Abstract
Background: LUS is a validated tool for the management of COVID-19 pneumonia. Chair positioning (CP) may have beneficial effects on oxygenation and lung aeration, and may be an easier alternative to PP. This study assessed the effects of a CP session on oxygenation and lung aeration (LA) changes in non-intubated COVID-19 patients. Methods: A retrospective multicenter study was conducted in an ICU. We analyzed data from LUS exams and SpO2:FiO2 performed before/after a CP session in non-intubated COVID-19 patients. Patients were divided into groups of responders or non-responders in terms of oxygenation or LA. Results: Thirty-three patients were included in the study; fourteen (44%) were oxygenation non-responders and eighteen (56%) were oxygenation responders, while thirteen (40.6%) and nineteen (59.4%) patients were classified as LA non-responders and responders, respectively. Changes in oxygenation and LA before/after a CP session were not correlated (r = −0.19, p = 0.3, 95% CI: −0.5–0.17). The reaeration scores did not differ between oxygenation responders and non-responders (1 (−0.75–3.75) vs. 4 (−1–6), p = 0.41). The LUS score was significantly correlated with SpO2:FiO2 before a CP session (r = 0.37, p = 0.04, 95% CI: 0.03–0.64) but not after (r = 0.17, p = 0.35, 95% CI: −0.19–0.50). Conclusion: A CP session was associated with improved oxygenation and LA in more than half of the non-intubated COVID-19 patients.
1. Introduction
Lung ultrasound (LUS) is a validated tool and possible alternative to chest computed tomography (CT) scans for the management of coronavirus disease 2019 (COVID-19)-related pneumonia and severity assessments [1,2]. Given its availability and feasibility, LUS could reduce the burden of overloaded CT departments. Furthermore, LUS is available at the bedside without requiring the transport of critically ill patients [3], thus reducing healthcare professionals’ risk of exposure to COVID-19 [4] and, in particular, patient-related adverse effects [5] during this pandemic. As a result, LUS is routinely used for triage and the management of intensive care unit (ICU) COVID-19 patients with acute respiratory failure. As prone positioning (PP) improves the outcomes of patients with moderate to severe acute respiratory distress syndrome (ARDS) [6], this positioning has been tested in non-intubated spontaneously breathing patients. In non-intubated COVID-19 patients, awake PP is associated with oxygenation improvement and a reduction in the need for mechanical ventilation [7]. However, the effect of PP is time-dependent, and the tolerance of prolonged awake PP is a major limitation of the procedure [8,9].
However, different positions, such as sitting/being upright, may have beneficial effects on respiratory mechanics and oxygenation in selected ARDS patients under (invasive) mechanical ventilation [10,11]. Therefore, chair positioning (CP) or Rodin’s Thinker CP could be an alternative to awake PP [12]. In addition, a recent study suggested an oxygenation improvement similar to that following awake PP after a Rodin’s Thinker positioning session [11]. The tolerability of CP may be better than that of PP in awake patients [11].
However, changes in positioning-related pathophysiological effects are always complex. During PP, LUS makes it possible to monitor lung aeration changes at the bedside [13,14]. A pilot study emphasized the potential role of LUS during awake PP [15].
Our study aimed to assess the effects of a CP session on oxygenation (using the pulse oxygen saturation:fractional inspired oxygen ratio (SpO2:FiO2)) and lung aeration (using lung reaeration scores) changes in non-intubated COVID-19 patients.
2. Materials and Methods
2.1. Study Design
A multicenter observational study was conducted in three intensive care units (ICUs) of French university hospitals (North Hospital of Marseille, La Timone Hospital Marseille, and South Hospital Lyon) using routine LUS examinations of COVID-19 patients. We retrospectively analyzed the collected data from LUS exams and the SpO2:FiO2 ratio performed before and after a CP session [13]. The study period was from January 2021 to April 2021. In our centers, a CP session was a common clinical use.
2.2. Ethical Considerations
The study protocol was approved by the Committee for Research Ethics of the French Society of Anesthesia and Intensive Care Medicine (IRB 00010254-2021-157). The patients received formal information on the use of their data. The different treatment strategies are considered standard care, and the analyses were performed retrospectively, so informed consent was waived according to French law [16].
