Cognitive Behavioral Therapy for Chronic Insomnia in Outpatients with Major Depression—A Randomised Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Inclusion Criteria
- Aged 18–67;
- Major depression, single or recurrent episode [25] with a score above 17 on the 17-item Hamilton Depression Rating Scale;
- Sleep Onset Latency (SOL) or Wake After Sleep Onset (WASO) lasting more than 30 min or early morning awakenings at least three nights a week despite sufficient opportunity to sleep and impaired daytime functioning;
- Insomnia for at least three months, but some participants had suffered from insomnia for years [26].
2.4. Exclusion Criteria
- Medical disorders considerably affecting sleep;
- Schizophrenia or bipolar disorders;
- Ongoing psychological treatment;
- Suicidality equivalent to level three on the 17-item Hamilton Depression Rating Scale;
- Current substance abuse;
- Pregnancy;
- Working at night shifts;
- Unable to speak or understand Danish;
- Other sleep disorders (e.g., severe sleep apnea (AHI (apnea-hypnopnea index) above 14) or restless legs syndrome) were excluded.
2.5. Randomisation and Blinding
2.6. Interventions
2.6.1. TAU
2.6.2. CBT-I
2.6.3. Measures
2.7. Ethics and Registration
2.8. Statistics
3. Results
3.1. Baseline Patient Characteristics
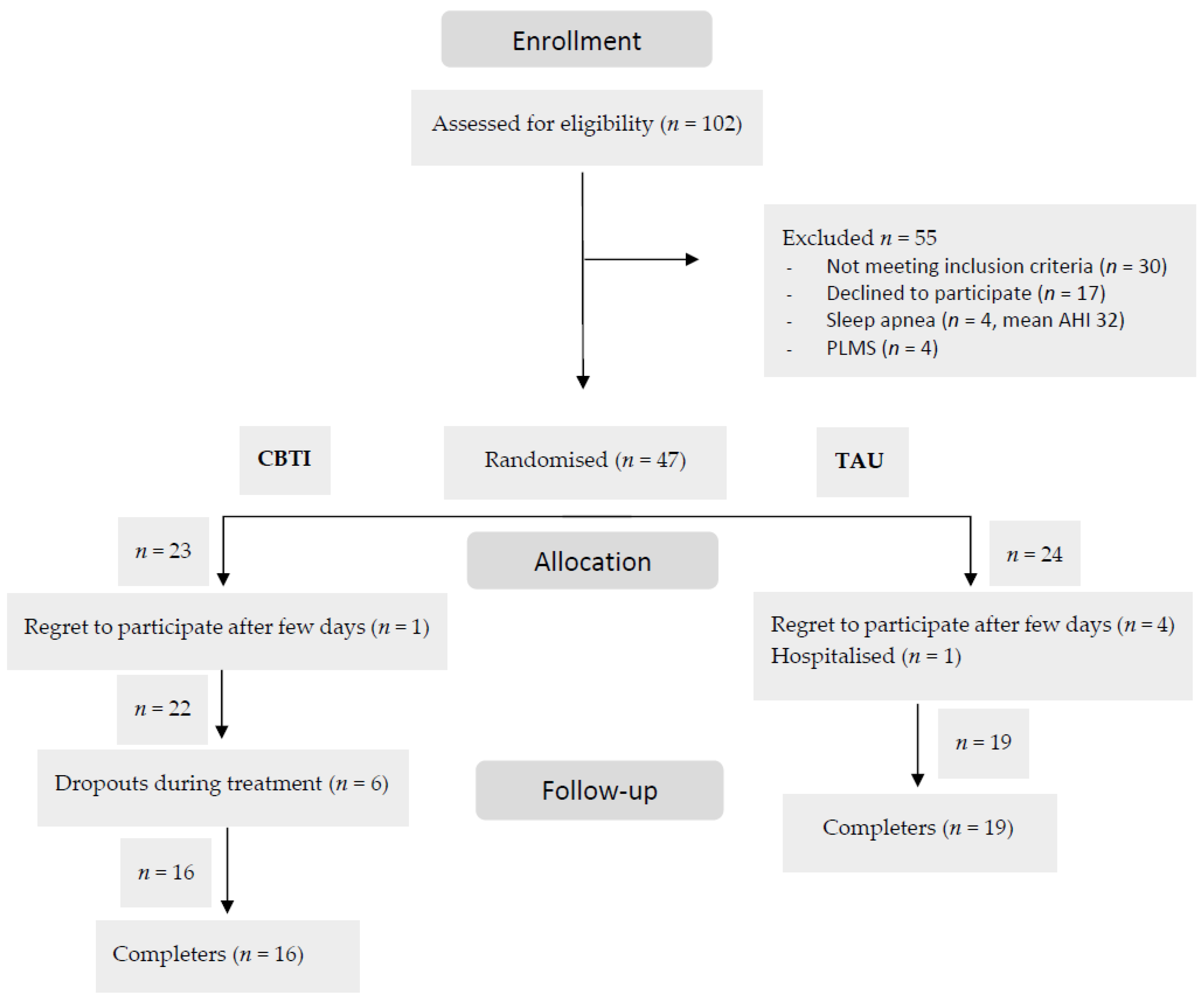
3.2. Completion and Attendance
3.3. Sleep Outcome Measures
Data from PSG Recordings
3.4. HAM-D17, HAM-D6 and WHO-5
3.5. DBAS-16
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutka, P.; Krivosova, M.; Muchova, Z.; Tonhajzerova, I.; Hamrakova, A.; Mlyncekova, Z.; Mokry, J.; Ondrejka, I. Association of Sleep Architecture and Physiology with Depressive Disorder and Antidepressants Treatment. Int. J. Mol. Sci. 2021, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Asarnow, L.D.; Manber, R. Cognitive Behavioral Therapy for Insomnia in Depression. Sleep Med. Clin 2019, 14, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Chelminski, I.; Young, D.; Dalrymple, K.; Hrabosky, J.; Zimmerman, M. Severe insomnia is associated with more severe presentation and greater functional deficits in depression. J. Psychiatr. Res. 2011, 45, 1101–1105. [Google Scholar] [CrossRef]
- McCall, W.V.; Blocker, J.N.; D’Agostino, R.; Kimball, J.; Boggs, N.; Lasater, B.; Rosenquist, P.B. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010, 11, 822–827. [Google Scholar] [CrossRef]
