Antidepressant Use and Its Association with 28-Day Mortality in Inpatients with SARS-CoV-2: Support for the FIASMA Model against COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Cohort Assembly
2.2. Variables Assessed
2.3. Antidepressant Use
2.4. Study Baseline and Outcomes
2.5. Potential Mechanisms
2.6. Statistical Analysis
3. Results
3.1. Prevalence of Antidepressant Use in Adult Patients Hospitalized with and without COVID-19
3.2. Antidepressant Use and 28-Day Mortality in Adult Patients Hospitalized with COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoertel, N.; Blachier, M.; Blanco, C.; Olfson, M.; Massetti, M.; Sánchez-Rico, M.; Limosin, F.; Leleu, H. A Stochastic Agent-Based Model of the SARS-CoV-2 Epidemic in France. Nat. Med. 2020, 26, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Blachier, M.; Sánchez-Rico, M.; Limosin, F.; Leleu, H. Impact of the Timing and Adherence to Face Mask Use on the Course of the COVID-19 Epidemic in France. J. Travel Med. 2021, 28, taab016. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Wiernik, E.; Robineau, O.; Carrat, F.; Touvier, M.; Severi, G.; de Lamballerie, X.; Blanché, H.; Deleuze, J.-F.; Gouraud, C.; et al. Association of Self-Reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern. Med. 2021, 2021, 6454. [Google Scholar] [CrossRef] [PubMed]
- Chevance, A.; Gourion, D.; Hoertel, N.; Llorca, P.-M.; Thomas, P.; Bocher, R.; Moro, M.-R.; Laprévote, V.; Benyamina, A.; Fossati, P.; et al. Ensuring mental health care during the SARS-CoV-2 epidemic in France: A narrative review. L’Encephale 2020, 46, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Tignanelli, C.J.; Hoertel, N.; Boulware, D.R.; Usher, M.G. Prevalence of Medical Contraindications to Nirmatrelvir/Ritonavir in a Cohort of Hospitalized and Nonhospitalized Patients With COVID-19. Open. For. Infect. Dis. 2022, 9, ofac389. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Infectious Diseases Unmet Need for COVID-19 Therapies in Community Settings. Lancet Infect. Dis. 2021, 21, 1471. [CrossRef]
- Hoertel, N. Do the Selective Serotonin Reuptake Inhibitor Antidepressants Fluoxetine and Fluvoxamine Reduce Mortality Among Patients With COVID-19? JAMA Netw. Open. 2021, 4, e2136510. [Google Scholar] [CrossRef] [PubMed]
- Tham, A.; Jonsson, U.; Andersson, G.; Söderlund, A.; Allard, P.; Bertilsson, G. Efficacy and Tolerability of Antidepressants in People Aged 65 Years or Older with Major Depressive Disorder—A Systematic Review and a Meta-Analysis. J. Affect. Disord. 2016, 205, 12. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Stingl, J.C. Antidepressant Drug Treatment Protecting from COVID-19: One More Piece in the Repurposing Puzzle. BJPsych Open 2021, 8, e20. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Cougoule, C.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Becker, K.A.; Reiersen, A.M.; Lenze, E.J.; Seftel, D.; et al. Repurposing Antidepressants Inhibiting the Sphingomyelinase Acid/Ceramide System against COVID-19: Current Evidence and Potential Mechanisms. Mol. Psychiatry 2021, 26, 7098–7099. [Google Scholar] [CrossRef]
- Kornhuber, J.; Hoertel, N.; Gulbins, E. The Acid Sphingomyelinase/Ceramide System in COVID-19. Mol. Psychiatry 2022, 27, 307–314. [Google Scholar] [CrossRef]
- Brunotte, L.; Zheng, S.; Mecate-Zambrano, A.; Tang, J.; Ludwig, S.; Rescher, U.; Schloer, S. Combination Therapy with Fluoxetine and the Nucleoside Analog GS-441524 Exerts Synergistic Antiviral Effects against Different SARS-CoV-2 Variants In Vitro. Pharmaceutics 2021, 13, 1400. [Google Scholar] [CrossRef]
- Fred, S.M.; Kuivanen, S.; Ugurlu, H.; Casarotto, P.C.; Levanov, L.; Saksela, K.; Vapalahti, O.; Castrén, E. Antidepressant and Antipsychotic Drugs Reduce Viral Infection by SARS-CoV-2 and Fluoxetine Shows Antiviral Activity Against the Novel Variants in Vitro. Front. Pharmacol. 2022, 12, 755600. [Google Scholar] [CrossRef]
- Dechaumes, A.; Nekoua, M.P.; Belouzard, S.; Sane, F.; Engelmann, I.; Dubuisson, J.; Alidjinou, E.K.; Hober, D. Fluoxetine Can Inhibit SARS-CoV-2 In Vitro. Microorganisms 2021, 9, 339. [Google Scholar] [CrossRef]
- Schloer, S.; Brunotte, L.; Mecate-Zambrano, A.; Zheng, S.; Tang, J.; Ludwig, S.; Rescher, U. Drug Synergy of Combinatory Treatment with Remdesivir and the Repurposed Drugs Fluoxetine and Itraconazole Effectively Impairs SARS-CoV-2 Infection in Vitro. Br. J. Pharmacol. 2021, 178, 2339–2350. [Google Scholar] [CrossRef]
- Schloer, S.; Brunotte, L.; Goretzko, J.; Mecate-Zambrano, A.; Korthals, N.; Gerke, V.; Ludwig, S.; Rescher, U. Targeting the Endolysosomal Host-SARS-CoV-2 Interface by Clinically Licensed Functional Inhibitors of Acid Sphingomyelinase (FIASMA) Including the Antidepressant Fluoxetine. Emerg. Microb. Infect. 2020, 9, 2245–2255. [Google Scholar] [CrossRef]
