Intraoral Scanning as an Alternative to Evaluate the Accuracy of Dental Implant Placements in Partially Edentate Situations: A Prospective Clinical Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Preoperative Data Acquisition
2.3. Virtual Implant Planning
2.4. Implant Placement
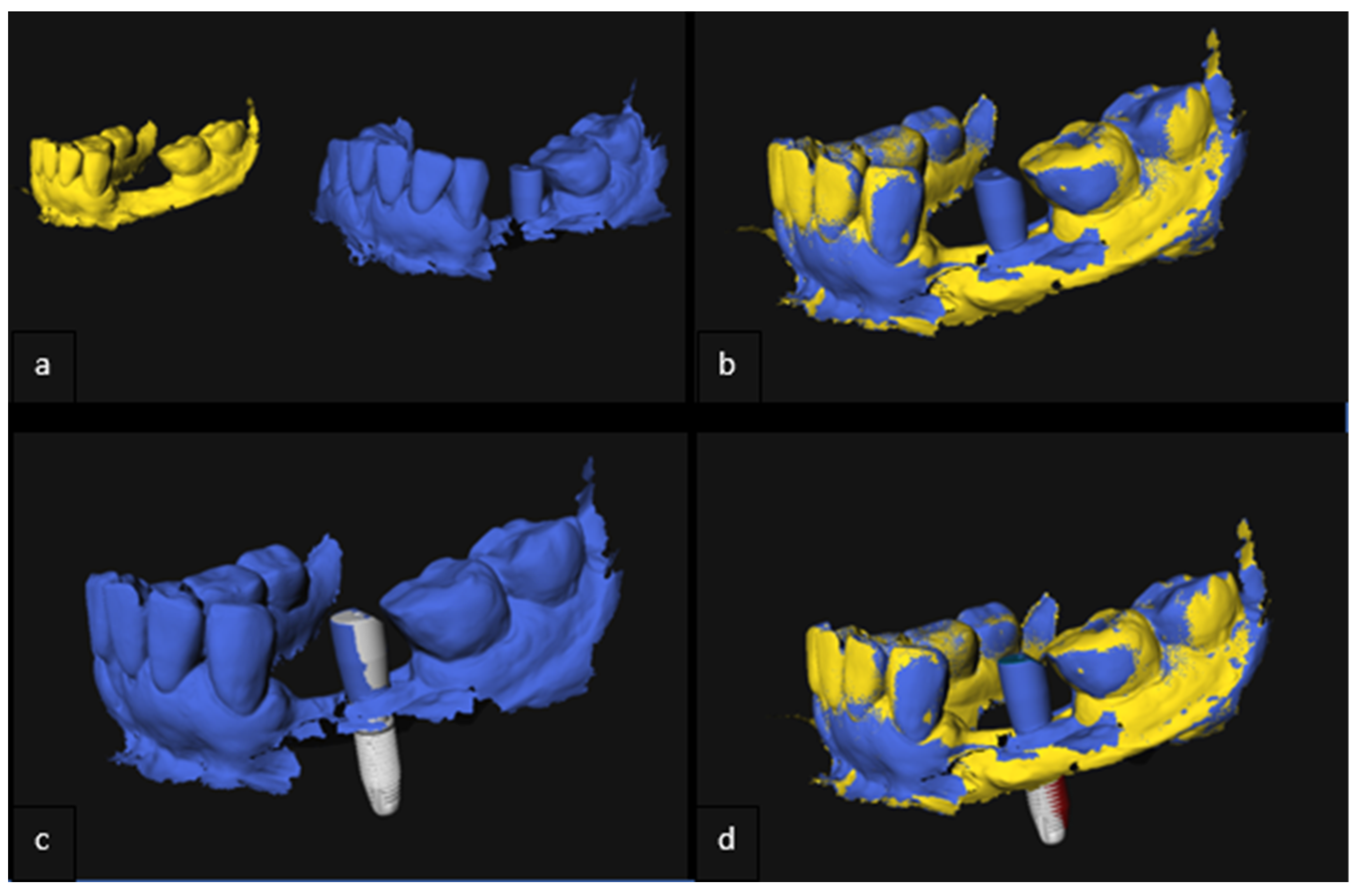
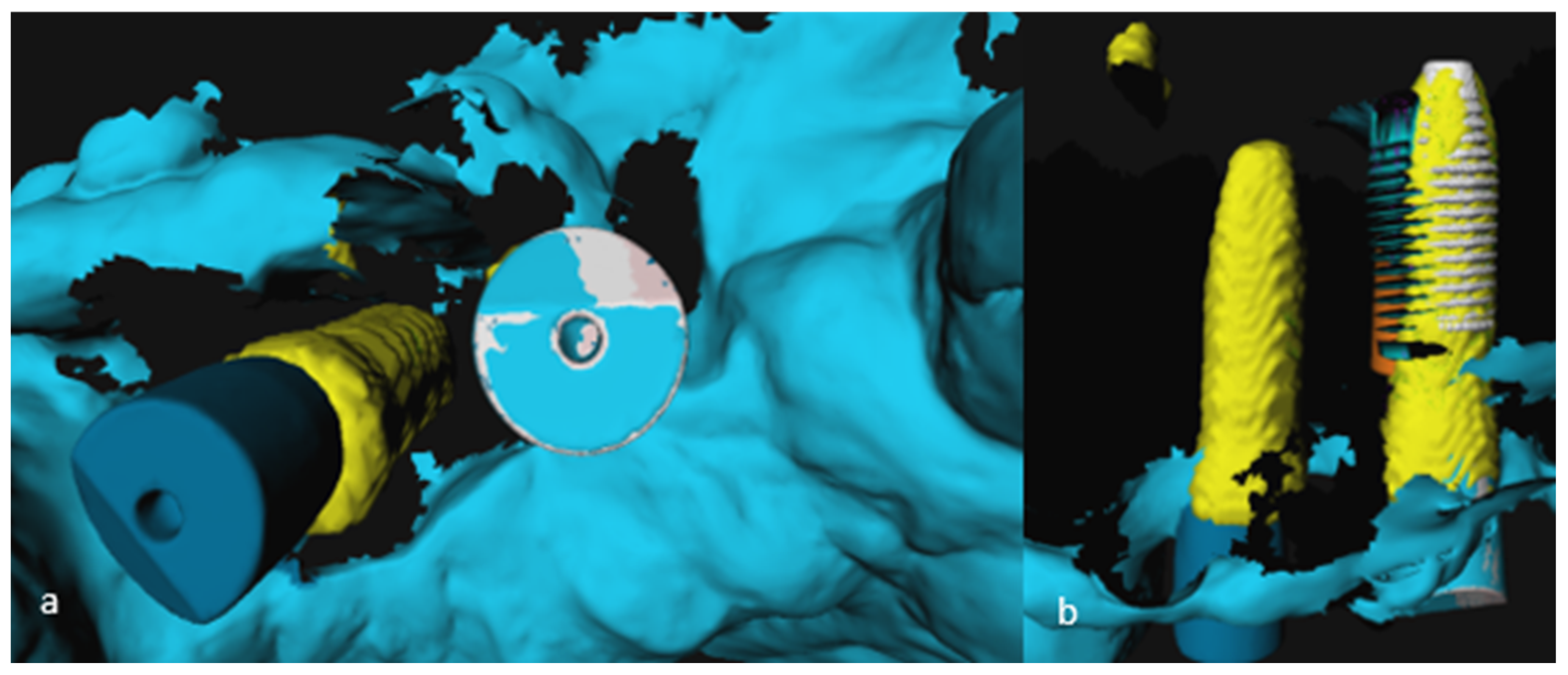
2.5. Analysis of 3D Imaging Based on Postoperative CBCT and IOS
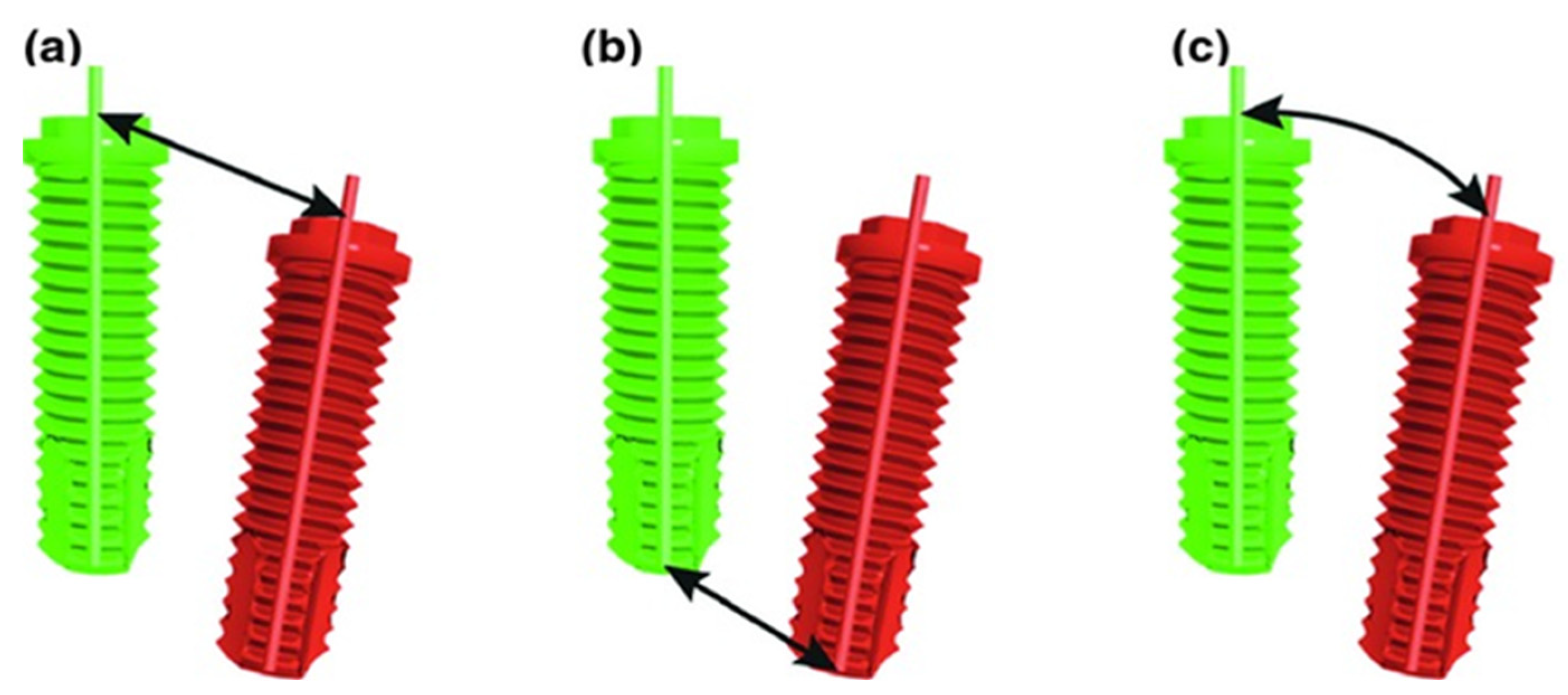
2.6. Implant Validation
- (a)
- Deviation in implant shoulder in millimeters (mm): three-dimensional distance between shoulder of planned and placed implant, measured from the axis;
- (b)
- Deviation in implant tip in millimeters (mm): three-dimensional distance between tip of planned and placed implant, measured from the axis;
- (c)
- Angular deviation in degrees (°): largest angle between the central, longitudinal axis of planned and realized implant positions.