2.3. Population
The COVID-19 patients admitted to ICUs with acute respiratory failure were included if they met the following criteria: (i) age of 18 or older, (ii) polymerase chain reaction (PCR)-documented severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal samples upon ICU admission, (iii) spontaneous pulse oxygen saturation (breathing with no previous tracheal intubation during their ICU stay), and (iv) the need for oxygen to maintain SpO2 above 90%. A CP session was performed if patients presented all of the inclusion criteria in our standard care. The exclusion criteria were the presence of subcutaneous emphysema, a CP contraindication (altered vigilance, vomiting, hemoptysis, and spinal injury), need for immediate invasive mechanical ventilation upon ICU admission, symptomatic pericardial effusion, abdominal surgery (<1 month), pregnant or lactating woman, and a follow-up of less than 24 h.
2.4. Study Protocol
At ICU admission, each patient’s features (demographic and clinical history), Simplified Acute Physiology Score II (SAPS II) [17], and Sepsis-related Organ Failure Assessment (SOFA) score [18] were collected. Each patient’s SAPS II underwent a medical examination, arterial blood gas analysis, and monitoring of the heart rate, blood pressure, respiratory rate, and SpO2. For each patient, conventional oxygen therapy, high-flow nasal cannula therapy, or non-invasive ventilation were started to maintain SpO2 above 90%. In patients undergoing conventional oxygen therapy, the FiO2 was calculated as follows: FiO2 = (21 + 3 × oxygen flow (L/min))/100 [19]. The SpO2:FiO2 ratio was previously determined, and its use was correlated to the arterial pressure in oxygen (PaO2):FiO2 ratio in the respiratory component of the SOFA [20]. The SpO2:FiO2 ratio was collected according to the standard nurse monitoring protocol. In our standard of care, each patient underwent an LUS examination in a supine semi-recumbent position (constant 40 to 45°) at ICU admission, less than 10 min before CP (LUS1), and less than 10 min after CP (LUS2). Each eligible patient was installed on a medical armchair with the assistance of caregivers for at least three consecutive hours. The patients were positioned in a vertical trunk position. LUS was performed by imaging the 12 lung regions as described elsewhere [21]. To examine the posterior areas, the patients were placed in lateral decubitus. A 1–5 MHz convex probe with standard devices for ultrasound was used (Supplementary Figure S1).
The regional aeration score featuring the anterior, lateral, and posterior lung areas was calculated. Points were allocated according to the worst ultrasound pattern observed. The LUS score corresponds to the sum of each examined area score (maximum score = 36) [22]. An ultrasound reaeration score was calculated as previously described [23] from the variation in the ultrasound pattern of each area examined between LUS1 and LUS2 (Supplementary Table S1). A positive reaeration score indicates an aeration gain, and a negative reaeration score indicates an aeration loss.
The LUS examinations were performed by senior (level 2 or 3) intensivists in charge of the patients [24,25]. The skill-levels of the operators were rated as follows [24]:
- -
- Level 2 operator: More than 25 supervised procedures and 200 non-supervised procedures.
- -
- Level 3 operator: LUS academic teacher with several publications in the field.
We collected the SpO2:FiO2 ratio at LUS1 and LUS2 [20]. Retrospectively, we defined responders and non-responders to CP sessions based on the reaeration score and SpO2:FiO2 ratio variations, respectively, as follows:
- -
- Oxygenation responders: Positive difference of SpO2:FiO2 ratios measured at LUS2 and LUS1.
- -
- Oxygenation non-responders: Negative difference of SpO2:FiO2 ratios measured at LUS2 and LUS1.
- -
- Lung aeration responders: A positive reaeration score between LUS2 and LUS1.
- -
- Lung aeration non-responders: A negative reaeration score between LUS2 and LUS1.
2.5. Outcomes
Our study aimed to assess the effects of a CP session on oxygenation (using the SpO2:FiO2 ratio) and lung aeration (using lung reaeration scores) changes in non-intubated COVID-19 patients.