- Zimniak, M.; Kirschner, L.; Hilpert, H.; Geiger, N.; Danov, O.; Oberwinkler, H.; Steinke, M.; Sewald, K.; Seibel, J.; Bodem, J. The Serotonin Reuptake Inhibitor Fluoxetine Inhibits SARS-CoV-2 in Human Lung Tissue. Sci. Rep. 2021, 11, 5890. [Google Scholar] [CrossRef]
- Clelland, C.L.; Ramiah, K.; Steinberg, L.; Clelland, J.D. Analysis of the Impact of Antidepressants and Other Medications on COVID-19 Infection Risk in a Chronic Psychiatric in-Patient Cohort. BJPsych Open. 2021, 8, e6. [Google Scholar] [CrossRef]
- Lenze, E.J.; Mattar, C.; Zorumski, C.F.; Stevens, A.; Schweiger, J.; Nicol, G.E.; Miller, J.P.; Yang, L.; Yingling, M.; Avidan, M.S.; et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2292–2300. [Google Scholar] [CrossRef]
- Seftel, D.; Boulware, D.R. Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19. Open. For Infect. Diseases 2021, 8, ofab050. [Google Scholar] [CrossRef]
- Reis, G.; dos Santos Moreira-Silva, E.A.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; dos Santos, C.V.Q.; de Souza Campos, V.H.; Nogueira, A.M.R.; de Almeida, A.P.F.G.; et al. Effect of Early Treatment with Fluvoxamine on Risk of Emergency Care and Hospitalisation among Patients with COVID-19: The TOGETHER Randomised, Platform Clinical Trial. Lancet Glob. Health 2021, 10, e42–e51. [Google Scholar] [CrossRef]
- Bramante, C.T.; Huling, J.D.; Tignanelli, C.J.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Cohen, K.; Puskarich, M.A.; Belani, H.K.; Proper, J.L.; et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for COVID-19. N. Engl. J. Med. 2022, 387, 599–610. [Google Scholar] [CrossRef]
- Fritz, B.; Hoertel, N.; Lenze, E.; Jalali, F.; Reiersen, A. Association Between Antidepressant Use and ED or Hospital Visits in Outpatients with SARS-CoV-2. Trans.Psychiatry 2022, Accepted. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.-S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between Antidepressant Use and Reduced Risk of Intubation or Death in Hospitalized Patients with COVID-19: Results from an Observational Study. Mol. Psychiatry 2021, 26, 5199–5212. [Google Scholar] [CrossRef]
- Németh, Z.K.; Szûcs, A.; Vitrai, J.; Juhász, D.; Németh, J.P.; Holló, A. Fluoxetine Use Is Associated with Improved Survival of Patients with COVID-19 Pneumonia: A Retrospective Case-Control Study. Ideggyogy Sz 2021, 74, 389–396. [Google Scholar] [CrossRef]
- Oskotsky, T.; Marić, I.; Tang, A.; Oskotsky, B.; Wong, R.J.; Aghaeepour, N.; Sirota, M.; Stevenson, D.K. Mortality Risk Among Patients With COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants. JAMA Netw. Open. 2021, 4, e2133090. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Lenze, E.J.; Reiersen, A.M.; Abellán, M.; de la Muela, P.; Vernet, R.; et al. Association Between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: An Observational Multicenter Study. Clin Pharmacol Ther 2021, 110, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Sánchez-Rico, M.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Abellán, M.; de la Muela, P.; Vernet, R.; Beeker, N.; Neuraz, A.; et al. Association between FIASMA Psychotropic Medications and Reduced Risk of Intubation or Death in Individuals with Psychiatric Disorders Hospitalized for Severe COVID-19: An Observational Multicenter Study. Transl Psychiatry 2022, 12, 90. [Google Scholar] [CrossRef]
- Calusic, M.; Marcec, R.; Luksa, L.; Jurkovic, I.; Kovac, N.; Mihaljevic, S.; Likic, R. Safety and Efficacy of Fluvoxamine in COVID-19 ICU Patients: An Open Label, Prospective Cohort Trial with Matched Controls. Br. J. Clin. Pharmacol. 2021, 88, 2065–2073. [Google Scholar] [CrossRef]
- Sánchez-Rico, M.; de la Muela, P.; Herrera-Morueco, J.J.; Geoffroy, P.A.; Limosin, F.; Hoertel, N.; AP-HP/Université de Paris/INSERM COVID-19 Research Collaboration/AP-HP COVID CDR Initiative/Entrepôt de Données de Santé AP-HP Consortium. Melatonin Does Not Reduce Mortality in Adult Hospitalized Patients with COVID-19: A Multicenter Retrospective Observational Study. J. Travel Med. 2022, 29, taab195. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Sánchez-Rico, M.; de la Muela, P.; Abellán, M.; Blanco, C.; Leboyer, M.; Cougoule, C.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; et al. Risk of Death in Individuals Hospitalized for COVID-19 with and without Psychiatric Disorders: An Observational Multicenter Study in France. Biol. Psychiatry Glob. Open. Sci. 2022, 110, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rico, M.; Limosin, F.; Hoertel, N. Is a Diagnosis of Schizophrenia Spectrum Disorder Associated With Increased Mortality in Patients With COVID-19? AJP 2022, 179, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rico, M.; Limosin, F.; Vernet, R.; Beeker, N.; Neuraz, A.; Blanco, C.; Olfson, M.; Lemogne, C.; Meneton, P.; Daniel, C.; et al. Hydroxyzine Use and Mortality in Patients Hospitalized for COVID-19: A Multicenter Observational Study. J. Clin. Med. 2021, 10, 5891. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Sánchez-Rico, M.; Herrera-Morueco, J.J.; de la Muela, P.