2.7. Statistical Analysis
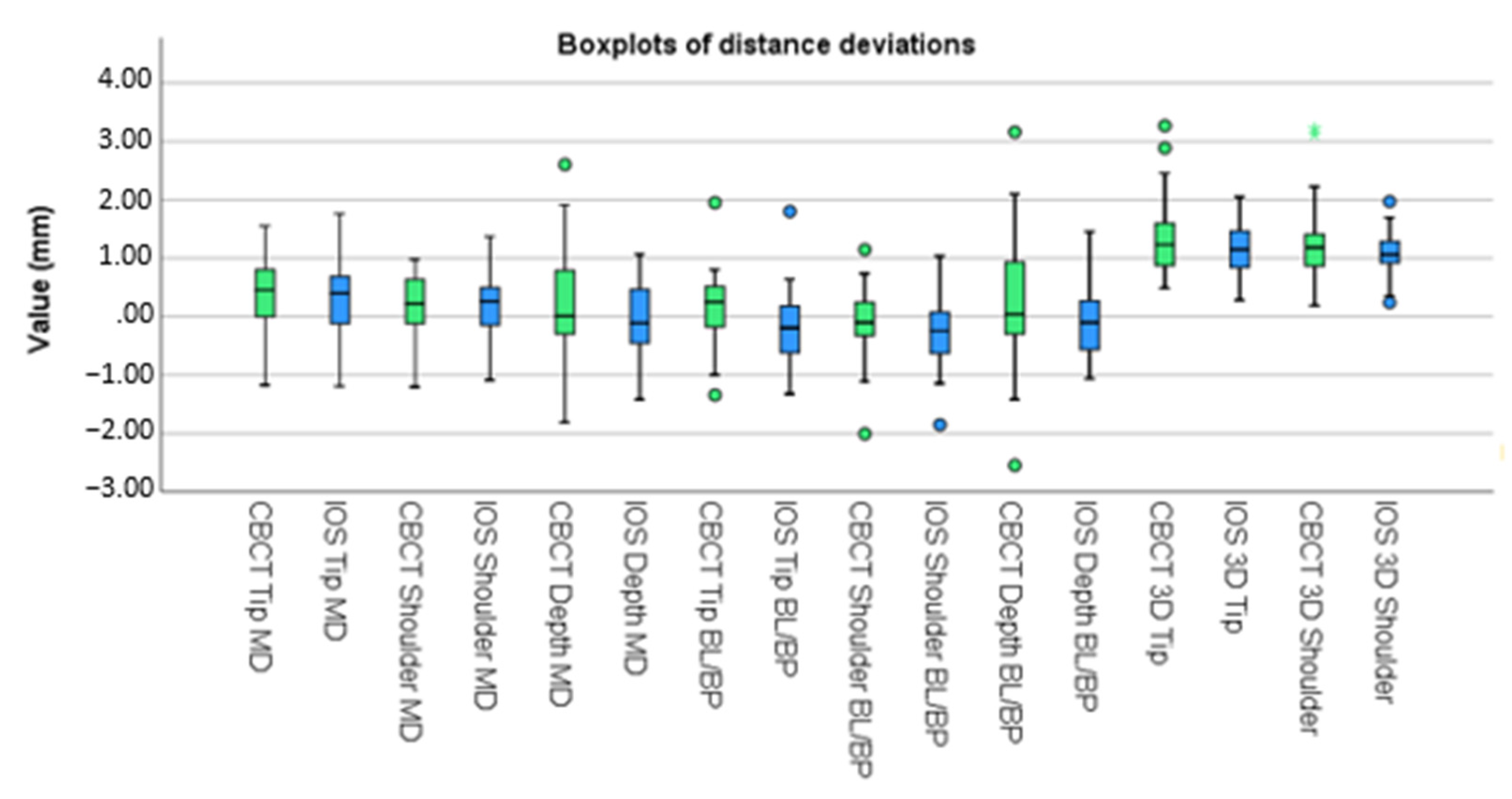
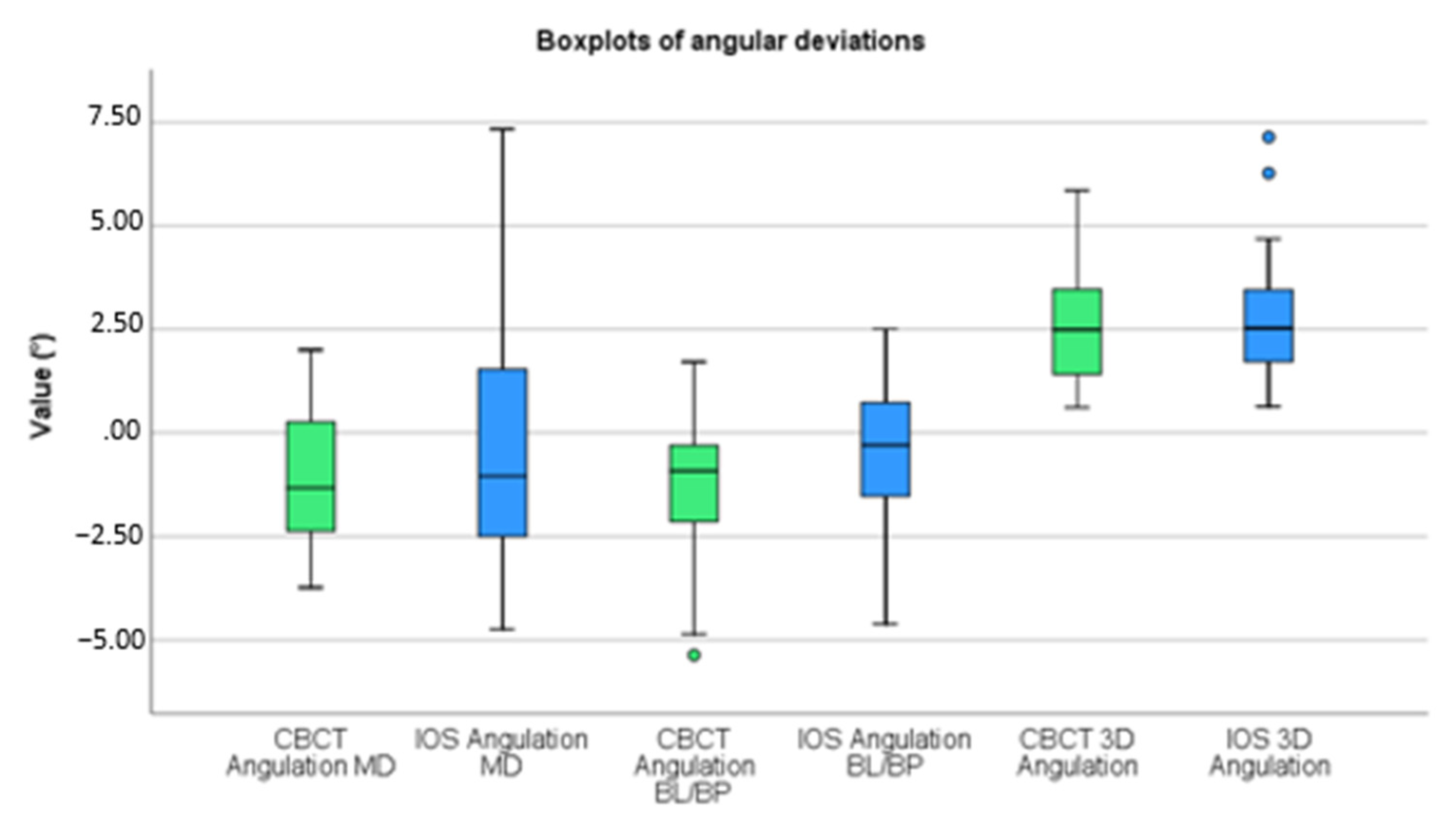
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Haese, J.; Ackhurst, J.; Wismeijer, D.; De Bruyn, H.; Tahmaseb, A. Current state of the art of computer-guided implant surgery. Periodontology 2000 2017, 73, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Jorba-García, A.; González-Barnadas, A.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. Accuracy assessment of dynamic computer–aided implant placement: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2479–2494. [Google Scholar] [CrossRef] [PubMed]
- Smitkarn, P.; Subbalekha, K.; Mattheos, N.; Pimkhaokham, A. The accuracy of single-tooth implants placed using fully digital-guided surgery and freehand implant surgery. J. Clin. Periodontol. 2019, 46, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Nada, R.M.; Maal, T.J.J.; Breuning, K.H.; Bergé, S.J.; Mostafa, Y.A.; Kuijpers-Jagtman, A.M. Accuracy and Reproducibility of Voxel Based Superimposition of Cone Beam Computed Tomography Models on the Anterior Cranial Base and the Zygomatic Arches. PLoS ONE 2011, 6, e16520. [Google Scholar] [CrossRef] [PubMed]
- Almukhtar, A.; Ju, X.; Khambay, B.; McDonald, J.; Ayoub, A. Comparison of the Accuracy of Voxel Based Registration and Surface Based Registration for 3D Assessment of Surgical Change following Orthognathic Surgery. PLoS ONE 2014, 9, e93402. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone beam computed tomography in implant dentistry: Recommendations for clinical use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef]
