Virtual Reality during Intrathecal Pump Refills in Children: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Outcome Measurements
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Primary Outcome Measurement: Pain
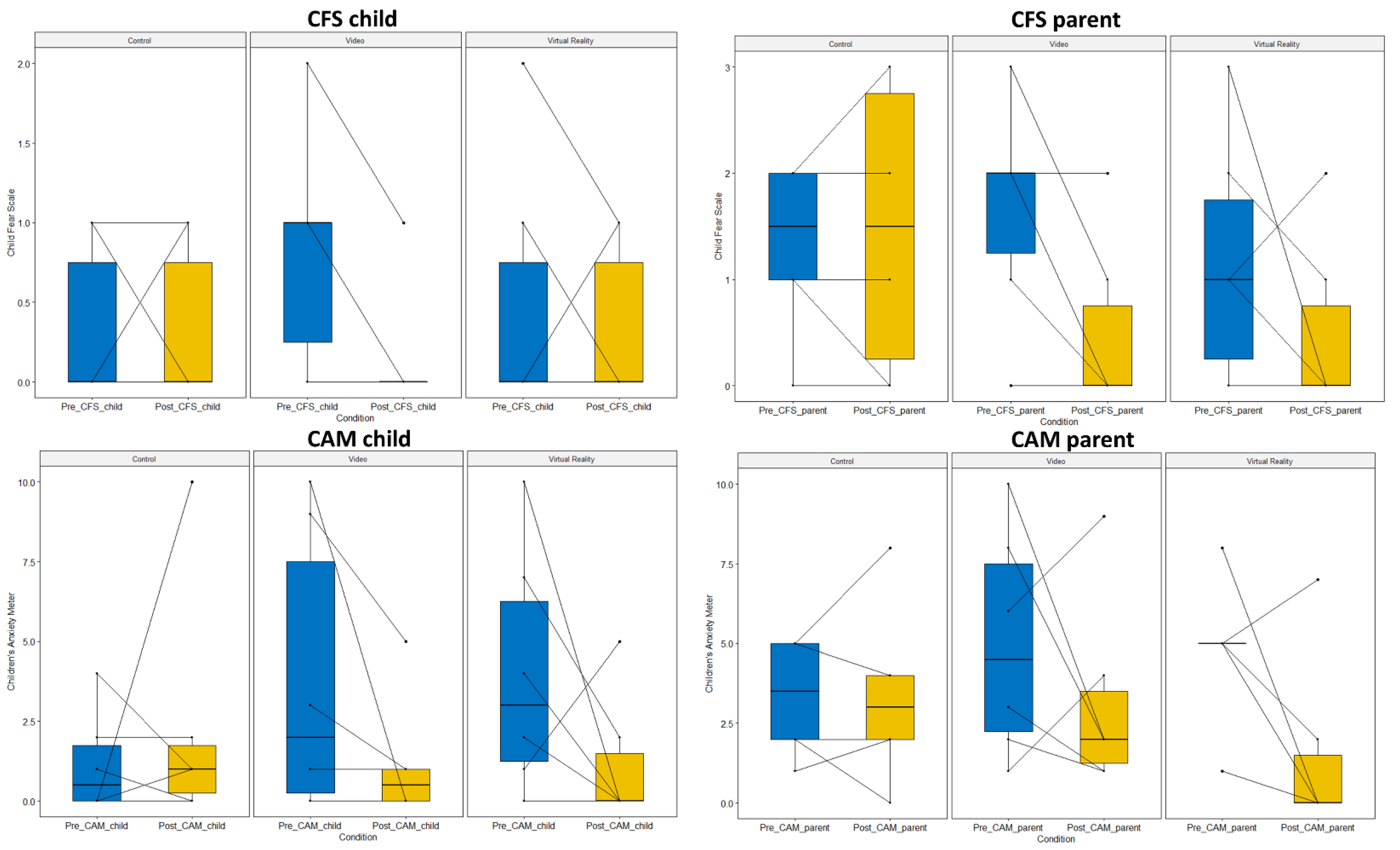
3.3. Secondary Outcome Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piskorz, J.; Czub, M. Effectiveness of a virtual reality intervention to minimize pediatric stress and pain intensity during venipuncture. J. Spec. Pediatric Nurs. 2018, 23, e12201. [Google Scholar] [CrossRef] [PubMed]
- Kleye, I.; Heden, L.; Karlsson, K.; Sundler, A.J.; Darcy, L. Children’s individual voices are required for adequate management of fear and pain during hospital care and treatment. Scand. J. Caring Sci. 2021, 35, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, B.M. Intervention studies involving parents of hospitalized young children: An analysis of the past and future recommendations. J. Pediatric Nurs. 2000, 15, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Schlechter, A.K.; Whitaker, W.; Iyer, S.; Gabriele, G.; Wilkinson, M. Virtual reality distraction during pediatric intravenous line placement in the emergency department: A prospective randomized comparison study. Am. J. Emerg. Med. 2021, 44, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Heden, L.; von Essen, L.; Ljungman, G. Children’s self-reports of fear and pain levels during needle procedures. Nurs. Open 2020, 7, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Chorney, J.M.; Kain, Z.N. Behavioral analysis of children’s response to induction of anesthesia. Anesth. Analg. 2009, 109, 1434–1440. [Google Scholar] [CrossRef]
- Kain, Z.N.; Wang, S.M.; Mayes, L.C.; Caramico, L.A.; Hofstadter, M.B. Distress during the induction of anesthesia and postoperative behavioral outcomes. Anesth. Analg. 1999, 88, 1042–1047. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Lim, H.; Son, J.S.; Lee, J.R.; Kim, D.C.; Ko, S. Cartoon distraction alleviates anxiety in children during induction of anesthesia. Anesth. Analg. 2012, 115, 1168–1173. [Google Scholar] [CrossRef]
- Mifflin, K.A.; Hackmann, T.; Chorney, J.M. Streamed video clips to reduce anxiety in children during inhaled induction of anesthesia. Anesth. Analg. 2012, 115, 1162–1167. [Google Scholar] [CrossRef]