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Becker, K.A.; Cougoule, C.; Limosin, F.; et al. Comorbid Medical Conditions Are a Key Factor to Understand the Relationship between Psychiatric Disorders and COVID-19-Related Mortality: Results from 49,089 COVID-19 Inpatients. Mol. Psychiatry 2021, 27, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Jannot, A.-S.; Neuraz, A.; Blanco, C.; Lemogne, C.; Airagnes, G.; Paris, N.; Daniel, C.; et al. Observational Study of Haloperidol in Hospitalized Patients with COVID-19. PLoS ONE 2021, 16, e0247122. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Jannot, A.-S.; Neuraz, A.; Blanco, C.; Lemogne, C.; Airagnes, G.; Paris, N.; Daniel, C.; et al. Observational Study of Chlorpromazine in Hospitalized Patients with COVID-19. Clin. Drug. Investig. 2021, 41, 221–233. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Neuraz, A.; Alvarado, J.M.; Daniel, C.; Paris, N.; Gramfort, A.; Lemaitre, G.; et al. Dexamethasone Use and Mortality in Hospitalized Patients with Coronavirus Disease 2019: A Multicentre Retrospective Observational Study. Br. J. Clin. Pharmacol. 2021, 87, 3766–3775. [Google Scholar] [CrossRef]
- Haut Conseil de La Santé Publique. Statement on the Management at Home or in a Care Facility of Suspected or Confirmed COVID-19 Patients. 8 April 2020. Available online: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=825 (accessed on 15 May 2020).
- Lagunas-Rangel, F.A. Neutrophil-to-lymphocyte Ratio and Lymphocyte-to-C-reactive Protein Ratio in Patients with Severe Coronavirus Disease 2019 (COVID-19): A Meta-analysis. J. Med. Virol. 2020, 92, 1733–1734. [Google Scholar] [CrossRef] [Green Version]
- Hayasaka, Y.; Purgato, M.; Magni, L.R.; Ogawa, Y.; Takeshima, N.; Cipriani, A.; Barbui, C.; Leucht, S.; Furukawa, T.A. Dose Equivalents of Antidepressants: Evidence-Based Recommendations from Randomized Controlled Trials. J. Affect. Disord. 2015, 180, 179–184. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Suzuki, T.; Hashimoto, K. Mechanisms of Action of Fluvoxamine for COVID-19: A Historical Review. Mol. Psychiatry 2022, 27, 1898–1907. [Google Scholar] [CrossRef]
- Sukhatme, V.P.; Reiersen, A.M.; Vayttaden, S.J.; Sukhatme, V.V. Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19. Front. Pharmacol. 2021, 12, 652688. [Google Scholar] [CrossRef]
- Facente, S.N.; Reiersen, A.M.; Lenze, E.J.; Boulware, D.R.; Klausner, J.D. Fluvoxamine for the Early Treatment of SARS-CoV-2 Infection: A Review of Current Evidence. Drugs 2021, 81, 2081–2089. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Gripp, B.; Hoffmann, M.; Pöhlmann, S.; Hoertel, N.; Edwards, M.J.; Kamler, M.; Kornhuber, J.; Becker, K.A.; Gulbins, E. Inhibition of Acid Sphingomyelinase by Ambroxol Prevents SARS-CoV-2 Entry into Epithelial Cells. J. Biol. Chem. 2021, 296, 100701. [Google Scholar] [CrossRef]
- Pashaei, Y. Fluoxetine and Molnupiravir: A Synergistic Combination for COVID-19 Treatment? Hosp. Pharm. 2022, 57, 603–604. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Roweth, H.G.; Yan, R.; Bedwani, N.H.; Chauhan, A.; Fowler, N.; Watson, A.H.; Malcor, J.-D.; Sage, S.O.; Jarvis, G.E. Citalopram Inhibits Platelet Function Independently of SERT-Mediated 5-HT Transport. Sci. Rep. 2018, 8, 3494. [Google Scholar] [CrossRef] [Green Version]
- Kornhuber, J.; Muehlbacher, M.; Trapp, S.; Pechmann, S.; Friedl, A.; Reichel, M.; Mühle, C.; Terfloth, L.; Groemer, T.W.; Spitzer, G.M.; et al. Identification of Novel Functional Inhibitors of Acid Sphingomyelinase. PLoS ONE 2011, 6, e23852. [Google Scholar] [CrossRef] [Green Version]
- Pashaei, Y. Drug Repurposing of Selective Serotonin Reuptake Inhibitors: Could These Drugs Help Fight COVID-19 and Save Lives? J. Clin. Neurosci. 2021, 88, 163–172. [Google Scholar] [CrossRef]
- Hansen, B.B.; Klopfer, S.O. Optimal Full Matching and Related Designs via Network Flows. J. Comput. Graphical Statis. 2006, 15, 609–627. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. Using the Standardized Difference to Compare the Prevalence of a Binary Variable between Two Groups in Observational Research. Commun. Statis. Simulat. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
- McAlister, F.A.; Wang, T.; Wang, X.; Chu, A.; Goodman, S.G.; van Diepen, S.; Jackevicius, C.A.; Kaul, P.; Udell, J.; Ko, D.T.; et al. Statins and SARS-CoV-2 Infection: Results of a Population-Based Prospective Cohort Study of 469 749 Adults From 2 Canadian Provinces. JAHA 2021, 10, e022330. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19—Preliminary Report. N. Engl. J. Med. 2020, 384, 693–704. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical Primer: Propensity Score Matching and Its Alternatives. Eur. J. Cardio Thoracic Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [Green Version]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268. [Google Scholar] [CrossRef] [Green Version]