- Ortorp, A.; Jemt, T.; Bäck, T. Photogrammetry and Conventional Impressions for Recording Implant Positions: A Comparative Laboratory Study. Clin. Implant Dent. Relat. Res. 2005, 7, 43–50. [Google Scholar] [CrossRef]
- Maes, F.; Collignon, A.; Vandermeulen, D.; Marchal, G.; Suetens, P. Multimodality image registration by maximization of mutual information. IEEE Trans. Med. Imaging 1997, 16, 187–198. [Google Scholar] [CrossRef]
- Verhamme, L.M.; Meijer, G.J.; Boumans, T.; Schutyser, F.; Bergé, S.J.; Maal, T.J.J. A clinically relevant validation method for implant placement after virtual planning. Clin. Oral Implant. Res. 2013, 24, 1265–1272. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Hanak, B.W.; Yang, T.-C.; Wilson, T.A.; Hsia, J.M.; Walsh, H.E.; Shih, H.-C.; Nagatomo, K.J. Computer-Assisted Surgery in Medical and Dental Applications. Expert Rev. Med. Devices 2021, 18, 669–696. [Google Scholar] [CrossRef]
- Pellegrino, G.; Taraschi, V.; Andrea, Z.; Ferri, A.; Marchetti, C. Dynamic navigation: A prospective clinical trial to evaluate the accuracy of implant placement. Int. J. Comput. Dent. 2019, 22, 139–147. [Google Scholar] [PubMed]
- Stefanelli, L.V.; DeGroot, B.S.; I Lipton, D.; A Mandelaris, G. Accuracy of a Dynamic Dental Implant Navigation System in a Private Practice. Int. J. Oral Maxillofac. Implant. 2019, 34, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, L.; Yang, J.; Zhang, B.; Lin, Y.; Chen, J.; Huang, W.; Chen, Y. Accuracy of dynamic navigation compared to static surgical guide for dental implant placement. Int. J. Implant Dent. 2020, 6, 78. [Google Scholar] [CrossRef]