The secondary objectives were to assess the following:
- -
- The mechanisms of change in oxygenation during a CP session are reflected by the correlation between oxygenation and lung aeration changes.
- -
- The performance of baseline LUS can be used to predict the effect of a CP session on oxygenation and lung aeration.
- -
- The effect of oxygenation and lung aeration responses on the outcomes (need for invasive mechanical ventilation, length of stay, ICU mortality, etc.).
2.6. Statistical Analysis
No sample size was calculated a priori, given the exploratory nature of our study. Patients were included based on convenience sampling. Quantitative variables are expressed in median and interquartile ranges. Qualitative variables are expressed in numbers and percentages. Missing values were omitted from the analysis, as no data imputation was planned. The primary outcome was assessed using a comparison of the lung aeration index (total LUS score) between the two time points with a non-parametric Mann–Whitney U test. The secondary outcomes were assessed using the same test. The correlations between quantitative variables were studied via Pearson’s correlation coefficient, its p-value, and the 95% confidence interval (CI). The quantitative variables were compared between groups using a non-parametric Mann–Whitney U test. The qualitative variables were compared between groups using Fisher’s exact test or a chi-squared test in cases of more than two classes. This observational study was reported in compliance with the STROBE guidelines [26]. All the comparisons were two-tailed. A p-value < 0.05 was required for statistical significance. Data analysis was performed using R software (Version: R 4.1.2. Vienna, Austria) [27].
3. Results
3.1. Enrolled Patients’ Characteristics
The final analysis was performed on a population of 33 patients (Figure 1). The patients’ features are illustrated in Table 1. Our cohort consisted of 58% males (42% females) with a median age of 67 (range: 53–74) years with a SAPS II of 29 (range: 24–32). Twenty-five (76%) patients received oxygen via a high-flow nasal cannula, and the median SpO2:FiO2 rate was 140 (range: 111–195).
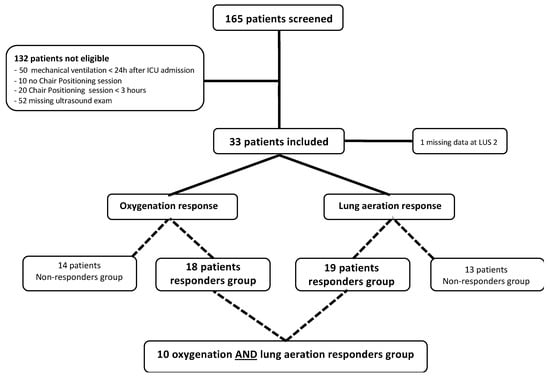
Figure 1.
Flow chart of the study.

Table 1.
Baseline clinical data of patients in the whole cohort and according to their response to chair positioning in terms of oxygenation and lung aeration.
3.2. Primary Outcome
Based on the SpO2:FiO2 ratio variations, 14 (44%) oxygenation non-responders and 18 (56%) oxygenation responders were identified. The SpO2:FiO2 ratios were 140 (range: 110–190) before CP and 160 (range: 120–200) after CP (p = 0.4) (Figure 2). Based on the reaeration score, 13 (40.6%) and 19 (59.4%) patients were classified as lung aeration non-responders and responders, respectively. The median reaeration score was 2 (interquartile range (IQR): −2, 6), with a median reaeration score of −1 (IQR: −5, −1) in non-responders and 5 (IQR: 3, 7) in responders (Table 1). The individual values of oxygenation and lung aeration changes during a CP session are presented in Figure 3. One missing value in the reaeration score explains the discrepancy in the final analysis of 32 patients.
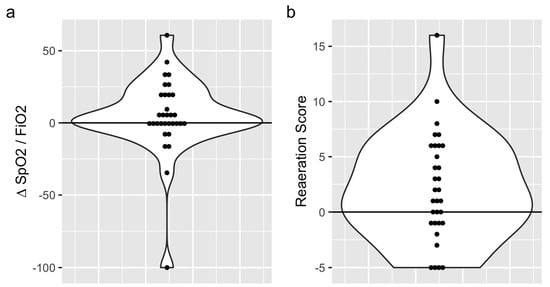
Figure 2.