- Chaurasia, B.; Jain, D.; Mehta, S.; Gandhi, K.; Mathew, P.J. Incentive-Based Game for Allaying Preoperative Anxiety in Children: A Prospective, Randomized Trial. Anesth. Analg. 2019, 129, 1629–1634. [Google Scholar] [CrossRef]
- Li, A.; Montaño, Z.; Chen, V.J.; Gold, J.I. Virtual reality and pain management: Current trends and future directions. Pain Manag. 2011, 1, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Scott, K.; Dukewich, M. Innovative technology using virtual reality in the treatment of pain: Does it reduce pain via distraction, or is there more to it? Pain Med. 2018, 19, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Eijlers, R.; Utens, E.; Staals, L.M.; de Nijs, P.F.A.; Berghmans, J.M.; Wijnen, R.M.H.; Hillegers, M.H.J.; Dierckx, B.; Legerstee, J.S. Systematic Review and Meta-analysis of Virtual Reality in Pediatrics: Effects on Pain and Anxiety. Anesth. Analg. 2019, 129, 1344–1353. [Google Scholar] [CrossRef]
- Goudman, L.; Jansen, J.; Billot, M.; Vets, N.; De Smedt, A.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e34402. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Houdayer, E.; Emedoli, D.; Locatelli, M.; Mortini, P.; Mandelli, C.; Raggi, A.; Iannaccone, S. Efficacy of virtual reality to reduce chronic low back pain: Proof-of-concept of a non-pharmacological approach on pain, quality of life, neuropsychological and functional outcome. PLoS ONE 2019, 14, e0216858. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Ma, W.; Guo, L.; Feng, H. Virtual reality in preoperative preparation of children undergoing general anesthesia: A randomized controlled study. Anaesthesiologie 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, D.; Dorman, D.; Brown, S.; Files, A.; Graves, T.; Kirk, E.; Meredith-Neve, S.; Sanders, J.; White, B.; Swearingen, C.J. Effect of virtual reality on adolescent pain during burn wound care. J. Burn Care Res. 2014, 35, 395–408. [Google Scholar] [CrossRef]
- Jones, T.; Moore, T.; Choo, J. The Impact of Virtual Reality on Chronic Pain. PLoS ONE 2016, 11, e0167523. [Google Scholar] [CrossRef]
- Trost, Z.; France, C.; Anam, M.; Shum, C. Virtual reality approaches to pain: Toward a state of the science. Pain 2021, 162, 325–331. [Google Scholar] [CrossRef]