- Sherrard, J.F.; Rossouw, P.E.; Benson, B.W.; Carrillo, R.; Buschang, P.H. Accuracy and reliability of tooth and root lengths measured on cone-beam computed tomographs. Am. J. Orthod. Dentofac. Orthop. 2010, 137, S100–S108. [Google Scholar] [CrossRef]
- Torres, M.G.G.; Campos, P.S.F.; Segundo, N.P.N.; Navarro, M.; Crusoé-Rebello, I. Accuracy of Linear Measurements in Cone Beam Computed Tomography With Different Voxel Sizes. Implant Dent. 2012, 21, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Baan, F.; Sabelis, J.F.; Schreurs, R.; van de Steeg, G.; Xi, T.; van Riet, T.C.T.; Becking, A.G.; Maal, T.J.J. Validation of the OrthoGnathicAnalyser 2.0-3D accuracy assessment tool for bimaxillary surgery and genioplasty. PLoS ONE 2021, 16, e0246196. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Li, J.; Wang, S.; Wang, L.; Zhou, Y.; Liu, Y. A comparison of voxel- and surface-based cone-beam computed tomography mandibular superimposition in adult orthodontic patients. J. Int. Med. Res. 2021, 49, 300060520982708. [Google Scholar] [CrossRef]
- Pattamavilai, S.; Ongthiemsak, C. Accuracy of intraoral scanners in different complete arch scan patterns. J. Prosthet. Dent. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Amornvit, P.; Rokaya, D.; Sanohkan, S. Comparison of Accuracy of Current Ten Intraoral Scanners. BioMed Res. Int. 2021, 2021, 2673040. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, H.; Li, S.-Y.; Geng, Y.-M. Dental implant location via surface scanner: A pilot study. BMC Oral Health 2020, 20, 306. [Google Scholar] [CrossRef]