Variations in the SpO2:FiO2 ration (a) and reaeration score (b) after the chair trial in the whole cohort.
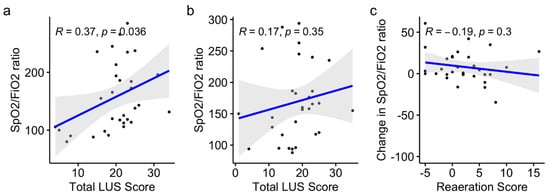
Figure 3.
Variations in the SpO2:FiO2 ratio and reaeration score before (a) and after (b) the CP session, and variation of oxygenation and reaeration after CP session (c) in the whole cohort.
3.3. Secondary Outcomes
The changes in lung aeration and oxygenation (defined by the SpO2:FiO2 ratio) before and after a CP session were not correlated. The reaeration scores did not differ between responders and non-responders in terms of oxygenation (1 (−0.75–3.75) vs. 4 (−1–6), p = 0.41). The LUS score was significantly correlated with the SpO2:FiO2 ratio before a CP session (Pearson’s r = 0.37, p = 0.04, 95% CI: 0.03–0.64) but not after (Pearson’s r = 0.17, p = 0.35, 95% CI: −0.19–0.50), as shown in Figure 3a,b. Oxygenation changes and reaeration scores after the CP sessions were not correlated (Pearson’s r = −0.19, p = 0.3, 95% CI: −0.5–0.17) (Figure 3c).
In terms of predicting a CP session response, the baseline LUS score was similar in oxygenation responders and non-responders (median: 20, IQR: (17–24) vs. 22 (20–23), p = 0.8) (Supplementary Figure S2A). The changes in the SpO2:FiO2 ratio did not significantly differ in patients with or without consolidations before the CP session (0.02, (−0.01–0.2) vs. 0.04 (−0.01–0.1), p = 0.9) (Supplementary Figure S2B). Similarly, the baseline LUS score was similar in the lung aeration responders and non-responders (median: 22, IQR: (20–24) vs. 19 (18–23), respectively, p = 0.2) (Supplementary Figure S3A). The reaeration score did not differ between patients with and without consolidations before the CP session (0.5, (−1.0–6.0) vs. 2.5 (−0.5–4.75), respectively, p = 0.09) (Supplementary Figure S3B). Regional LUS scores before and after the CP session are presented in Supplementary Figure S4.
The outcomes, including the need for invasive mechanical ventilation, duration of ICU and hospital stay, and mortality rates in the ICU, in hospital, and at day 28, were similar between the responders and non-responders in terms of the oxygenation (Supplementary Table S2A) and lung aeration responses (Supplementary Table S2B).
4. Discussion
Our study showed that a CP session was associated with an improvement in oxygenation and pulmonary aeration in more than half of our COVID-19 patients. However, the results did not show a significant correlation between oxygenation and lung aeration changes.
To the best of our knowledge, this study is the first to report the effects of a CP session on oxygenation and lung aeration in awake COVID-19 patients. A single-center study including 25 patients found that Rodin’s Thinker positioning was associated with a significant subsequent improvement in oxygenation of more than 40 mmHg of PaO2 measured immediately at the end of the session [12]. Rodin’s Thinker positioning may be theoretically more efficient than classical CP because it reverses the gravitational gradient in a manner similar to PP. Nevertheless, the mechanism underlying this effect was not assessed in this study. Furthermore, the mean PaCO2 was not altered after a Rodin’s Thinker positioning session, suggesting an insignificant effect on lung aeration. To summarize, CP and Rodin’s Thinker positioning are feasible and may improve oxygenation in selected patients, but larger studies are needed to confirm these findings.
The pathophysiological effects of positioning in patients with acute respiratory failure are difficult to assess [28].