- Dew, M.A.; Reynolds, C.F.; Houck, P.R.; Hall, M.; Buysse, D.J.; Frank, E.; Kupfer, D.J. Temporal Profiles of the Course of Depression During Treatment: Predictors of Pathways Toward Recovery in the Elderly. Arch. Gen. Psychiatry 1997, 54, 1016–1024. [Google Scholar] [CrossRef]
- Dombrovski, A.Y.; Mulsant, B.H.; Houck, P.R.; Mazumdar, S.; Lenze, E.J.; Andreescu, C.; Cyranowski, J.M.; Reynolds, C.F., III. Residual symptoms and recurrence during maintenance treatment of late-life depression. J. Affect. Disord. 2007, 103, 77–82. [Google Scholar] [CrossRef]
- Boland, E.M.; Vittengl, J.R.; Clark, L.A.; Thase, M.E.; Jarrett, R.B. Is sleep disturbance linked to short- and long-term outcomes following treatments for recurrent depression? J. Affect. Disord. 2020, 262, 323–332. [Google Scholar] [CrossRef]
- Jindal, R.D. Insomnia in Patients with Depression: Some Pathophysiological and Treatment Considerations. CNS Drugs 2009, 23, 309–329. [Google Scholar] [CrossRef]
- Carney, C.; Posner, D. Cognitive Behavior Therapy for Insomnia in Those with Depression: A Guide for Clinicians; Routledge, Taylor & Francis Group: New York, NY, USA, 2016; p. 226. [Google Scholar]
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Kumar, L. A Systematic Review and Meta-Analysis of the Efficacy of Cognitive Behavioral Therapy for the Management of Pediatric Migraine. Headache 2017, 57, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Goncalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Geiger-Brown, J.M.; Rogers, V.E.; Liu, W.; Ludeman, E.M.; Downton, K.D.; Diaz-Abad, M. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep Med. Rev. 2015, 23, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Han, M.; Li, X.; Geng, L.; Miao, Y. The Clinical Effectiveness of Cognitive Behavioral Therapy for Patients with Insomnia and Depression: A Systematic Review and Meta-Analysis. Evid Based Complement Altern. Med. 2020, 2020, 8071821. [Google Scholar] [CrossRef] [PubMed]
- Gebara, M.A.; Siripong, N.; DiNapoli, E.A.; Maree, R.D.; Germain, A.; Reynolds, C.F.; Kasckow, J.W.; Weiss, P.M.; Karp, J.F. Effect of insomnia treatments on depression: A systematic review and meta-analysis. Depress. Anxiety 2018, 35, 717–731. [Google Scholar] [CrossRef]