- Franchina, A.; Stefanelli, L.V.; Maltese, F.; Mandelaris, G.A.; Vantaggiato, A.; Pagliarulo, M.; Pranno, N.; Brauner, E.; De Angelis, F.; Di Carlo, S. Validation of an Intra-Oral Scan Method Versus Cone Beam Computed Tomography Superimposition to Assess the Accuracy between Planned and Achieved Dental Implants: A Randomized In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 9358. [Google Scholar] [CrossRef] [PubMed]
- Skjerven, H.; Olsen-Bergem, H.; Rønold, H.J.; Riis, U.H.; Ellingsen, J.E. Comparison of postoperative intraoral scan versus cone beam computerised tomography to measure accuracy of guided implant placement—A prospective clinical study. Clin. Oral Implants Res. 2019, 30, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Derksen, W.; Wismeijer, D.; Flügge, T.; Hassan, B.; Tahmaseb, A. The accuracy of computer-guided implant surgery with tooth-supported, digitally designed drill guides based on CBCT and intraoral scanning. A prospective cohort study. Clin. Oral Implants Res. 2019, 30, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Orban, K.; Varga, E.; Windisch, P.; Braunitzer, G.; Molnar, B. Accuracy of half-guided implant placement with machine-driven or manual insertion: A prospective, randomized clinical study. Clin. Oral Investig. 2022, 26, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
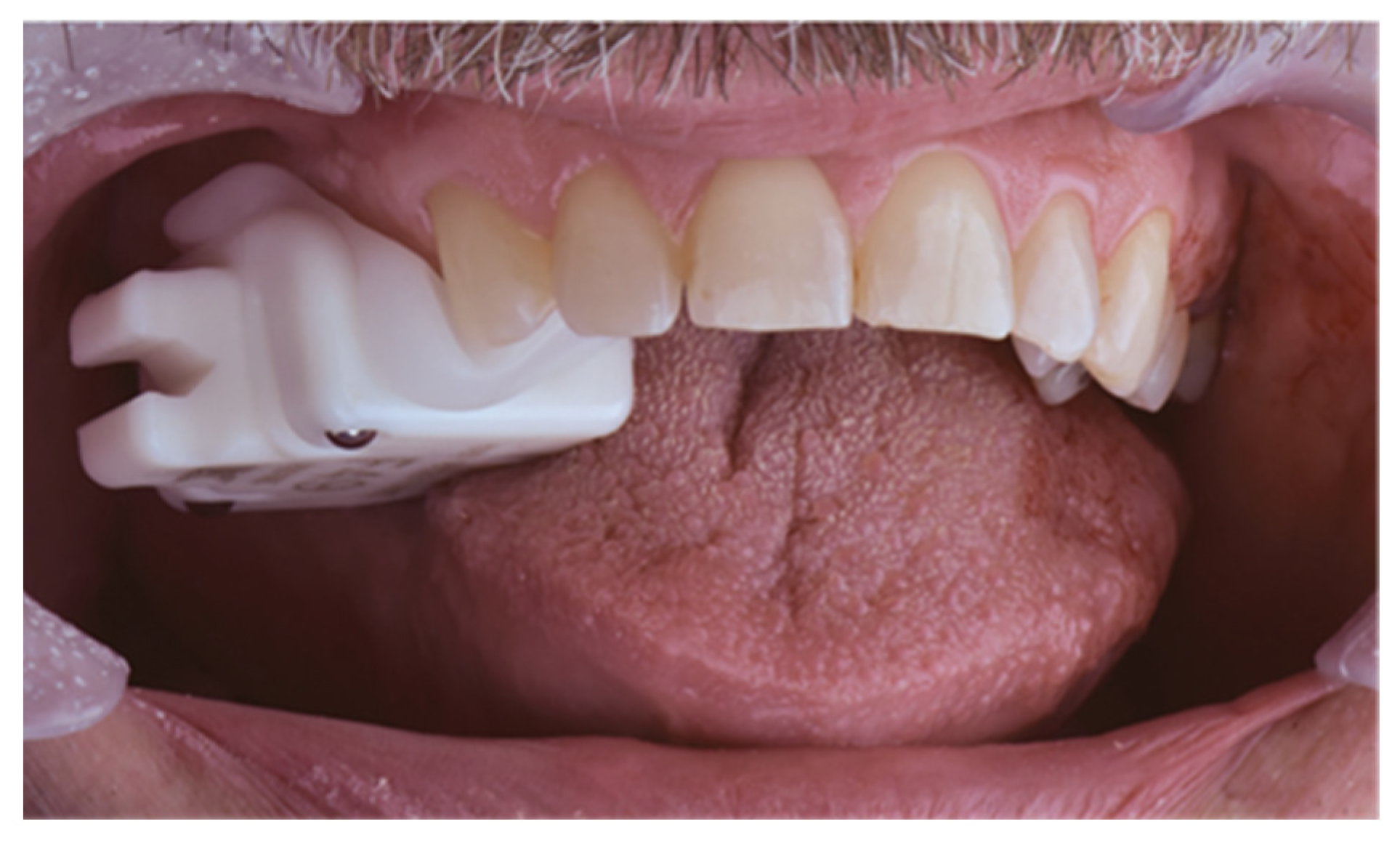
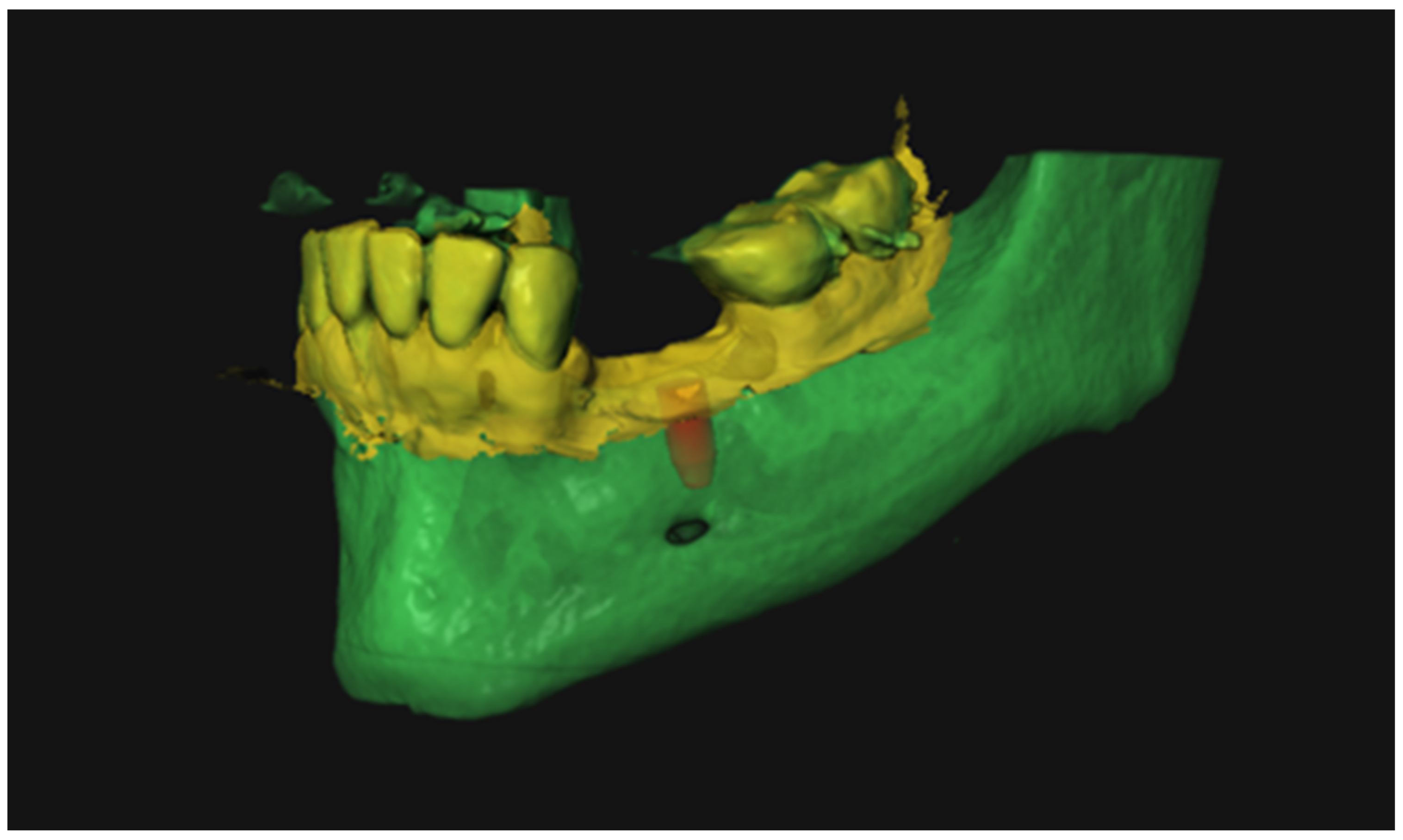
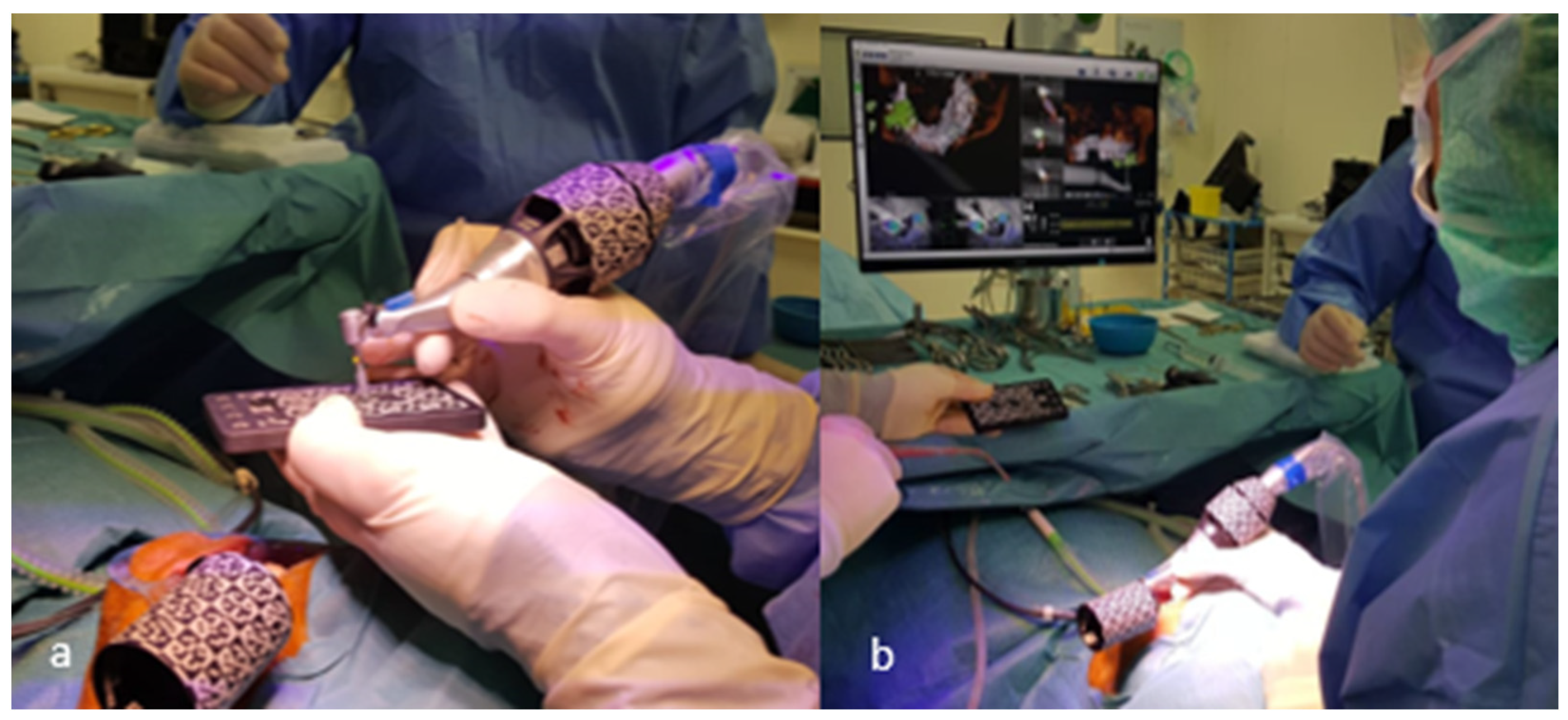
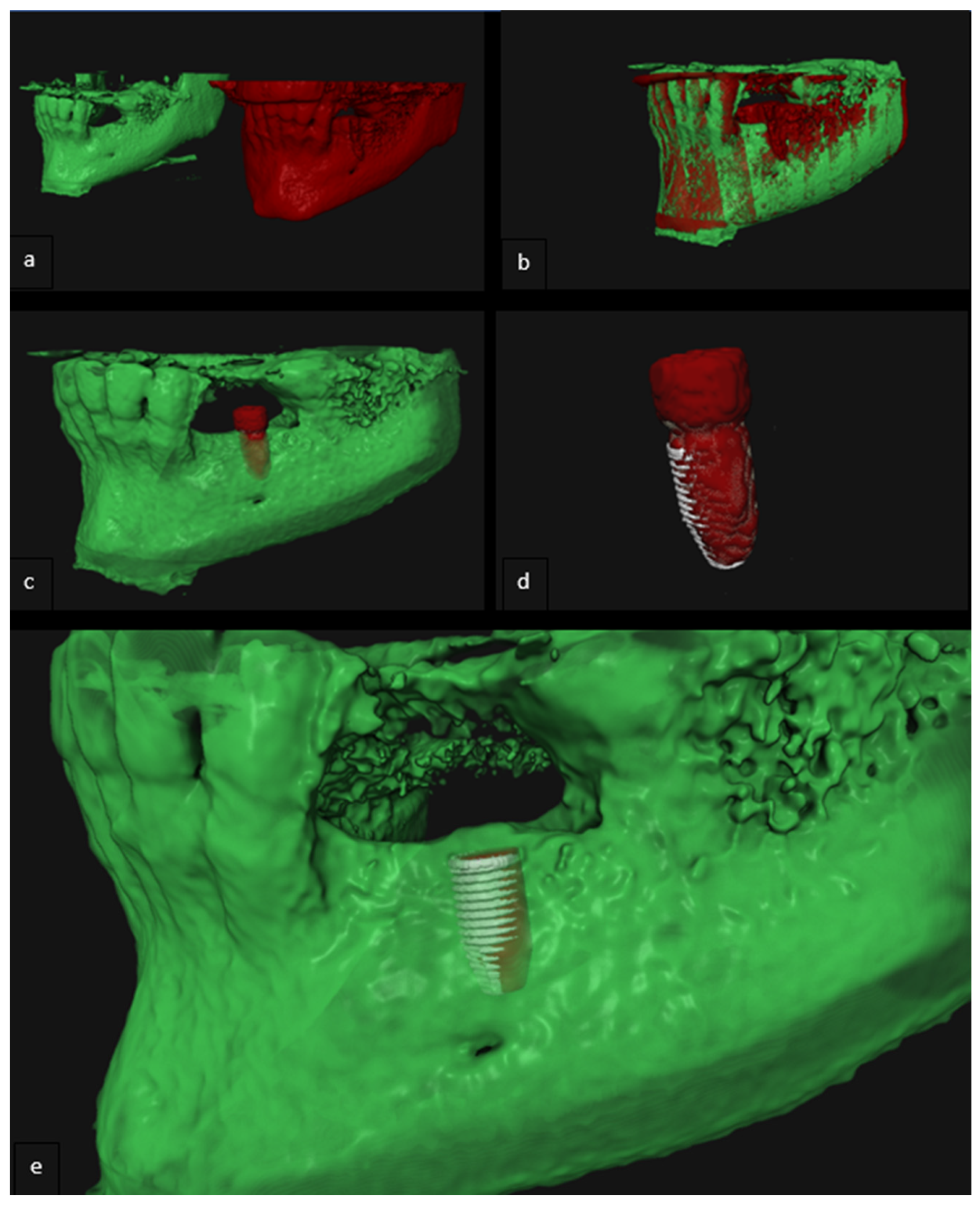
| Case | Mandible/Maxilla | Implant Location |
|---|---|---|
| 1 | Maxilla | 11 |
| 2 | Mandible | 36 |
| 3 | Maxilla | 15 |
| 4 | Maxilla | 16 |
| 5.1 | Maxilla | 13 |