- Goslinga-van der Gaag, S.M.E.; Delhaas, E.M.; Frankema, S.P.G.; Huygen, F. Efficiency and Safety of Aftercare With Intrathecal Baclofen on Location. Neuromodulation 2019, 22, 828–833. [Google Scholar] [CrossRef]
- Patel, A.K.; Dowling, M.; Purcell, A.; O’Brien, J.; Moore, D.M. Managing a National Intrathecal Pump Service During the COVID-19 Pandemic. Neuromodulation 2020, 23, 922–925. [Google Scholar] [CrossRef]
- Chau, B.; Chi, B.; Wilson, T. Decreasing pediatric pain and agitation during botulinum toxin injections for spasticity with virtual reality: Lessons learned from clinical use. J. Pediatr. Rehabil. Med. 2018, 11, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Gerceker, G.O.; Binay, S.; Bilsin, E.; Kahraman, A.; Yilmaz, H.B. Effects of Virtual Reality and External Cold and Vibration on Pain in 7- to 12-Year-Old Children During Phlebotomy: A Randomized Controlled Trial. J. Perianesth. Nurs. 2018, 33, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Finnstrom, B.; Kokinsky, E. The FLACC behavioral scale for procedural pain assessment in children aged 5–16 years. Paediatr. Anaesth. 2008, 18, 767–774. [Google Scholar] [CrossRef] [PubMed]
- McKinley, S.; Madronio, C. Validity of the Faces Anxiety Scale for the assessment of state anxiety in intensive care patients not receiving mechanical ventilation. J. Psychosom. Res. 2008, 64, 503–507. [Google Scholar] [CrossRef]
- McMurtry, C.M.; Noel, M.; Chambers, C.T.; McGrath, P.J. Children’s fear during procedural pain: Preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011, 30, 780–788. [Google Scholar] [CrossRef]
- Kleiber, C.; McCarthy, A.M. Evaluating instruments for a study on children’s responses to a painful procedure when parents are distraction coaches. J. Pediatr. Nurs. 2006, 21, 99–107. [Google Scholar] [CrossRef]
- Ersig, A.L.; Kleiber, C.; McCarthy, A.M.; Hanrahan, K. Validation of a clinically useful measure of children’s state anxiety before medical procedures. J. Spec. Pediatr. Nurs. 2013, 18, 311–319. [Google Scholar] [CrossRef]
- Caruso, T.J.; George, A.; Menendez, M.; De Souza, E.; Khoury, M.; Kist, M.N.; Rodriguez, S.T. Virtual reality during pediatric vascular access: A pragmatic, prospective randomized, controlled trial. Paediatr. Anaesth. 2020, 30, 116–123. [Google Scholar] [CrossRef]