- Gee, B.; Orchard, F.; Clarke, E.; Joy, A.; Clarke, T.; Reynolds, S. The effect of non-pharmacological sleep interventions on depression symptoms: A meta-analysis of randomised controlled trials. Sleep Med. Rev. 2019, 43, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.E.A.; Shapiro, C.M. Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: A systematic review. J. Psychosom. Res. 2018, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Blom, K.; Jernelöv, S.; Rück, C.; Lindefors, N.; Kaldo, V. Three-Year Follow-Up Comparing Cognitive Behavioral Therapy for Depression to Cognitive Behavioral Therapy for Insomnia, for Patients With Both Diagnoses. Sleep 2017, 40, zsx108. [Google Scholar] [CrossRef]
- Ballesio, A.; Aquino, M.; Feige, B.; Johann, A.F.; Kyle, S.D.; Spiegelhalder, K.; Lombardo, C.; Rücker, G.; Riemann, D.; Baglioni, C. The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: A systematic review and network meta-analysis. Sleep Med. Rev. 2018, 37, 114–129. [Google Scholar] [CrossRef]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, Mr000030. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33, quiz 34–57. [Google Scholar] [PubMed]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef] [PubMed]
- The ICD-10 Classification of Mental and Behavioural Disorders Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993.
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; Volume xliv, 947p.
- RADS—Rådet for Anvendelse af dyr Sygehusmedicin: Kort og Godt 2012; Dampfærgevej 27-29, 2100 København Ø. Available online: https://rads.dk (accessed on 28 August 2022).
- Spielman, A.J.; Saskin, P.; Thorpy, M.J. Treatment of chronic insomnia by restriction of time in bed. Sleep 1987, 10, 45–56. [Google Scholar] [PubMed]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993; p. 238. [Google Scholar]
- Jacobson, E. Progressive Relaxation: A Physiological and Clinical Investigation of Muscular States and Their Significance in Psychology and Medical Practice, 2nd ed.University of Chicago Press: Chicago, IL, USA, 1959; p. 494. [Google Scholar]
- Bech, P. Clinical Psychometrics, 1st ed.; Wiley-Blackwell: Somerset, UK, 2012. [Google Scholar]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.L.; Vaughn, B.V. The AASM manual for the scoring of sleep and associated events. Rules, Terminology and Technical Specifications, Darien, Illinois. Am. Acad. Sleep Med. 2015, 176, 2012. [Google Scholar]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Belanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Yang, M.; Morin, C.M.; Schaefer, K.; Wallenstein, G.V. Interpreting score differences in the Insomnia Severity Index: Using health-related outcomes to define the minimally important difference. Curr. Med. Res. Opin. 2009, 25, 2487–2494. [Google Scholar] [CrossRef]