- Khodadoust, M.M. Inferring a Causal Relationship between Ceramide Levels and COVID-19 Respiratory Distress. Sci. Rep. 2021, 11, 20866. [Google Scholar] [CrossRef]
- Marín-Corral, J.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Pascual-Guardia, S.; Muñoz-Bermúdez, R.; Salazar-Degracia, A.; Pérez-Terán, P.; Restrepo, M.I.; Khymenets, O.; Haro, N.; et al. Metabolic Signatures Associated with Severity in Hospitalized COVID-19 Patients. Int. J. Mol. Sci. 2021, 22, 4794. [Google Scholar] [CrossRef]
- Torretta, E.; Garziano, M.; Poliseno, M.; Capitanio, D.; Biasin, M.; Santantonio, T.A.; Clerici, M.; Lo Caputo, S.; Trabattoni, D.; Gelfi, C. Severity of COVID-19 Patients Predicted by Serum Sphingolipids Signature. Int. J. Mol. Sci. 2021, 22, 10198. [Google Scholar] [CrossRef] [PubMed]
- Mühle, C.; Kremer, A.; Vetter, M.; Schmid, J.; Achenbach, S.; Schumacher, F.; Lenz, B.; Cougoule, C.; Hoertel, N.; Carpinteiro, A.; et al. COVID-19 and Its Clinical Severity Are Associated with Alterations of Plasma Sphingolipids and Enzyme Activities of Sphingomyelinase and Ceramidase. MedRxiv 2022. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ottolenghi, S.; Morano, C.; Rinaldo, R.; Roda, G.; Chiumello, D.; Centanni, S.; Samaja, M.; Paroni, R. Link between Serum Lipid Signature and Prognostic Factors in COVID-19 Patients. Sci. Rep. 2021, 11, 21633. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Tripal, P.; Reichel, M.; Terfloth, L.; Bleich, S.; Wiltfang, J.; Gulbins, E. Identification of New Functional Inhibitors of Acid Sphingomyelinase Using a Structure- Property-Activity Relation Model. J. Med. Chem. 2008, 51, 219–237. [Google Scholar] [CrossRef]
- Eugene, A.R. Fluoxetine Pharmacokinetics and Tissue Distribution Quantitatively Supports a Therapeutic Role in COVID-19 at a Minimum Dose of 20 Mg per Day. F1000Research 2022, 10, 477. [Google Scholar] [CrossRef]
- Le Strat, Y.; Hoertel, N. Correlation Is No Causation: Gymnasium Proliferation and the Risk of Obesity. Addiction 2011, 106, 1871–1872. [Google Scholar] [CrossRef]
- Mazereel, V.; Vanbrabant, T.; Desplenter, F.; De Hert, M. COVID-19 Vaccine Uptake in Patients with Psychiatric Disorders Admitted to or Residing in a University Psychiatric Hospital. Lancet Psychiatry 2021, 8, 860–861. [Google Scholar] [CrossRef]
- Poly, T.N.; Islam, M.M.; Yang, H.C.; Lin, M.C.; Jian, W.-S.; Hsu, M.-H.; Jack Li, Y.-C. Obesity and Mortality Among Patients Diagnosed With COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 620044. [Google Scholar] [CrossRef]
- Patten, S.B.; Williams, J.V.A.; Lavorato, D.H.; Brown, L.; McLaren, L.; Eliasziw, M. Major Depression, Antidepressant Medication and the Risk of Obesity. Psychother. Psychosom. 2009, 78, 182–186. [Google Scholar] [CrossRef]
- Sawada, N.; Uchida, H.; Suzuki, T.; Watanabe, K.; Kikuchi, T.; Handa, T.; Kashima, H. Persistence and Compliance to Antidepressant Treatment in Patients with Depression: A Chart Review. BMC Psychiatry 2009, 9, 38. [Google Scholar] [CrossRef]
- Alaeddin, N.; Stingl, J.C.; Breteler, M.M.B.; Vries, F.M. Validation of Self-reported Medication Use Applying Untargeted Mass Spectrometry-based Metabolomics Techniques in the Rhineland Study. Brit. J. Clin. Pharm. 2022, 88, 2380–2395. [Google Scholar] [CrossRef]
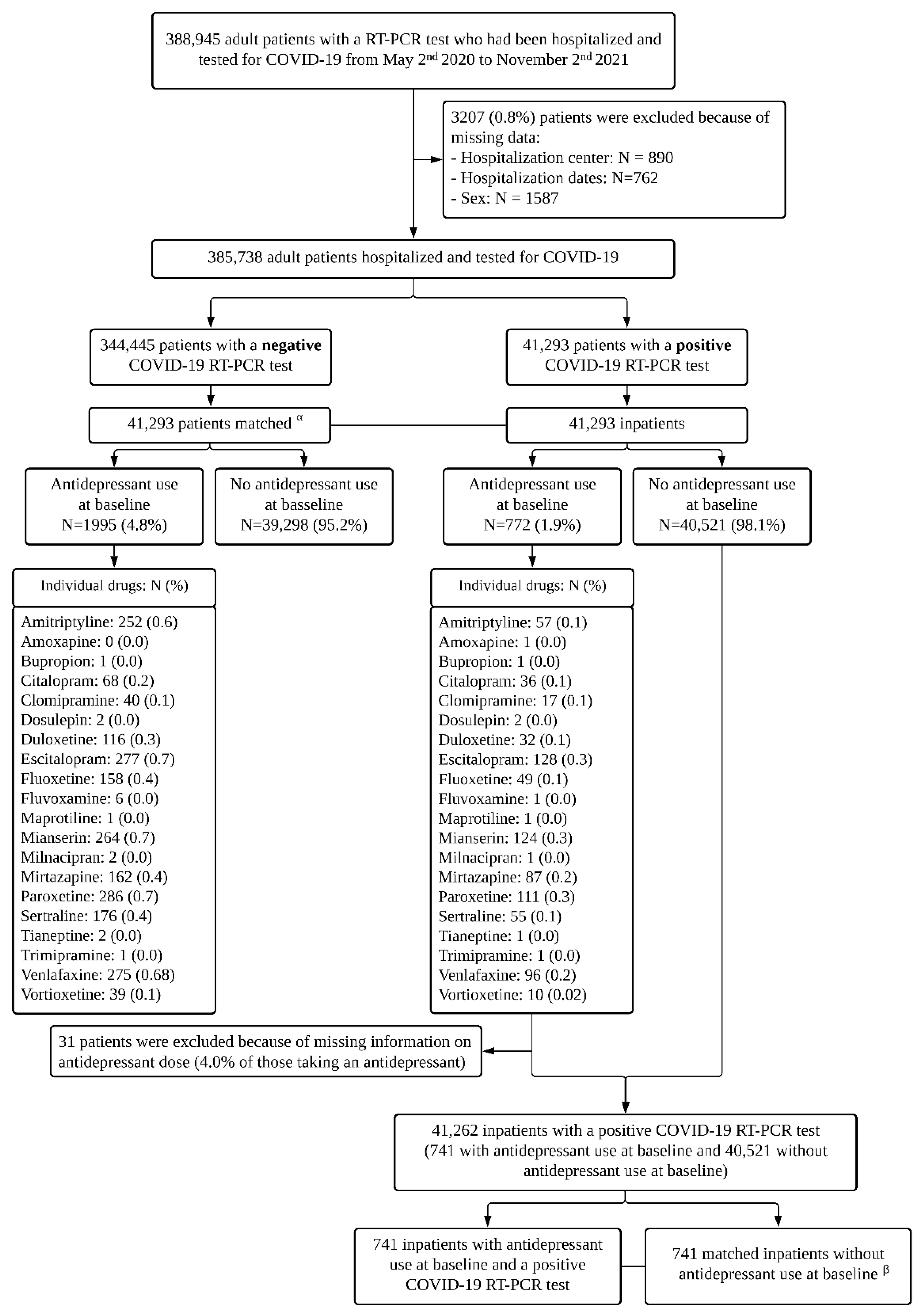
| Patients Hospitalized with COVID-19 (N = 41,293) | Patients Hospitalized without COVID-19 (N = 41,293) | Hospitalized with COVID-19 versus without COVID-19 in a 1:1 Ratio Matched Analytic Sample | |
|---|---|---|---|
| N (%) | N (%) | OR (95%CI; Two-Sided p-Value) | |
| No antidepressant | 40,521 (98.1%) | 39,298 (95.2%) | Ref. |
| Any antidepressant | 772 (1.9%) | 1988 (4.8%) | 0.38 (0.35–0.41; <0.001) |
| Stratification by age, sex, period of hospitalization, and diagnosis of any psychiatric disorder | |||
| Men | |||
| Without antidepressants | 20,456 (98.7%) | 19,920 (96.0%) | Ref. |
| Any antidepressant | 276 (1.33%) | 821 (3.96%) | 0.33 (0.29–0.38; <0.001 ***) |
| Women | |||
| Without antidepressants | 20,065 (97.6%) | 19,378 (94.3%) | Ref. |
| Any antidepressant | 496 (2.41%) | 1174 (5.71%) | 0.41 (0.37–0.45; <0.001 ***) |
| Younger patients (≤53) | |||
| Without antidepressants | 20,422 (99.7%) | 20,289 (97.5%) | Ref. |
| Any antidepressant | 53 (0.26%) | 529 (2.54%) | 0.10 (0.08–0.13; <0.001 ***) |
| Older patients (>53) | |||
| Without antidepressants | 20,099 (96.5%) | 19,009 (92.8%) | Ref. |
| Any antidepressant | 719 (3.45%) | 1466 (7.16%) | 0.46 (0.42–0.51; <0.001 ***) |
| Hospitalization from 2 May 2020–29 January 2021 | |||
| Without antidepressants | 19,910 (98.0%) | 20,081 (94.6%) | Ref. |
| Any antidepressant | 396 (1.95%) | 1149 (5.41%) | 0.35 (0.31–0.39; <0.001 ***) |
| Hospitalization from 30 January 2021–2 November 2021 | |||
| Without antidepressants | 20,611 (98.2%) | 19,217 (95.8%) | Ref. |
| Any antidepressant | 376 (1.79%) | 846 (4.22%) | 0.41 (0.37–0.47; <0.001 ***) |