- Jarvis, L. Narrative as virtual reality 2: Revisiting immersion and interactivity in literature and electronic media. Int. J. Perform. Arts Digit. Media 2019, 15, 239–241. [Google Scholar] [CrossRef]
- Witmer, B.G.; Singer, M.J. Measuring Presence in Virtual Environments: A Presence Questionnaire. Presence Teleoperators Virtual Environ. 1998, 7, 225–240. [Google Scholar] [CrossRef]
- Mütterlein, J. The Three Pillars of Virtual Reality? Investigating the Roles of Immersion, Presence, and Interactivity. In Proceedings of the HICSS, Hilton, Waikoloa Village, HI, USA, 3–6 January 2018. [Google Scholar]
- Chan, E.; Foster, S.; Sambell, R.; Leong, P. Clinical efficacy of virtual reality for acute procedural pain management: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0200987. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.M.O.; Spadoni, V.S. Is virtual reality useful for pain management in patients who undergo medical procedures? Einstein 2019, 17, eMD4837. [Google Scholar] [CrossRef]
- Georgescu, R.; Fodor, L.A.; Dobrean, A.; Cristea, I.A. Psychological interventions using virtual reality for pain associated with medical procedures: A systematic review and meta-analysis. Psychol. Med. 2020, 50, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Indovina, P.; Barone, D.; Gallo, L.; Chirico, A.; De Pietro, G.; Giordano, A. Virtual Reality as a Distraction Intervention to Relieve Pain and Distress During Medical Procedures: A Comprehensive Literature Review. Clin. J. Pain 2018, 34, 858–877. [Google Scholar] [CrossRef] [PubMed]
- Windich-Biermeier, A.; Sjoberg, I.; Dale, J.C.; Eshelman, D.; Guzzetta, C.E. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. J. Pediatr. Oncol. Nurs. 2007, 24, 8–19. [Google Scholar] [CrossRef]
- Dahlquist, L.M.; Weiss, K.E.; Clendaniel, L.D.; Law, E.F.; Ackerman, C.S.; McKenna, K.D. Effects of videogame distraction using a virtual reality type head-mounted display helmet on cold pressor pain in children. J. Pediatr. Psychol. 2009, 34, 574–584. [Google Scholar] [CrossRef]

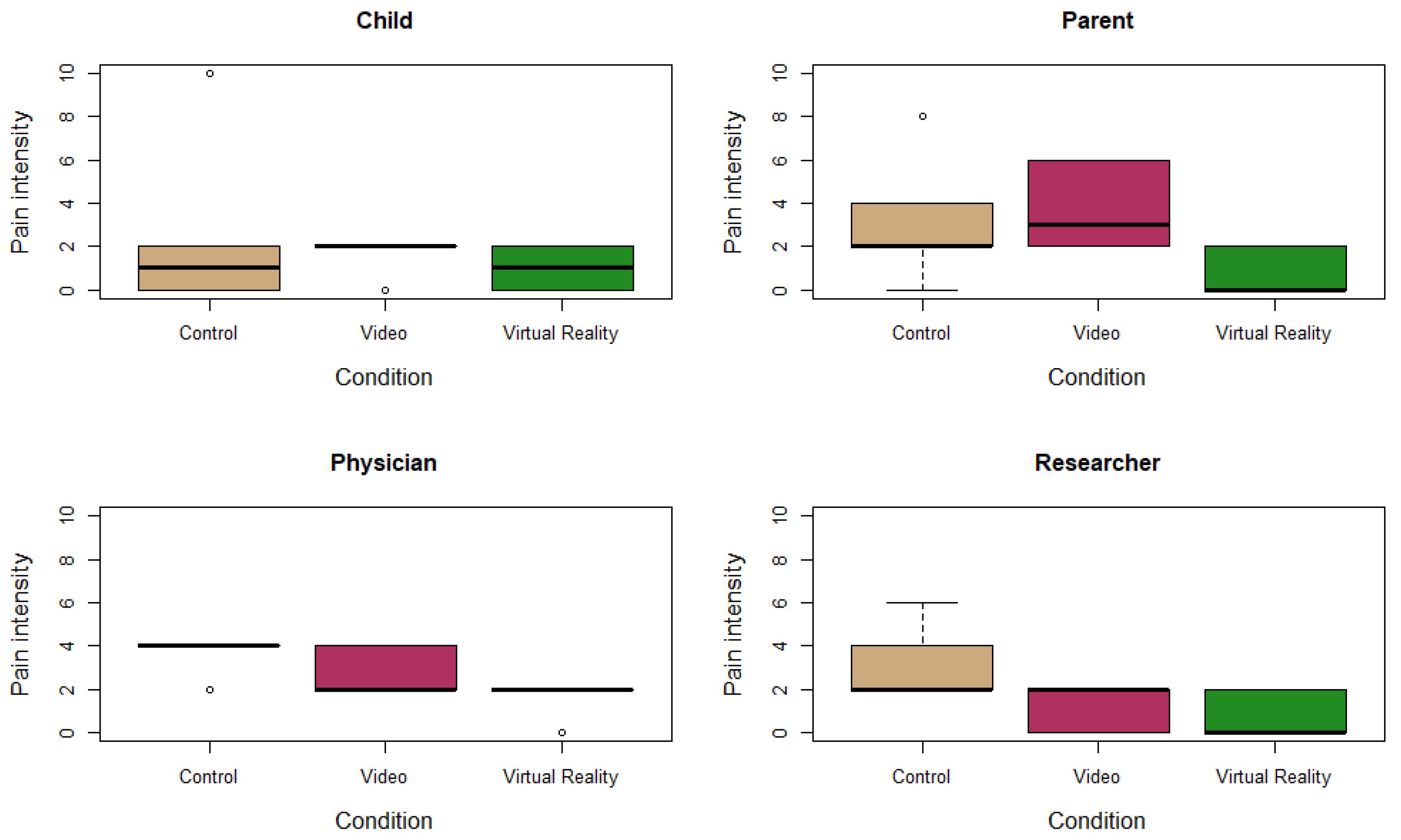
| Patient ID | Sex | Age | Condition | Pain Score Child | Pain Score Parent | Pain Score Physician | Pain Score Researcher |
|---|---|---|---|---|---|---|---|
| 1 | Male | 14 | Control | 0 | 2 | 4 | 4 |
| Video | 2 | 4 | 4 | 2 | |||
| Virtual Reality | 2 | 0 | 2 | 2 | |||
| 2 | Female | 14 | Control | 2 | 4 | 4 | 2 |