- Morin, C.M.; Vallieres, A.; Ivers, H. Dysfunctional beliefs and attitudes about sleep (DBAS): Validation of a brief version (DBAS-16). Sleep 2007, 30, 1547–1554. [Google Scholar] [CrossRef]
- Hall, T.; Krahn, G.L.; Horner-Johnson, W.; Lamb, G.; The Rehabilitation Research and Training Center Expert Panel on Health Measurement. Examining functional content in widely used Health-Related Quality of Life scales. Rehabil. Psychol. 2011, 56, 94–99. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Fisher, R.A. On the interpretation of χ 2 from contingency tables, and the calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]
- William, S. The probable error of a mean. Biometrika 1908, 6, 1–25. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples)†. Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Vickers, A.J.; Altman, D.G. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Norell-Clarke, A.; Jansson-Fröjmark, M.; Tillfors, M.; Holländare, F.; Engström, I. Group cognitive behavioural therapy for insomnia: Effects on sleep and depressive symptomatology in a sample with comorbidity. Behav. Res. Ther. 2015, 74, 80–93. [Google Scholar] [CrossRef]
- Amidi, A.; Buskbjerg, C.R.; Damholdt, M.F.; Dahlgaard, J.; Thorndike, F.P.; Ritterband, L.; Zachariae, R. Changes in sleep following internet-delivered cognitive-behavioral therapy for insomnia in women treated for breast cancer: A 3-year follow-up assessment. Sleep Med. 2022, 96, 35–41. [Google Scholar] [CrossRef]
| Baseline Characteristics | All (n = 41) | TAU (n = 19) | CBT-I (n = 22) | p-Value | ||
|---|---|---|---|---|---|---|
| Sex Female | 28 (68.3%) | 14 (73.7%) | 14 (63.6%) | 0.52 | ||
| Age Mean (Range) | 37.07 (20-67) | 38.26 (20-67) | 36.05 (20-66) | 0.62 | ||
| Civil Status Living alone Living together | 11 (26.8%) 30 (73.2%) | 6 (31.6%) 13 (68.4%) | 5 (22.7%) 17 (77.3%) | 0.17 | ||
| Employment Job Retired Student Unemployed | 7 (17.7%) 6 (14.6%) 15 (36.6%) 13 (31.7%) | 3 (15.8%) 3 (15.8%) 6 (31.6%) 7 (36.8%) | 4 (18.2%) 3 (13.6%) 9 (40.9%) 6 (27.3%) | 0.90 | ||
| Years of Schooling 7–14 14–20 | 30 (73.2%) 11 (26.8%) | 13 (68.4%) 6 (31.6%) | 17 (56.7%) 5 (22.7%) | 0.73 | ||