Recently, Giosa et al. [29] found that orthodeoxia is frequent in COVID-19 patients and might partly explain the benefits of awake prone position. We cannot exclude that this phenomenon was present in the oxygenation non-responder patients of our cohort. The use of LUS allows for the bedside assessment of regional lung aeration. Thus, it represents a useful tool for assessing changes in patients with acute respiratory failure [13]. The LUS reaeration score was recently validated in comparison to a gold standard method—end-expiratory lung volume measured by an automated nitrogen washout/washing technique—for PP-induced lung inflation [14]. In a previous study, we assessed regional aeration changes during a PP session in 51 ARDS patients [13]. We found that changes in aeration and oxygenation were not correlated, suggesting that both ventilation and perfusion were critical determinants of oxygenation. In fact, using a different tool, our current results are in line with several physiopathological recent studies on awake PP effects during COVID-19. In a recent case report on a COVID-19 patient on PP using thoracic Electrical Impedance Tomography (EIT), Zarantonello et al. concluded that oxygenation gain was related to ventilation:perfusion matching improvement [30]. Combining a CT scan and EIT, Fossali et al. showed that PP effects on COVID-19 patients are mainly driven by improved ventilation:perfusion matching [31]. Finally, using EIT and an intrapulmonary shunt calculation (based on a modified Berggren equation), a recent study confirmed that PP is associated with a reduction in an intrapulmonary shunt [32]. Thus, the pulmonary blood flow could be diverted away from the reaerated lung regions in PP, resulting in ventilation:perfusion matching alteration [33].
Similar to PP, CP affects the gravitational gradient, end-expiratory lung volume, and hemodynamics. However, the magnitude of these changes is poorly described. In our patients, the lack of a correlation between oxygenation and lung aeration changes also suggests ventilation:perfusion matching alteration during a CP session, as reported during PP in mechanically ventilated COVID-19 patients [34]. It is worth noting that Langer et al. [35] showed that, in patients under invasive MV, PP was associated with improved oxygenation without any modification of respiratory system compliance, suggesting that lung recruitment was not the major mechanism. Previous studies using the ultrasound monitoring of lung aeration during PP found similar results [13,14]. In our study, lung involvement morphology assessed with baseline LUS scores did not predict subsequent changes in lung aeration or oxygenation. Only limited previous data have suggested that lung involvement morphology could predict oxygenation responses to PP in intubated or non-intubated patients [15,36]. However, larger studies have shown that baseline lung involvement profiles, either evaluated with LUS or chest computed tomography, were not predictive of responses to PP [13,37]. Thus, it seems that the complex mechanisms induced by changes in positioning are not predictable with the assessment of baseline lung involvement morphology, regardless of the imaging technique. Even if less helpful in clinical practice, changes in LUS scores during the early phase of a PP session could more accurately predict the PP response and outcome [14,38]. Future studies should determine if an early response to a CP session is predictive of oxygenation, lung aeration, or outcome improvement.
Our study has several limitations. As a retrospective analysis, we included only a convenience sample of patients who met the inclusion criteria. Due to its observational nature, we did not perform a systematic blood gas analysis at different time points. As in many other studies, we used the SpO2:FiO2 ratio is a validated surrogate of the PaO2:FiO2 ratio [20]. However, both PaO2:FiO2 and SpO2:FiO2 ratio may have intrinsic limitations during COVID-19 pneumonia management [39,40]. As our study was non-interventional, we did not perform any systematic chest CT scan. Thus, not all of the patients had a chest CT scan, and, more importantly, the delay between the chest CT scan and CP was highly variable. Consequently, we could not compare the LUS examination with chest CT scan. The number of patients was relatively small, and we did not include a control group, which precluded the analysis of clinical outcomes. In our population, the median delay between hospital admission and CP session in ICU was 8 days indicating a well-established pneumonia which may have impacted our results. Thus, the effect of a CP session on the outcome, including the need for invasive ventilation, duration of ICU and hospital stay, and mortality rate, remains to be assessed with further well-designed studies. However, this multicenter study reflects a global approach used by different teams. Finally, we included only non-intubated patients at the onset of their ICU stays. Therefore, as the response to PP and recruitment decreases over time due to progressive lung consolidations versus atelectasis [34], we cannot expand our results to patients previously intubated or those in a late stage of their disease progression.