| 5.2 | Maxilla | 12 |
| 6 | Maxilla | 21 |
| 7.1 | Maxilla | 12 |
| 7.2 | Maxilla | 14 |
| 8.1 | Mandible | 46 |
| 8.2 | Mandible | 47 |
| 9 | Maxilla | 12 |
| 10 | Mandible | 35 |
| 11.1 | Maxilla | 13 |
| 11.2 | Maxilla | 23 |
| 12.1 | Mandible | 36 |
| 12.2 | Mandible | 37 |
| 13 | Maxilla | 13 |
| 14.1 | Maxilla | 11 |
| 14.2 | Maxilla | 21 |
| 15.1 | Maxilla | 24 |
| 15.2 | Maxilla | 25 |
| 16 | Maxilla | 21 |
| Mean (CBCT) | Standard Deviation (CBCT) | Mean (IOS) | Standard Deviation (CBCT) | ||
|---|---|---|---|---|---|
| Mesio-Distal plane | Tip (mm) | 0.601 | 0.460 | 0.685 | 0.466 |
| Shoulder (mm) | 0.473 | 0.350 | 0.486 | 0.348 | |
| Angular (°) | 1.643 | 1.220 | 2.288 | 1.608 | |
| Depth (mm) | 0.151 | 1.016 | −0.045 | 0.692 | |
| Bucco-Lingual/Bucco-palatal plane | Tip (mm) | 0.535 | 0.455 | 0.552 | 0.454 |
| Shoulder (mm) | 0.500 | 0.489 | 0.549 | 0.451 | |
| Angular (°) | 1.755 | 1.555 | 1.421 | 1.169 | |
| Depth (mm) | 0.209 | 1.206 | −0.045 | 0.680 | |
| 3D plane | Tip (mm) | 1.369 | 0.746 | 1.186 | 0.484 |
| Shoulder (mm) | 1.265 | 0.773 | 1.057 | 0.429 | |
| Angular (°) | 2.625 | 1.494 | 2.835 | 1.595 |
| Mean | Standard Deviation | 95% Confidence Interval of the Difference | p-Value | |||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Mesio-Distal Plane | Tip (mm) | 0.09 | 0.54 | −0.14 | 0.33 | 0.419 |
| Shoulder (mm) | 0.01 | 0.35 | −0.14 | 0.16 | 0.910 | |
| Angular (°) | −0.55 | 2.34 | −1.56 | 0.46 | 0.273 | |
| Depth | 0.20 | 0.85 | −0.17 | 0.57 | 0.280 | |
| Bucco-Lingual/Bucco-palatal plane | Tip (mm) | 0.25 | 0.23 | 0.15 | 0.34 | 0.000 * |
| Shoulder (mm) | 0.12 | 0.20 | 0.03 | 0.21 | 0.011 * | |