| Video | 2 | 6 | 2 | 0 | |||
| Virtual Reality | 2 | 0 | 0 | 0 | |||
| 3 | Female | 16 | Control | 2 | 2 | 2 | 2 |
| Video | 2 | 2 | 2 | 2 | |||
| Virtual Reality | 0 | 0 | 2 | 0 | |||
| 4 | Male | 10 | Control | 0 | 2 | 4 | 2 |
| Video | 0 | 2 | 2 | 0 | |||
| Virtual Reality | 0 | 2 | 2 | 0 | |||
| 5 | Female | 10 | Control | 10 | 8 | 4 | 6 |
| Video | 2 | 6 | 4 | 2 | |||
| Virtual Reality | 2 | 2 | 2 | 2 | |||
| 6 | Female | 15 | Control | 0 | 0 | 4 | 2 |
| Video | 2 | 2 | 2 | 2 | |||
| Virtual Reality | 0 | 0 | 2 | 0 |
| Topic | Condition | Rating | Child | Parent | Physician |
|---|---|---|---|---|---|
| Next refill with video/VR | Video | Completely disagree | 1 | ||
| Disagree | |||||
| Neutral | 1 | 1 | |||
| Agree | 2 | 2 | 4 | ||
| Completely agree | 2 | 3 | 2 | ||
| Virtual reality | Completely disagree | ||||
| Disagree | |||||
| Neutral | 1 | ||||
| Agree | 2 | 1 | 5 | ||
| Completely agree | 4 | 5 | |||
| Interest of the child in the video/VR | Video | Completely disagree | |||
| Disagree | |||||
| Neutral | 1 | ||||
| Agree | 2 | 4 | 4 | ||
| Completely agree | 4 | 2 | 1 | ||
| Virtual reality | Completely disagree | ||||
| Disagree | |||||
| Neutral | |||||
| Agree | 2 | 1 | 6 | ||
| Completely agree | 4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goudman, L.; Jansen, J.; De Smedt, A.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality during Intrathecal Pump Refills in Children: A Case Series. J. Clin. Med. 2022, 11, 5877. https://doi.org/10.3390/jcm11195877
Goudman L, Jansen J, De Smedt A, Billot M, Roulaud M, Rigoard P, Moens M. Virtual Reality during Intrathecal Pump Refills in Children: A Case Series. Journal of Clinical Medicine. 2022; 11(19):5877. https://doi.org/10.3390/jcm11195877
Chicago/Turabian StyleGoudman, Lisa, Julie Jansen, Ann De Smedt, Maxime Billot, Manuel Roulaud, Philippe Rigoard, and Maarten Moens. 2022. "Virtual Reality during Intrathecal Pump Refills in Children: A Case Series" Journal of Clinical Medicine 11, no. 19: 5877. https://doi.org/10.3390/jcm11195877
APA StyleGoudman, L., Jansen, J., De Smedt, A., Billot, M., Roulaud, M., Rigoard, P., & Moens, M. (2022). Virtual Reality during Intrathecal Pump Refills in Children: A Case Series. Journal of Clinical Medicine, 11(19), 5877. https://doi.org/10.3390/jcm11195877