5. Conclusions
In this pilot study, a CP session was found to be associated with improved oxygenation and lung aeration in more than half of the non-intubated COVID-19 patients. However, oxygenation and lung aeration changes were not associated, suggesting that a CP session induces ventilation:perfusion matching alteration. These preliminary results suggest that a CP session may be associated with improved oxygenation and lung aeration in select patients. The prediction of CP session responses and their effects on outcomes need to be addressed in future larger studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm11195885/s1. Supplementary Figure S1: Study protocol, Supplementary Figure S2: a/LUS scores before chair positioning session in oxygenation responders and non-responders; b/∆ SpO2:FiO2 ratios in patients with and without ultrasonographic consolidation before chair trial session, Supplementary Figure S3: a/LUS scores before chair trial session in lung aeration responders and non-responders in terms; b/Reaeration score in patients with and without ultrasonographic consolidations before chair trial, Supplementary Figure S4: Regional LUS scores before and after chair positioning session: a/Posterior LUS scores, b/Lateral LUS scores, c/Anterior LUS scores, Supplementary Table S1: Lung ultrasound reaeration score aimed at evaluating the effects of CP session on lung aeration, Supplementary Table S2: Outcomes according to oxygenation and lung aeration responders and non-responders groups a/oxygenation response, b/lung aeration response.
Author Contributions
A.L., L.Z. and M.L. were responsible for the study design, data acquisition, analysis, and interpretation, and for preparing the first draft of the manuscript. P.S., C.A., I.L., L.V., B.A., G.D. and S.H. were responsible for conducting the study. B.P., S.D., E.J. and L.J. were responsible for recruiting study participants and clinical input in the study design. L.D. and K.B. were responsible for the statistical analysis. S.H., B.A. and P.S. helped write the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no funding for this work.
Institutional Review Board Statement
This study was conducted with the support of the French Society of Anesthesia and Resuscitation (SFAR), the French College of Anesthesia and Resuscitation (CFAR), regional university representatives, and unions.
Informed Consent Statement
According to French law, people were informed of their initial inclusion in the database.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors: alexandre.lopez@ap-hm.fr (A.L.); pierre.simeone@ap-hm.fr (P.S.); marc.leone@ap-hm.fr (M.L.); laurent.zieleskiewicz@ap-hm.fr (L.Z.).
Acknowledgments
The authors are indebted to the nurses and the ICU staff managing SARS-CoV-2 patients’ bedsides.
Conflicts of Interest
L.Z. received fees from General Electric Healthcare for teaching ultrasound to General Electric Healthcare customers. The other authors do not have any link of interest related to this work.
References
- Zieleskiewicz, L.; Markarian, T.; Lopez, A.; Taguet, C.; Mohammedi, N.; Boucekine, M.; Baumstarck, K.; Besch, G.; Mathon, G.; Duclos, G.; et al. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020, 46, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Via, G.; Melniker, L.; Goffi, A.; Tavazzi, G.; Neri, L.; Villen, T.; Hoppmann, R.; Mojoli, F.; Noble, V.; et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): International expert consensus. Crit. Care 2020, 24, 702. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, M.; Forel, J.-M.; De Bourmont, S.; Jung, B.; Thomas, G.; Mahul, M.; Bisbal, M.; Nougaret, S.; Hraiech, S.; Roch, A.; et al. Diagnostic yield and safety of CT scans in ICU. Intensive Care Med. 2015, 41, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Pata, D.; Chiaretti, A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir. Med. 2020, 8, e27. [Google Scholar] [CrossRef]