| Patients with any psychiatric disorder | |||
| Without antidepressants | 3059 (88.7%) | 6105 (87.3%) | Ref. |
| Any antidepressant | 389 (11.3%) | 890 (12.7%) | 0.87 (0.77–0.99; 0.035 *) |
| Patients without psychiatric disorders | |||
| Without antidepressants | 37,462 (99.0%) | 33,154 (96.7%) | Ref. |
| Any antidepressant | 383 (1.01%) | 1145 (3.34%) | 0.30 (0.26–0.33; <0.001 ***) |
| Antidepressant classes and individual molecules | |||
| SSRIs | 388 (0.9%) | 1002 (2.43%) | 0.38 (0.34–0.43; <0.001 ***) |
| Escitalopram | 128 (0.3%) | 277 (0.7%) | 0.46 (0.37–0.57; <0.001 ***) |
| Paroxetine | 111 (0.3%) | 286 (0.7%) | 0.39 (0.31–0.48; <0.001 ***) |
| Sertraline | 55 (0.1%) | 176 (0.4%) | 0.31 (0.23–0.42; <0.001 ***) |
| Fluoxetine | 49 (0.1%) | 158 (0.4%) | 0.31 (0.22–0.43; <0.001 ***) |
| Citalopram | 36 (0.1%) | 68 (0.2%) | 0.53 (0.35–0.79; 0.002**) |
| Vortioxetine | 10 (0.0%) | 39 (0.1%) | 0.26 (0.13–0.51; <0.001 ***) |
| Fluvoxamine | 1 (0.0%) | 6 (0.0%) | 0.17 (0.02–1.38; 0.097) |
| Fluoxetine or fluvoxamine | 50 (0.1%) | 164 (0.4%) | 0.30 (0.22–0.42; <0.001 ***) |
| Non-SSRI antidepressants | 384 (0.93%) | 993 (2.40%) | 0.38 (0.34–0.43; <0.001 ***) |
| SNRIs | 128 (0.31%) | 392 (0.95%) | 0.32 (0.27–0.4; <0.001 ***) |
| Venlafaxine | 96 (0.2%) | 275 (0.7%) | 0.35 (0.28–0.44; <0.001 ***) |
| Duloxetine | 32 (0.1%) | 116 (0.3%) | 0.28 (0.19–0.41; <0.001 ***) |
| Milnacipran | 1 (0.0%) | 2 (0.0%) | NA |
| Tricyclic antidepressants | 78 (0.2%) | 295 (0.7%) | 0.26 (0.2–0.34; <0.001 ***) |
| Amitriptyline | 57 (0.1%) | 252 (0.6%) | 0.23 (0.17–0.30; <0.001 ***) |
| Clomipramine | 17 (0.1%) | 40 (0.1%) | 0.42 (0.24–0.75; 0.003 **) |
| Dosulepin | 2 (0.0%) | 2 (0.0%) | NA |
| Maprotiline | 1 (0.0%) | 1 (0.0%) | NA |
| Trimipramine | 1 (0.0%) | 1 (0.0%) | NA |
| Amoxapine | 1 (0.0%) | 0 (0.0%) | NA |
| Imipramine | 0 (0.0%) | 1 (0.0%) | NA |
| Other antidepressants | 211 (0.51%) | 423 (1.02%) | 0.50 (0.42–0.59; <0.001 ***) |
| Mianserin | 124 (0.3%) | 264 (0.6%) | 0.47 (0.38–0.58; <0.001 ***) |
| Mirtazapine | 87 (0.2%) | 162 (0.4%) | 0.54 (0.41–0.70; <0.001 ***) |
| Tianeptine | 1 (0.0%) | 2 (0.0%) | NA |
| Bupropion | 1 (0.0%) | 1 (0.0%) | NA |
| Number of antidepressants | |||
| 1 | 719 (1.74%) | 1820 (4.41%) | 0.38 (0.35–0.42; <0.001 ***) |
| 2+ | 53 (0.13%) | 175 (0.42%) | 0.29 (0.22–0.40; <0.001 ***) |
| Comparing 2+ versus 1 antidepressant | |||
| 1 | 719 (1.74%) | 1820 (4.41%) | Ref. |
| 2+ | 53 (0.13%) | 175 (0.42%) | 0.77 (0.56–1.05; 0.103) |
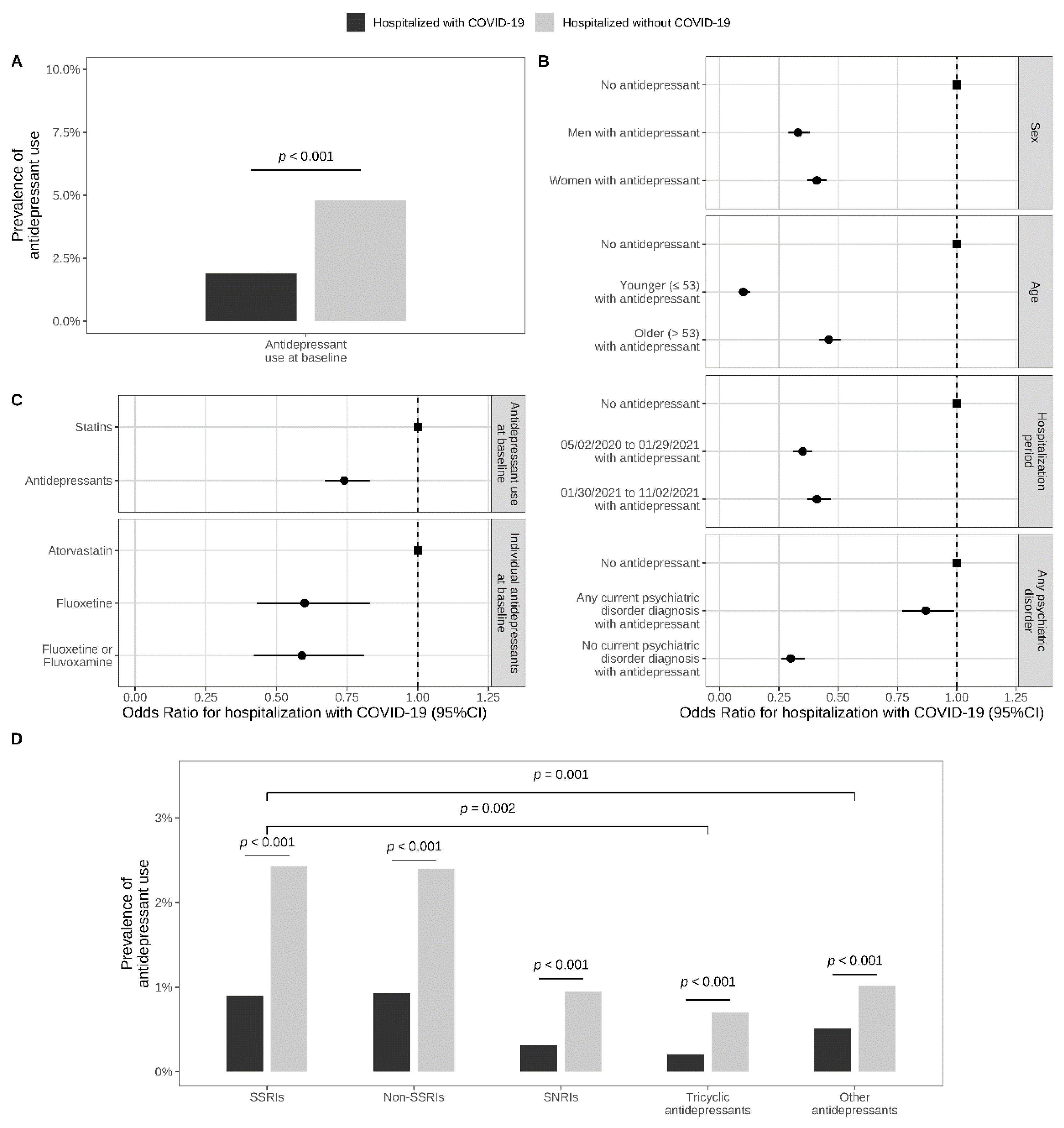
| Patients hospitalized with COVID-19 | Patients hospitalized without COVID-19 | Hospitalized with COVID-19 versus without COVID-19 | |
| N (%) | N (%) | OR (95%CI; p-value) β | |
| Antidepressants grouped by class, FIASMA class, and S1R affinity class | |||
| Comparing antidepressant classes α | N = 709 | N = 1788 | |
| SSRIs | 358 (50.5%) | 902 (50.4%) | Ref. |
| Non-SSRI antidepressants | 351 (49.5%) | 886 (49.6%) | 1.00 (0.84–1.19; 0.983) |
| SNRIs | 110 (15.5%) | 331 (18.5%) | 0.84 (0.65–1.07; 0.161) |
| Tricyclic antidepressants | 60 (8.5%) | 245 (13.7%) | 0.62 (0.45–0.84; 0.002 **) |
| Other antidepressants | 181 (25.5%) | 310 (17.3%) | 1.47 (1.18–1.83; 0.001 **) |
| FIASMA classes α | N = 41,209 | N = 41,289 | |
| No antidepressant | 40,521 (98.1%) | 39,298 (95.2%) | Ref. |
| High FIASMA activity | 311 (0.8%) | 1006 (2.4%) | 0.30 (0.27–0.35; <0.001 ***) |
| Lower FIASMA activity | 452 (1.1%) | 985 (2.4%) | 0.45 (0.40–0.51; <0.001 ***) |
| Comparing FIASMA classes α | N = 688 | N = 1693 | |
| High FIASMA activity | 266 (38.7%) | 827 (48.8%) | 0.66 (0.55–0.79; <0.001 ***) |
| Lower FIASMA activity | 422 (61.3%) | 866 (51.2%) | Ref. |
| S1R affinity classes | N = 40,910 | N = 40,303 | |
| No antidepressant | 40,521 (99.0%) | 39,298 (97.5%) | Ref. |
| High S1R affinity (agonist) | 50 (0.1%) | 164 (0.4%) | 0.30 (0.22–0.42; <0.001 ***) |
| Intermediate S1R affinity | 163 (0.4%) | 341 (0.8%) | 0.48 (0.39–0.57; <0.001 ***) |
| Low S1R affinity | 111 (0.3%) | 286 (0.7%) | 0.39 (0.31–0.48; <0.001 ***) |
| High S1R affinity (antagonist) | 65 (0.2%) | 214 (0.5%) | 0.30 (0.23–0.40; <0.001 ***) |
| Comparing S1R affinity classes α | N = 387 | N = 999 | |
| High S1R affinity (agonist) | 50 (12.9%) | 164 (16.4%) | 0.78 (0.53–1.15; 0.213) |
| Intermediate S1R affinity | 162 (41.9%) | 339 (33.9%) | 1.23 (0.92–1.64; 0.164) |
| Low S1R affinity | 111 (28.7%) | 285 (28.5%) | Ref. |
| High S1R affinity (antagonist) | 64 (16.5%) | 211 (21.1%) | 0.78 (0.55–1.11; 0.168) |
| Comparing antidepressant classes among antidepressants with high FIASMA activity α | N = 256 | N = 798 | |
| SSRIs | 198 (77.3%) | 554 (69.4%) | Ref. |
| Non-SSRI antidepressants | 58 (22.7%) | 244 (30.6%) | 0.67 (0.48–0.92; 0.015 *) |
| SNRIs | 0 (0.0%) | 0 (0.0%) | NA |
| Tricyclic antidepressants | 58 (22.7%) | 244 (30.6%) | 0.67 (0.48–0.92; 0.015 *) |
