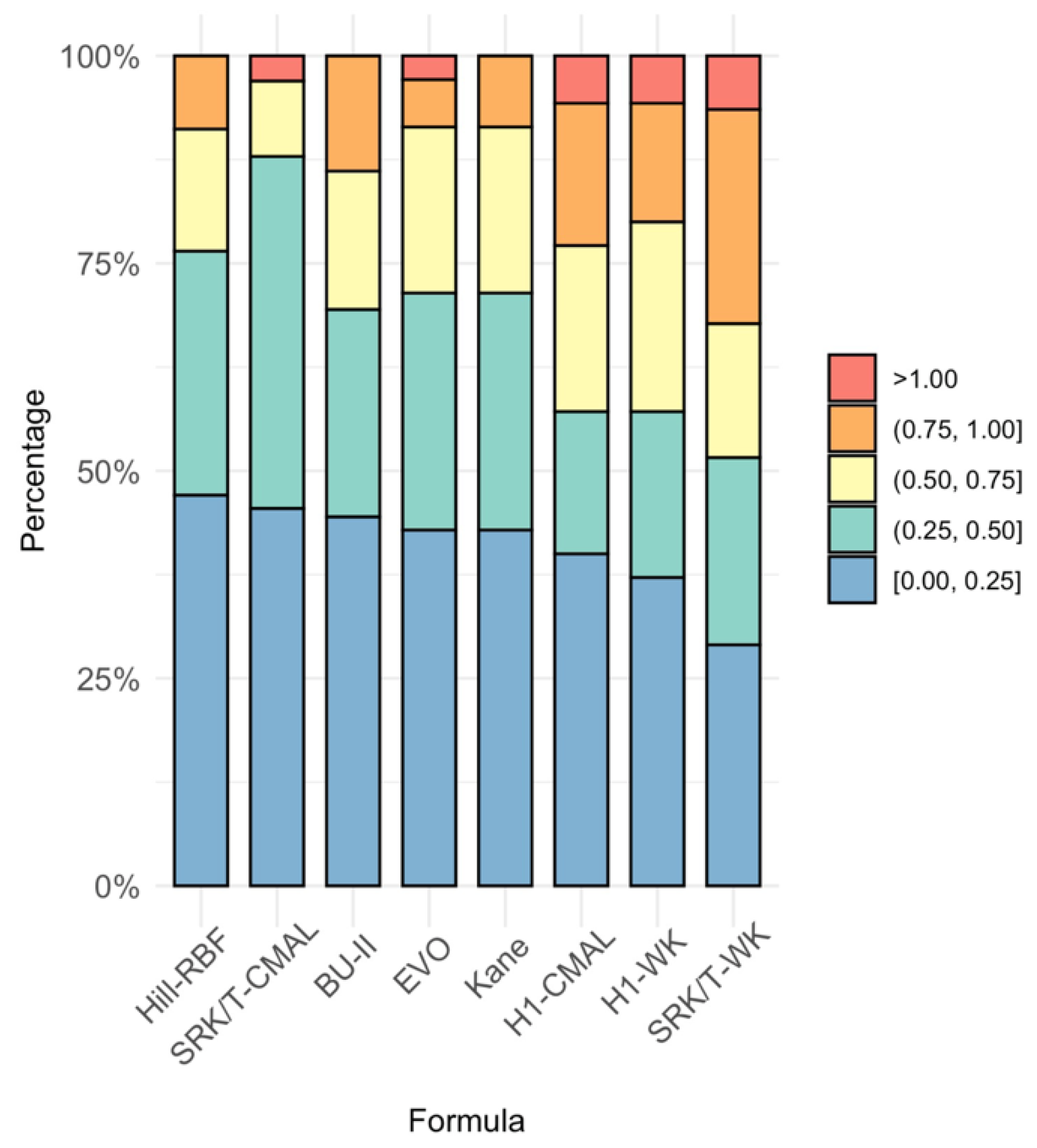
| Formula | MPE | SD | MAE | MedAE | Max AE | ± 0.25 D a | ± 0.50 D a | ± 0.75 D a | ± 1.00 D a |
|---|---|---|---|---|---|---|---|---|---|
| BU-II | 0.146 | 0.451 | 0.379 | 0.295 | 0.895 | 45.71 | 68.57 | 85.71 | 100.00 |
| EVO | 0.147 | 0.416 | 0.361 | 0.285 | 1.005 | 42.86 | 71.43 | 91.43 | 97.14 |
| Hill-RBF | 0.136 | 0.407 | 0.333 | 0.288 | 0.965 | 47.06 | 76.47 | 91.18 | 100.00 |
| H1-CMAL | 0.352 | 0.393 | 0.419 | 0.370 | 1.010 | 40.00 | 57.14 | 77.14 | 94.29 |
| H1-WK | −0.396 | 0.401 | 0.450 | 0.430 | 1.380 | 37.14 | 57.14 | 80.00 | 94.29 |
| Kane | 0.082 | 0.418 | 0.346 | 0.270 | 0.810 | 42.86 | 68.57 | 91.43 | 100.00 |
| SRK/T-CMAL | −0.015 | 0.385 | 0.303 | 0.310 | 1.100 | 45.45 | 87.88 | 96.97 | 96.97 |
| SRK/T-WK | −0.442 | 0.411 | 0.474 | 0.450 | 1.410 | 33.33 | 54.45 | 69.70 | 93.94 |
| Formulas | BU-II | EVO | Hill-RBF | H1-CMAL | H1-WK | Kane | SRK/T-CMAL | SRK/T-WK |
|---|---|---|---|---|---|---|---|---|
| BU-II | - | - | - | - | - | - | - | - |
| EVO | 0.762 | - | - | - | - | - | - | - |
| Hill-RBF | 0.189 | 0.442 | - | - | - | - | - | - |
| H1-CMAL | 0.09 | 0.114 | 0.097 | - | - | - | - | - |
| H1-WK | 0.408 | 0.207 | 0.156 | 0.801 | - | - | - | - |
| Kane | 0.158 | 0.172 | 0.974 | 0.073 | 0.164 | - | - | - |
| SRK/T-CMAL | 0.073 | 0.153 | 0.562 | 0.125 | 0.012 † | 0.376 | - | - |