| BMI (kg/m2) Mean (SD) | 25.5 (6.7) | 25.7 (8.3) | 25.3 (5.1) | 0.84 | ||
| Height (cm) Mean (SD) | 173.7 (10.0) | 173.63 (11.1) | 173.75 (9.2) | 0.14 | ||
| Weight (kg) Mean (SD) | 76.73 (20.3) | 77.4 (25.3) | 76.14 (15.4) | 0.84 | ||
| Previous Depressive episode Yes | 28 (68.3) | 16 (84.2) | 12 (54.5) | 0.052 | ||
| Use of Psychotropics Yes No | 34 (83.0%) 7 (17.0%) | 15 (78.9%) 4 (21.1%) | 19 (86.3%) 3 (13.6%) | 0.54 | ||
| Medication Name of medication Dose range Some participants used more than one psychotropic | 29 non-unique users | 31 non-unique users | ||||
| Sertraline | 100–150 mg | Sertraline | 100 mg | |||
| Escitalopram | 20 mg | Escitalopram | 30 mg | |||
| Citalopram | 40 mg | Citalopram | 40 mg | |||
| Venlafaxine | 150–225 mg | Fluoxetine | 60 mg | |||
| Duloxetine | 60–120 mg | Venlafaxine | 150 mg | |||
| Nortriptyline | 100–325 mg | Duloxetine | 30–120 mg | |||
| Clomipramine | 1125 mg | Mirtazapine | 30 mg | |||
| Mirtazapine | 7.5–30 mg | Nortriptyline | 100–150 mg | |||
| Quetiapine | 25–50 mg | Valdoxane | 25–50 mg | |||
| Melatonin | 6 mg | Quetiapin | 25–300 mg | |||
| Z-hypnotics | 7.5–10 mg | Phenergan | 25 mg | |||
| Chlorprothixen | 50 mg | Melatonin | 9 mg | |||
| Z-hypnotics | 10 mg | |||||
| Benzodiazepines | 10–22.5 mg | |||||
| Age (Years) | Sex Females % | HAM-D17 | ISI | Sleep Efficiency Sleep Diary % | SOL Sleep Diary (min) | WASO Sleep Diary (min) | Total Sleep Time Sleep Diary (min) | |
|---|---|---|---|---|---|---|---|---|
| Dropouts of CBTI, n = 6 | ||||||||
| Mean | 25.17 | 50% | 20.33 | 21.80 | 73.81 | 84.32 | 27.68 | 470.54 |
| SD | 4.22 | 1.86 | 2.39 | 7.21 | 36.34 | 16.75 | 39.78 | |
| Completers of CBTI, n = 16 | ||||||||
| Mean | 40.13 | 63.6% | 21.50 | 20.19 | 70.68 | 52.48 | 63.17 | 378.45 |
| SD | 14.62 | 6.02 | 2.80 | 10.53 | 29.42 | 51.50 | 63.65 | |
| Sign * | 0.001 | 0.54 | 0.25 | 0.26 | 0.51 | 0.80 | 0.15 | 0.007 |
| Df | 19,511 | 20 | 19 | 20 | 18 | 19 | 19 | |
| Dropouts of TAU, n = 5 | ||||||||
| Mean | 29.0 | 80.0% | 21.20 | 18.50 | 78.52 | 61.67 | 14.63 | 409.77 |
| SD | 9.8 | 3.03 | 3.54 | 7.80 | 34.05 | 12.95 | 66.78 | |
| Completers of TAU, n = 19 | ||||||||
| Mean | 38.3 | 73.7% | 21.1 | 20.7 | 68.3 | 82.2 | 50.6 | 362.7 |
| SD | 13.9 | 2.5 | 3.0 | 9.4 | 57.8 | 36.8 | 128.4 | |
| Sign * | 0.18 | 1.00 | 0.88 | 0.30 | 0.10 | 0.57 | 0.12 | 0.55 |
| Df | 22 | 1 | 19 | 19 | 16 | 17 | 17 | 16 |