| Other antidepressants | 0 (0.0%) | 0 (0.0%) | NA |
| Comparing antidepressant classes among antidepressants with lower FIASMA activity α | N = 416 | N = 549 | |
| SSRIs | 153 (36.8%) | 318 (37.1%) | Ref. |
| Non-SSRI antidepressants | 263 (63.2%) | 540 (62.9%) | 1.01 (0.79–1.29; 0.922) |
| SNRIs | 84 (20.2%) | 231 (26.9%) | 0.76 (0.55–1.04; 0.082) |
| Tricyclic antidepressants | 0 (0.0%) | 0 (0.0%) | NA |
| Other antidepressants | 179 (43.0%) | 309 (36.0%) | 1.20 (0.92–1.57; 0.172) |
| Antidepressant use versus statin use α | N = 2063 | N = 4473 | |
| Antidepressants | 772 (37.4%) | 1995 (44.6%) | 0.74 (0.67–0.83; <0.001 ***) |
| Statines | 1291 (62.6%) | 2478 (55.4%) | Ref. |
| Fluoxetine use versus atorvastatin use α | N = 831 | N = 1659 | |
| Atorvastatin | 782 (94.1%) | 1501 (90.5%) | Ref. |
| Fluoxetine | 49 (5.9%) | 158 (9.5%) | 0.60 (0.43–0.83; 0.002 **) |
| Fluoxetine or fluvoxamine use versus atorvastatin use α | N = 832 | N = 1665 | |
| Atorvastatin | 782 (94.0%) | 1501 (90.2%) | Ref. |
| Fluoxetine or fluvoxamine | 50 (6.0%) | 164 (9.8%) | 0.59 (0.42–0.81; 0.001 **) |
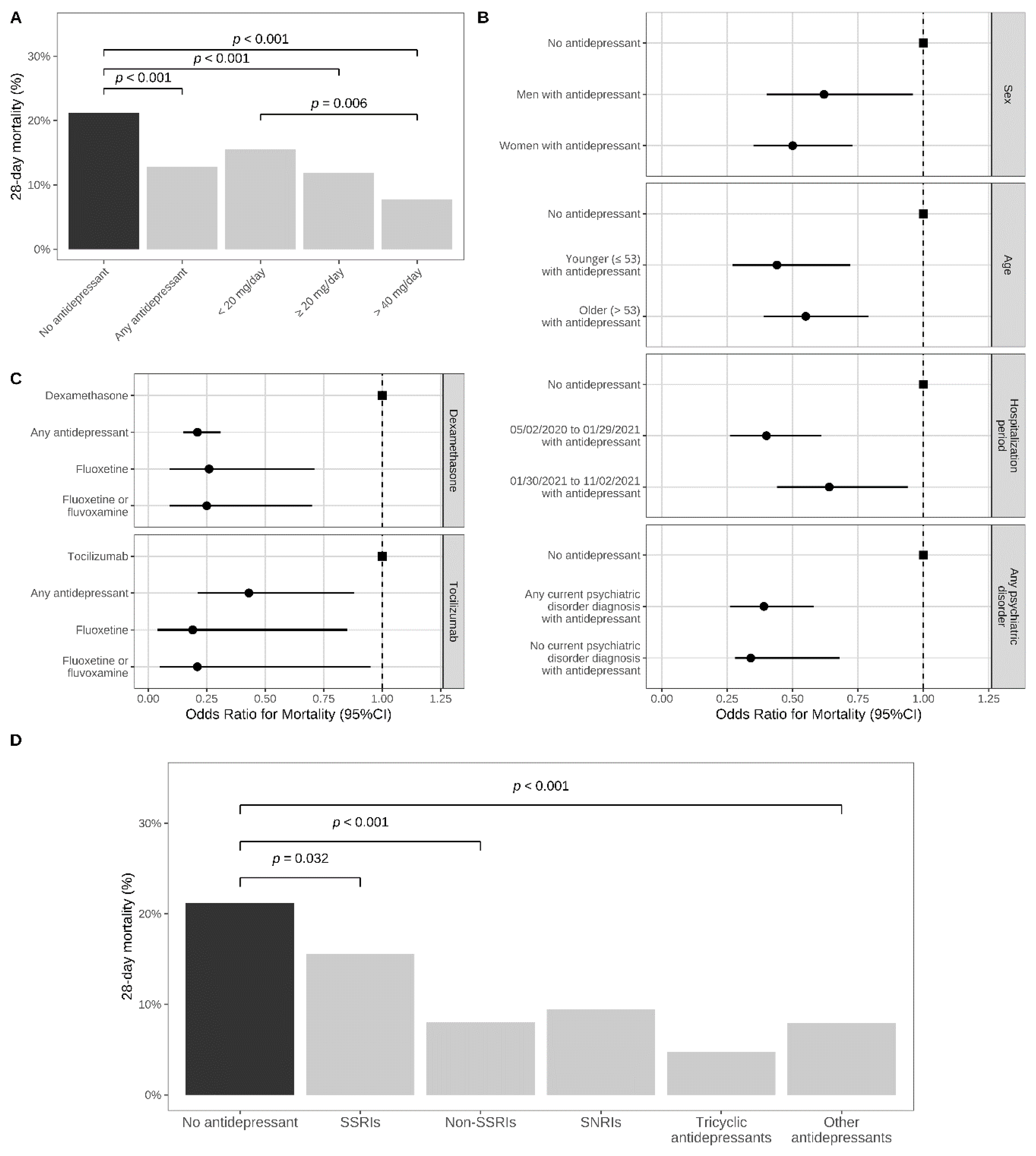
| Daily Antidepressant Dose | Antidepressant Use at Baseline | Matched Control Group Not Taking an Antidepressant at Baseline (1:1 ratio) | Crude Logistic Regression in the Matched Analytic Sample | Multivariable Logistic Regression Adjusted for Unbalanced Covariates | |
|---|---|---|---|---|---|
| Median (IQR) | Deaths/ Patients (%) | Deaths/ Patients (%) | OR (95%CI; p-Value) | AOR (95%CI; p-Value) | |
| Any antidepressant | 30.0 (19.0–49.5) | 95/741 (12.8%) | 157/741 (21.2%) | 0.55 (0.41–0.72; <0.001 ***) | - |
| Stratification by age, sex, period of hospitalization, and diagnosis of any psychiatric disorders | |||||
| Sex | |||||
| Women | 30.4 (17.5–48.0) | 54/477 (11.3%) | 91/454 (20.0%) | 0.51 (0.35–0.73; <0.001 ***) | 0.50 (0.35–0.73; <0.001 ***) a |
| Men | 25.0 (20–50.8) | 41/264 (15.5%) | 66/287 (23.0%) | 0.62 (0.40–0.95; 0.028 *) | 0.62 (0.40–0.96; 0.031 *) b |
| Age | |||||
| Younger patients (≤79 y) | 30.0 (20.0–52.1) | 25/341 (7.3%) | 58/378 (15.3%) | 0.44 (0.27–0.72; 0.001 **) | - |
| Older patients (>79 y) | 25.5 (15.0–47.4) | 70/400 (17.5%) | 99/363 (27.3%) | 0.57 (0.40–0.80; 0.001 **) | 0.55 (0.39–0.79; 0.001 **) c |
| Period of hospitalization | |||||
| 2 May 2020–29 January 2021 | 33.0 (20.0–50.9) | 38/373 (10.2%) | 76/368 (20.7%) | 0.44 (0.29–0.66; <0.001 ***) | 0.40 (0.26–0.61; <0.001 ***) d |
| 30 January 2021–2 November 2021 | 25.0 (16.5–45.0) | 57/368 (15.5%) | 81/373 (21.7%) | 0.44 (0.45–0.96; <0.001 ***) | 0.64 (0.44–0.94; 0.023 *) e |
| Psychiatric disorders | |||||
| Patients with any psychiatric disorder | 35.3 (20–60) | 45/388 (11.6%) | 102/405 (25.2%) | 0.39 (0.27–0.57; <0.001 ***) | 0.39 (0.26–0.58; <0.001 ***) f |
| Patients without any psychiatric disorder | 24.1 (15.9–40.5) | 50/353 (14.2%) | 94/336 (28%) | 0.42 (0.29–0.62; <0.001 ***) | 0.44 (0.28–0.68; <0.001 ***) g |
| Dose effect | |||||
| Fluoxetine-equivalent daily dose (mg) | |||||
| <20 mg | 10.1 (6.0–11.9) | 29/187 (15.5%) | 157/741 (21.2%) | 0.68 (0.44–1.05; 0.086) | - |
| ≥20 mg | 40.0 (23.7–60.0) | 66/553 (11.9%) | 157/741 (21.2%) | 0.50 (0.37–0.69; <0.001 ***) | - |
| 20 mg–60 mg | 40.0 (20.0–40.0) | 53/423 (12.5%) | 157/741 (21.2%) | 0.53 (0.38–0.75; <0.001 ***) | - |
| >40 mg | 64.0 (50.6–81.0) | 18/233 (7.7%) | 157/741 (21.2%) | 0.31 (0.19–0.52; <0.001 ***) | - |
| >60 mg | 80.0 (79.1–117.5) | 13/130 (10.0%) | 157/741 (21.2%) | 0.41 (0.23–0.75; 0.004 **) | - |
| Fluoxetine-equivalent daily dose (mg) | |||||
| <20 mg | 10.1 (6.0–11.9) | 29/187 (15.5%) | - | Ref. | - |
| ≥20 mg | 40.0 (23.7–60.0) | 66/553 (11.9%) | - | 0.74 (0.46–1.18; 0.208) | - |
| 20 mg–60 mg | 40.0 (20.0–40.0) | 53/423 (12.5%) | - | 0.78 (0.48–1.27; 0.321) | - |
| >40 mg | 64.0 (50.6–81.0) | 18/233 (7.7%) | - | 0.47 (0.27–0.80; 0.006 **) | - |
| >60 mg | 80.0 (79.1–117.5) | 13/130 (10.0%) | - | 0.72 (0.39–1.33; 0.289) | - |
| Number of antidepressants | |||||
| 1 | 26.2 (16.0–45.0) | 89/689 (12.9%) | 157/741 (21.2%) | 0.55 (0.42–0.73; <0.001 ***) | |
| 2+ | 48.1 (27.9–74.7) | 6/52 (11.5%) | 157/741 (21.2%) | 0.49 (0.20–1.16; 0.103) | |
| Comparing 2+ versus one antidepressant | |||||
| 1 | 26.2 (16.0–45.0) | 89/689 (12.9%) | - | Ref. | - |
| 2+ | 48.1 (27.9–74.7) | 6/52 (11.5%) | - | 0.87 (0.36–2.12; 0.774) | - |
| Daily antidepressant dose | Antidepressant use at baseline | Matched control group not taking an antidepressant at baseline (1:5 ratio) | Crude logistic regression in the matched analytic sample | Multivariable logistic regression adjusted for unbalanced covariates | |
| Median (IQR) | Deaths/ Patients (%) | Deaths/ Patients (%) | OR (95%CI; p-value) | AOR (95%CI; p-value) | |
| Individual antidepressants | |||||
| SSRIs | |||||
| Escitalopram | 30.0 (20.0–40.0) | 20/123 (16.3%) | 137/615 (22.3%) | 0.68 (0.40–1.13; 0.139) | 0.56 (0.33–0.95; 0.031 *) h |