| SRK/T-WK | 0.331 | 0.161 | 0.200 | 0.514 | 0.335 | 0.153 | 0.010 † | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshirfar, M.; Durnford, K.M.; Jensen, J.L.; Beesley, D.P.; Peterson, T.S.; Darquea, I.M.; Ronquillo, Y.C.; Hoopes, P.C. Accuracy of Six Intraocular Lens Power Calculations in Eyes with Axial Lengths Greater than 28.0 mm. J. Clin. Med. 2022, 11, 5947. https://doi.org/10.3390/jcm11195947
Moshirfar M, Durnford KM, Jensen JL, Beesley DP, Peterson TS, Darquea IM, Ronquillo YC, Hoopes PC. Accuracy of Six Intraocular Lens Power Calculations in Eyes with Axial Lengths Greater than 28.0 mm. Journal of Clinical Medicine. 2022; 11(19):5947. https://doi.org/10.3390/jcm11195947
Chicago/Turabian StyleMoshirfar, Majid, Kathryn M. Durnford, Jenna L. Jensen, Daniel P. Beesley, Telyn S. Peterson, Ines M. Darquea, Yasmyne C. Ronquillo, and Phillip C. Hoopes. 2022. "Accuracy of Six Intraocular Lens Power Calculations in Eyes with Axial Lengths Greater than 28.0 mm" Journal of Clinical Medicine 11, no. 19: 5947. https://doi.org/10.3390/jcm11195947
APA StyleMoshirfar, M., Durnford, K. M., Jensen, J. L., Beesley, D. P., Peterson, T. S., Darquea, I. M., Ronquillo, Y. C., & Hoopes, P. C. (2022). Accuracy of Six Intraocular Lens Power Calculations in Eyes with Axial Lengths Greater than 28.0 mm. Journal of Clinical Medicine, 11(19), 5947. https://doi.org/10.3390/jcm11195947