| Angular (°) | −0.81 | 1.10 | −1.28 | −0.33 | 0.002 * | |
| Depth | 0.17 | 0.88 | −0.21 | 0.55 | 0.372 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Hooft, J.; Kielenstijn, G.; Liebregts, J.; Baan, F.; Meijer, G.; D’haese, J.; Bronkhorst, E.; Verhamme, L. Intraoral Scanning as an Alternative to Evaluate the Accuracy of Dental Implant Placements in Partially Edentate Situations: A Prospective Clinical Case Series. J. Clin. Med. 2022, 11, 5876. https://doi.org/10.3390/jcm11195876
van Hooft J, Kielenstijn G, Liebregts J, Baan F, Meijer G, D’haese J, Bronkhorst E, Verhamme L. Intraoral Scanning as an Alternative to Evaluate the Accuracy of Dental Implant Placements in Partially Edentate Situations: A Prospective Clinical Case Series. Journal of Clinical Medicine. 2022; 11(19):5876. https://doi.org/10.3390/jcm11195876
Chicago/Turabian Stylevan Hooft, Jan, Guido Kielenstijn, Jeroen Liebregts, Frank Baan, Gert Meijer, Jan D’haese, Ewald Bronkhorst, and Luc Verhamme. 2022. "Intraoral Scanning as an Alternative to Evaluate the Accuracy of Dental Implant Placements in Partially Edentate Situations: A Prospective Clinical Case Series" Journal of Clinical Medicine 11, no. 19: 5876. https://doi.org/10.3390/jcm11195876
APA Stylevan Hooft, J., Kielenstijn, G., Liebregts, J., Baan, F., Meijer, G., D’haese, J., Bronkhorst, E., & Verhamme, L. (2022). Intraoral Scanning as an Alternative to Evaluate the Accuracy of Dental Implant Placements in Partially Edentate Situations: A Prospective Clinical Case Series. Journal of Clinical Medicine, 11(19), 5876. https://doi.org/10.3390/jcm11195876