| Paroxetine | 30.0 (20.0–40.0) | 13/107 (12.1%) | 132/535 (24.7%) | 0.42 (0.23–0.78; 0.006) | 0.43 (0.23–0.79; 0.007 **) i |
| Sertraline | 40.0 (20.0–50.0) | 8/55 (14.5%) | 57/275 (20.7%) | 0.65 (0.29–1.46; 0.296) | 0.58 (0.25–1.36; 0.210) j |
| Fluoxetine | 20.0 (20.0–40.0) | 5/45 (11.1%) | 61/225 (27.1%) | 0.34 (0.13–0.89; 0.028 *) | 0.36 (0.13–0.95; 0.040 *) k |
| Citalopram | 20.0 (20.0–40.0) | 7/36 (19.4%) | 39/180 (21.7%) | 0.87 (0.36–2.14; 0.766) | 0.72 (0.28–1.84; 0.489) l |
| Vortioxetine | 22.5 (15.0–30.0) | 1/9 (11.1%) | 9/45 (20%) | 0.50 (0.06–4.53; 0.538) | 0.45 (0.04–4.84; 0.511) m |
| Fluvoxamine | 42.0 (NA) | 0/1 (0.0%) | 0/5 (0.0%) | NA | NA |
| Fluoxetine or Fluvoxamine | 20.0 (20.0–40.0) | 5/46 (10.9%) | 61/230 (26.5%) | 0.34 (0.13–0.89; 0.029 *) | 0.36 (0.13–0.96; 0.040 *) n |
| SNRIs | |||||
| Venlafaxine | 20.2 (10.1–40.5) | 7/90 (7.8%) | 99/450 (22%) | 0.30 (0.13–0.67; 0.003 *) | 0.28 (0.13–0.64; 0.002 **) o |
| Duloxetin | 40.2 (40.2–60.3) | 1/30 (3.3%) | 24/150 (16%) | 0.18 (0.02–1.39; 0.101) | 0.29 (0.03–2.48; 0.258) p |
| Milnacipran | 30.0 (NA) | 0/1 (0.0%) | 0/5 (0.0%) | NA | NA |
| Tricyclic antidepressants | |||||
| Amitriptyline | 8.2 (3.4–19.0) | 6/54 (11.1%) | 51/270 (18.9%) | 0.54 (0.22–1.32; 0.176) | 0.62 (0.24–1.61; 0.328) q |
| Clomipramine | 31.5 (26.2–35.0) | 3/17 (17.6%) | 18/85 (21.2%) | 0.80 (0.21–3.08; 0.743) | 1.15 (0.27–4.87; 0.853) r |
| Dosulepine | 87.0 (NA) | 0/1 (0.0%) | 2/5 (40.0%) | NA | NA |
| Maprotiline | 51.0 (NA) | 0/1 (0.0%) | 0/5 (0.0%) | NA | NA |
| Trimipramine | 45.0 (NA) | 0/1 (0.0%) | 3/5 (60.0%) | NA | NA |
| Amoxapine | 30.0 (NA) | 0/1 (0.0%) | 1/5 (20.0%) | NA | NA |
| Other antidepressants | |||||
| Mianserin | 8.0 (4.0–12.0) | 24/122 (19.7%) | 139/610 (22.8%) | 0.83 (0.51–1.35; 0.451) | 0.66 (0.40–1.09; 0.106) s |
| Mirtazapine | 23.7 (11.9–35.6) | 6/85 (7.1%) | 97/425 (22.8%) | 0.26 (0.11–0.61; 0.002 *) | 0.21 (0.09–0.5; <0.001 ***) t |
| Tianeptine | 60.0 (NA) | 0/1 (0.0%) | 2/5 (40.0%) | NA | NA |
| Bupropion | 16.5 (NA) | 0/1 (0.0%) | 0/5 (0.0%) | NA | NA |
| Daily antidepressant dose | Antidepressant use at baseline | Matched control group not taking an antidepressant at baseline (1:1 ratio) | Crude logistic regression in the matched analytic sample | Multivariable logistic regression | |
| Median (IQR) | Deaths/ Patients (%) | Deaths/ Patients (%) | OR (95%CI; p-value) | AOR (95%CI; p-value) β | |
| Antidepressants prescribed at the usual fluoxetine-equivalent daily dose (20–60 mg) grouped by class, FIASMA, and S1R affinity | |||||
| Antidepressant classes α | N = 387 | N = 741 | |||
| SSRIs | 40.0 (20.0–40.0) | 39/250 (15.6%) | 157/741 (21.2%) | 0.69 (0.47–1.01; 0.056) | 0.63 (0.41–0.96; 0.032 *) |
| Non-SSRI antidepressants | 30.0 (23.7–40.5) | 11/137 (8.03%) | 157/741 (21.2%) | 0.32 (0.17–0.62; 0.001 ***) | 0.23 (0.12–0.47; <0.001 ***) |
| SNRIs | 30.4 (20.2–40.5) | 5/53 (9.43%) | 157/741 (21.2%) | 0.39 (0.15–0.99; 0.048 *) | 0.39 (0.14–1.06; 0.064) |
| Tricyclic antidepressants | 26.4 (24.8–35.0) | 1/21 (4.76%) | 157/741 (21.2%) | NA | NA |
| Other antidepressants | 26.0 (23.7–47.4) | 5/63 (7.94%) | 157/741 (21.2%) | 0.32 (0.13–0.81; 0.017 *) | 0.15 (0.06–0.42; <0.001 ***) |
| Comparing antidepressant classes α | N = 387 | ||||
| SSRIs | 40.0 (20.0–40.0) | 39/250 (15.6%) | - | Ref. | Ref. |
| Non-SSRI antidepressants | 30.0 (23.7–40.5) | 11/137 (8.03%) | - | 0.47 (0.23–0.96; 0.037 *) | 0.41 (0.18–0.92; 0.031 *) |
| SNRIs | 30.4 (20.2–40.5) | 5/53 (9.43%) | - | 0.56 (0.21–1.51; 0.253) | 0.74 (0.24–2.26; 0.593) |
| Tricyclic antidepressants | 26.4 (24.8–35.0) | 1/21 (4.76%) | - | NA | NA |
| Other antidepressants | 26.0 (23.7–47.4) | 5/63 (7.94%) | - | 0.47 (0.18–1.24; 0.125) | 0.27 (0.09–0.8; 0.018 *) |
| FIASMA classes α | N = 261 | N = 741 | |||
| High FIASMA | 31.5 (20.0–40.0) | 20/156 (12.8%) | 157/741 (21.2%) | 0.55 (0.33–0.90; 0.018 *) | 0.53 (0.31–0.91; 0.022 *) |
| Lower FIASMA | 40.0 (20.0–40.0) | 21/105 (20.0%) | 157/741 (21.2%) | 0.93 (0.56–1.55; 0.78) | 0.72 (0.40–1.28; 0.262) |
| Comparing FIASMA classes α | N = 261 | ||||
| High FIASMA | 31.5 (20.0–40.0) | 20/156 (12.8%) | - | 0.59 (0.30–1.15; 0.121) | 0.71 (0.32–1.59; 0.409) |
| Lower FIASMA | 40.0 (20.0–40.0) | 21/105 (20.0%) | - | Ref. | Ref. |
| S1R affinity classes α | N = 249 | N = 741 | |||
| High S1R affinity (agonist) | 20.0 (20.0–40.0) | 3/30 (10.0%) | 157/741 (21.2%) | 0.41 (0.12–1.38; 0.151) | 0.45 (0.13–1.58; 0.211) |
| Intermediate S1R affinity | 40.0 (20.0–40.0) | 19/89 (21.3%) | 157/741 (21.2%) | 1.01 (0.59–1.73; 0.972) | 0.88 (0.47–1.63; 0.685) |
| Low S1R affinity | 30.0 (20.0–40.0) | 11/85 (12.9%) | 157/741 (21.2%) | 0.55 (0.29–1.07; 0.077) | 0.51 (0.25–1.05; 0.068) |
| High S1R affinity (antagonist) | 30.0 (20.0–40.0) | 7/45 (15.6%) | 157/741 (21.2%) | 0.69 (0.3–1.56; 0.369) | 0.66 (0.27–1.61; 0.358) |
| Comparing S1R affinity classes α | N = 249 | ||||