- Jia, L.; Wang, H.; Gao, Y.; Liu, H.; Yu, K. High incidence of adverse events during intra-hospital transport of critically ill patients and new related risk factors: A prospective, multicenter study in China. Crit. Care 2016, 20, 12. [Google Scholar] [CrossRef]
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranieri, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, S280–S288. [Google Scholar] [CrossRef]
- Ehrmann, S.; Li, J.; Ibarra-Estrada, M.; Perez, Y.; Pavlov, I.; McNicholas, B.; Roca, O.; Mirza, S.; Vines, D.; Garcia-Salcido, R.; et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: A randomised, controlled, multinational, open-label meta-trial. Lancet Respir. Med. 2021, 9, 1387–1395. [Google Scholar] [CrossRef]
- Coppo, A.; Bellani, G.; Winterton, D.; Di Pierro, M.; Soria, A.; Faverio, P.; Cairo, M.; Mori, S.; Messinesi, G.; Contro, E.; et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir. Med. 2020, 8, 765–774. [Google Scholar] [CrossRef]
- Koeckerling, D.; Barker, J.; Mudalige, N.L.; Oyefeso, O.; Pan, D.; Pareek, M.; Thompson, J.P.; Ng, G.A. Awake prone positioning in COVID-19. Thorax 2020, 75, 833–834. [Google Scholar] [CrossRef]
- Robak, O.; Schellongowski, P.; Bojic, A.; Laczika, K.; Locker, G.J.; Staudinger, T. Short-term effects of combining upright and prone positions in patients with ARDS: A prospective randomized study. Crit. Care 2011, 15, R230. [Google Scholar] [CrossRef]
- Griffiths, M.J.D.; McAuley, D.F.; Perkins, G.D.; Barrett, N.; Blackwood, B.; Boyle, A.; Chee, N.; Connolly, B.; Dark, P.; Finney, S.; et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp. Res. 2019, 6, e000420. [Google Scholar] [CrossRef] [PubMed]
- Coppo, A.; Winterton, D.; Benini, A.; Monzani, A.; Aletti, G.; Cadore, B.; Isgrò, S.; Pizzagalli, J.; Bellani, G.; Foti, G. Rodin’s Thinker: An Alternative Position in Awake Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Haddam, M.; Zieleskiewicz, L.; Perbet, S.; Baldovini, A.; Guervilly, C.; Arbelot, C.; Noel, A.; Vigne, C.; Hammad, E.; Antonini, F.; et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016, 42, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Rousset, D.; Sarton, B.; Riu, B.; Bataille, B.; Silva, S. Bedside ultrasound monitoring of prone position induced lung inflation. Intensive Care Med. 2021, 47, 626–628. [Google Scholar] [CrossRef]
- Avdeev, S.N.; Nekludova, G.V.; Trushenko, N.V.; Tsareva, N.A.; Yaroshetskiy, A.I.; Kosanovic, D. Lung ultrasound can predict response to the prone position in awake non-intubated patients with COVID-19 associated acute respiratory distress syndrome. Crit. Care 2021, 25, 35. [Google Scholar] [CrossRef]
- Toulouse, E.; Lafont, B.; Granier, S.; Mcgurk, G.; Bazin, J.-E. French legal approach to patient consent in clinical research. Anaesth. Crit. Care Pain Med. 2020, 39, 883–885. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Frat, J.-P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Shintani, A.K.; Hagerman, H.E.; St Jacques, P.J.; Rice, T.W.; Sanders, N.W.; Ware, L.B.; Bernard, G.R.; Ely, E.W. Derivation and validation of SpO2/FiO2 ratio to impute for PaO2/FiO2 ratio in the respiratory component of the Sequential Organ Failure Assessment (SOFA) Score. Crit. Care Med. 2009, 37, 1317–1321. [Google Scholar] [CrossRef]
- Mojoli, F.; Bouhemad, B.; Mongodi, S.; Lichtenstein, D. Lung Ultrasound for Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2018, 199, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Bouhemad, B.; Zhang, M.; Lu, Q.; Rouby, J.-J. Clinical review: Bedside lung ultrasound in critical care practice. Crit. Care 2007, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Bouhemad, B.; Liu, Z.-H.; Arbelot, C.; Zhang, M.; Ferarri, F.; Le-Guen, M.; Girard, M.; Lu, Q.; Rouby, J.-J. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit. Care Med. 2010, 38, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Arbelot, C.; Neto, F.L.D.; Gao, Y.; Brisson, H.; Chunyao, W.; Lv, J.; Barbas, C.S.V.; Perbet, S.; Caltabellotta, F.P.; Gay, F.; et al. Lung Ultrasound in Emergency and Critically Ill PatientsNumber of Supervised Exams to Reach Basic Competence. Anesthesiology 2020, 132, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Richard, J.-C.M.; Maggiore, S.M.; Mancebo, J.; Lemaire, F.; Jonson, B.; Brochard, L. Effects of vertical positioning on gas exchange and lung volumes in acute respiratory distress syndrome. Intensive Care Med. 2006, 32, 1623–1626. [Google Scholar] [CrossRef]
- Giosa, L.; Payen, D.; Busana, M.; Mattei, A.; Brazzi, L.; Caironi, P. Orthodeoxia and its implications on awake-proning in COVID-19 pneumonia. Crit. Care 2021, 25, 429. [Google Scholar] [CrossRef]
- Zarantonello, F.; Andreatta, G.; Sella, N.; Navalesi, P. Prone Position and Lung Ventilation and Perfusion Matching in Acute Respiratory Failure due to COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 278–279. [Google Scholar] [CrossRef]
- Fossali, T.; Pavlovsky, B.; Ottolina, D.; Colombo, R.; Basile, M.C.; Castelli, A.; Rech, R.; Borghi, B.; Ianniello, A.; Flor, N.; et al. Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients with COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. Crit. Care Med. 2022, 50, 723–732. [Google Scholar] [CrossRef]
- Dos Santos Rocha, A.; Diaper, J.; Balogh, A.L.; Marti, C.; Grosgurin, O.; Habre, W.; Peták, F.; Südy, R. Effect of body position on the redistribution of regional lung aeration during invasive and non-invasive ventilation of COVID-19 patients. Sci. Rep. 2022, 12, 11085. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.; Gattinoni, L. Assessment of oxygenation response to prone position ventilation in ARDS by lung ultrasonography. Intensive Care Med. 2016, 42, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Palumbo, M.M.; Sverzellati, N.; Busana, M.; Malchiodi, L.; Bresciani, P.; Ceccarelli, P.; Sani, E.; Romitti, F.; Bonifazi, M.; et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. 2022, 48, 56–66. [Google Scholar] [CrossRef]
- Langer, T.; Brioni, M.; Guzzardella, A.; Carlesso, E.; Cabrini, L.; Castelli, G.; Dalla Corte, F.; De Robertis, E.; Favarato, M.; Forastieri, A.; et al. Prone position in intubated, mechanically ventilated patients with COVID-19: A multi-centric study of more than 1000 patients. Crit. Care 2021, 25, 128. [Google Scholar] [CrossRef] [PubMed]
- Prat, G.; Guinard, S.; Bizien, N.; Nowak, E.; Tonnelier, J.-M.; Alavi, Z.; Renault, A.; Boles, J.-M.; L’Her, E. Can lung ultrasonography predict prone positioning response in acute respiratory distress syndrome patients? J. Crit. Care 2016, 32, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Paladini, M.-H.; Bregeon, F.; Thirion, X.; Durieux, O.; Gainnier, M.; Huiart, L.; Agostini, S.; Auffray, J.-P. Can the tomographic aspect characteristics of patients presenting with acute respiratory distress syndrome predict improvement in oxygenation-related response to the prone position? Anesthesiology 2002, 97, 599–607. [Google Scholar] [CrossRef]
- Wang, X.-T.; Ding, X.; Zhang, H.-M.; Chen, H.; Su, L.-X.; Liu, D.-W. Chinese Critical Ultrasound Study Group (CCUSG) Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit. Care 2016, 20, 385. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Jubran, A.; Laghi, F. PaO2/FIO2 ratio: The mismeasure of oxygenation in COVID-19. Eur. Respir. J. 2021, 57, 2100274. [Google Scholar] [CrossRef]
- de Carvalho, E.B.; Leite, T.R.S.; de Sacramento, R.F.M.; do Nascimento, P.R.L.; Samary, C.D.S.; Rocco, P.R.M.; Silva, P.L. Rationale and limitations of the SpO2/FiO2 as a possible substitute for PaO2/FiO2 in different preclinical and clinical scenarios. Rev. Bras. Ter. Intensiv. 2022, 34, 185–196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).