| High S1R affinity (agonist) | 20.0 (20.0–40.0) | 3/30 (10.0%) | - | 0.75 (0.19–2.88; 0.673) | 1.85 (0.71–4.86; 0.211) |
| Intermediate S1R affinity | 40.0 (20.0–40.0) | 19/89 (21.3%) | - | 1.83 (0.81–4.11; 0.146) | 1.01 (0.23–4.42; 0.989) |
| Low S1R affinity | 30.0 (20.0–40.0) | 11/85 (12.9%) | - | Ref. | Ref. |
| High S1R affinity (antagonist) | 30.0 (20.0–40.0) | 7/45 (15.6%) | - | 1.24 (0.44–3.45; 0.682) | 1.29 (0.40–4.19; 0.668) |
| Comparing antidepressant classes among antidepressants with high FIASMA α | N = 178 | ||||
| SSRIs | 30.0 (20.0–40.0) | 19/158 (12.0%) | - | Ref. | Ref. |
| Non-SSRI antidepressants | 26.3 (24.8–35.0) | 1/20 (5.0%) | - | NA | NA |
| SNRIs | NA | NA | - | NA | NA |
| Tricyclic antidepressants | 26.3 (24.8–35.0) | 1/20 (5.0%) | - | NA | NA |
| Other antidepressants | NA | NA | - | NA | NA |
| Comparing antidepressant classes among antidepressants with lower FIASMA α | N = 289 | ||||
| SSRIs | 40.0 (20.0–40.0) | 19/89 (21.3%) | - | Ref. | Ref. |
| Non-SSRI antidepressants | 26.0 (23.7–40.5) | 9/100 (9.0%) | - | 0.36 (0.16–0.85; 0.020 *) | 0.22 (0.07–0.69; 0.010 *) |
| SNRIs | 30.4 (20.2–40.5) | 4/39 (10.3%) | - | 0.42 (0.13–1.33; 0.141) | 0.6 (0.12–2.89; 0.522) |
| Tricyclic antidepressants | NA | NA | - | NA | NA |
| Other antidepressants | 26.0 (23.7–47.4) | 5/61 (8.2%) | - | 0.33 (0.12–0.94; 0.037 *) | 0.13 (0.03–0.51; 0.003 **) |
| Daily antidepressant dose | Antidepressant use at baseline | Matched control group taking an active comparator at baseline (1:1 ratio) | Crude logistic regression in the matched analytic sample | Multivariable logistic regression adjusted for unbalanced covariates | |
| Median (IQR) | Deaths/ Patients (%) | Deaths/ Patients (%) | OR (95%CI; p-value) | AOR (95%CI; p-value) | |
| Antidepressant use versus dexamethasone | 30.0 (19.0–49.5) | 53/518 (10.2%) | 157/518 (30.3%) | 0.26 (0.19–0.37; <0.001 *) | 0.21 (0.15–0.31; <0.001 *) u |
| Antidepressant use versus tocilizumab | 23.7 (15.2–40.5) | 39/306 (12.7%) | 59/306 (19.3%) | 0.61 (0.39–0.95; 0.028 *) | 0.43 (0.21–0.88; 0.022 *) v |
| Daily antidepressant dose | Antidepressant use at baseline | Matched control group taking an active comparator at baseline (1:5 ratio) | Crude logistic regression in the matched analytic sample | Multivariable logistic regression adjusted for unbalanced covariates | |
| Median (IQR) | Deaths/ Patients (%) | Deaths/ Patients (%) | OR (95%CI; p-value) | AOR (95%CI; p-value) | |
| Fluoxetine use versus dexamethasone | 20.0 (20.0–40.0) | 5/45 (11.1%) | 73/225 (32.4%) | 0.26 (0.1–0.69; 0.007 **) | 0.26 (0.09–0.71; 0.009 **) w |
| Fluoxetine use versus tocilizumab | 20.0 (20.0–40.0) | 4/44 (9.1%) | 50/220 (22.7%) | 0.34 (0.12–1.00; 0.049 *) | 0.19 (0.04–0.85; 0.030 *) x |
| Fluoxetine or fluvoxamine use versus dexamethasone | 20.0 (20.0–40.0) | 5/46 (10.9%) | 74/230 (32.2%) | 0.26 (0.10–0.68; 0.006 **) | 0.25 (0.09–0.70; 0.008 **) y |
| Fluoxetine or fluvoxamine use versus tocilizumab | 20.0 (20.0–40.0) | 4/45 (8.9%) | 52/225 (23.1%) | 0.32 (0.11–0.95; 0.040 *) | 0.21 (0.05–0.95; 0.043 *) z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoertel, N.; Sánchez-Rico, M.; Kornhuber, J.; Gulbins, E.; Reiersen, A.M.; Lenze, E.J.; Fritz, B.A.; Jalali, F.; Mills, E.J.; Cougoule, C.; et al. Antidepressant Use and Its Association with 28-Day Mortality in Inpatients with SARS-CoV-2: Support for the FIASMA Model against COVID-19. J. Clin. Med. 2022, 11, 5882. https://doi.org/10.3390/jcm11195882
Hoertel N, Sánchez-Rico M, Kornhuber J, Gulbins E, Reiersen AM, Lenze EJ, Fritz BA, Jalali F, Mills EJ, Cougoule C, et al. Antidepressant Use and Its Association with 28-Day Mortality in Inpatients with SARS-CoV-2: Support for the FIASMA Model against COVID-19. Journal of Clinical Medicine. 2022; 11(19):5882. https://doi.org/10.3390/jcm11195882
Chicago/Turabian StyleHoertel, Nicolas, Marina Sánchez-Rico, Johannes Kornhuber, Erich Gulbins, Angela M. Reiersen, Eric J. Lenze, Bradley A. Fritz, Farid Jalali, Edward J. Mills, Céline Cougoule, and et al. 2022. "Antidepressant Use and Its Association with 28-Day Mortality in Inpatients with SARS-CoV-2: Support for the FIASMA Model against COVID-19" Journal of Clinical Medicine 11, no. 19: 5882. https://doi.org/10.3390/jcm11195882
APA StyleHoertel, N., Sánchez-Rico, M., Kornhuber, J., Gulbins, E., Reiersen, A. M., Lenze, E. J., Fritz, B. A., Jalali, F., Mills, E. J., Cougoule, C., Carpinteiro, A., Mühle, C., Becker, K. A., Boulware, D. R., Blanco, C., Alvarado, J. M., Strub-Wourgaft, N., Lemogne, C., Limosin, F., & on behalf of AP-HP/Université Paris Cité/INSERM COVID-19 Research Collaboration, AP-HP COVID CDR Initiative and “Entrepôt de Données de Santé” AP-HP Consortium. (2022). Antidepressant Use and Its Association with 28-Day Mortality in Inpatients with SARS-CoV-2: Support for the FIASMA Model against COVID-19. Journal of Clinical Medicine, 11(19), 5882. https://doi.org/10.3390/jcm11195882