| Measures | TAU | CBT-I | p-Value a | p-Value a | p-Value b | p-Value c |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | within TAU Groups | within CBT-I Groups | between Groups | between Groups | |
| n = 19 | n = 22 | Baseline vs. Follow Up | Baseline vs. Follow Up | Baseline and Follow Up | Difference (Ancova) | |
| Sleep diaries | ||||||
| TST baseline | 362.7 (128.4) | 400.4 (70.5) | 0.27 | |||
| TST follow up | 361.5 (112.8) | 379.3 (68.3) | 0.88 (df14) | 0.30 (df17) | 0.57 | |
| Change score | 2.93 (70.4) | −21.14 (83.0) | 0.50 (df 1) | |||
| SOL baseline | 82.2 (57.8) | 58.9 (32.6) | 0.14 | |||
| SOL follow up | 102.5 (131.9) | 36.1 (38.1) | 0.40 (df 15) | 0.002 (df 17) | 0.53 | |
| Change score | 29.2 (135.0) | −22.3 (25.2) | 0.83 (df 1) | |||
| WASO baseline | 50.6 (36.8) | 54.7 (47.8) | 0.78 | |||
| WASO follow up | 43.8 (41.1) | 19.0 (16.4) | 0.58 (df 15) | 0.002 (df 16) | 0.27 | |
| Change score | −4.1 (29.0) | −37.0 (41.5) | 0.003 (df 1) | |||
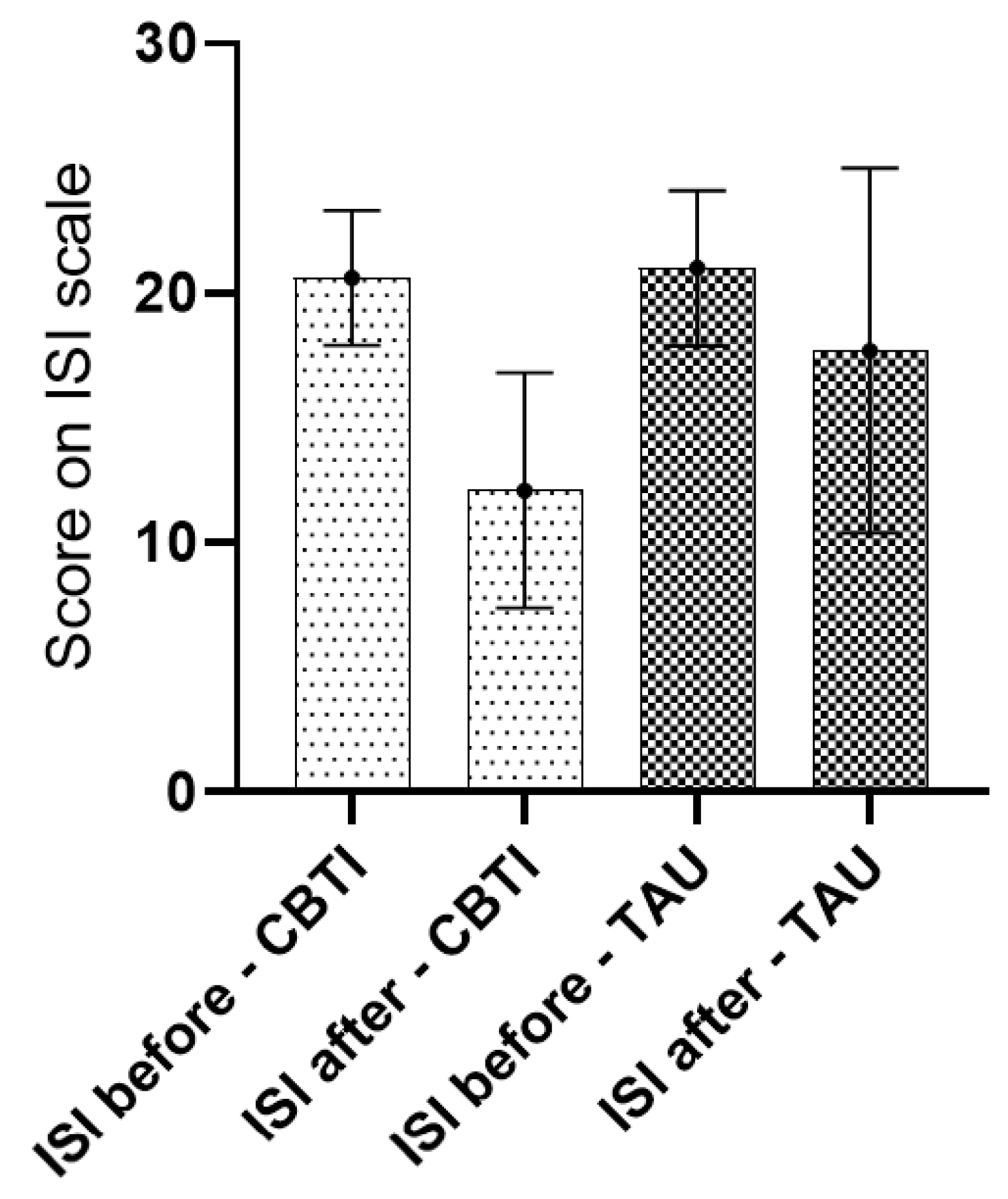
| ISI baseline | 21.0 (3.1) | 20.6 (2.7) | 0.64 | |||
| ISI follow up | 17.7 (7.3) | 12.1 (4.7) | 0.08 (df 17) | 0.001 (df 15) | 0.01 | |
| Change score | −3.1 (7.0) | −8.1 (5.6) | 0.001 (df 1) | |||
| Sleep efficiency baseline d | 69.7 (SE 2.3) | 71.6 (2.1) | 0.50 (df 40705) | |||
| Sleep efficiency follow-up d | 72.1 (SE 2.8) | 83.4 (2.8) | 0.006 (df 34915) | |||
| Polysomnography | ||||||
| TST baseline | 417.9 (92.8) | 421.0 (68.7) | 0.91 | |||
| TST follow up | 428.8 (99.7) | 385.1 (52.5) | 0.94(df 16) | 0.09(df 15) | 0.13 | |
| Change score | 1.8 (101.3) | −35.7 (78.4) | 0.29 (df 1) | |||
| Sleep efficiency baseline d | 83.6 (8.5) | 82.1 (10.9) | 0.69 | |||
| Sleep efficiency follow up d | 82.0 (9.8) | 85.8 (8.0) | 0.61(df 16) | 0.21(df 15) | 0.24 | |
| Change score | −0.99 (7.7) | 4.0 (12.3) | 0.40 (df 1) | |||
| SOL baseline | 23.4 (20.4) | 15.5 (12.1) | 0.15 | |||
| SOL follow up | 10.2 (12.7) | 9.0 (8.1) | 0.02 (df 16) | 0.17 (df 15) | 0.77 | |
| Change score | 29.2 (135.0) | −22.3 (25.2) | 0.83 (df 1) | |||
| WASO baseline | 57.7 (37.7) | 70.0 (70.5) | 0.5 | |||
| WASO follow up | 81.1 (59.4) | 49.3 (30.2) | 0.15(df 16) | 0.15(df 15) | 0.06 | |
| Change score | −4.1 (29.0) | −37.0 (41.5) | 0.003 (df 1) | |||
| AHI baseline | 2.2 (2.8) | 3.3 (4.8) | 0.38 | |||
| AHI follow up | 2.5 (3.5) | 2.8 (3.7) | 0.99(df 14) | 0.3(df 13) | 0.8 | |
| Change score | 0.01 (3.9) | −1.3 (4.6) | 0.42 (df 1) | |||
| Measures | TAU | CBT-I | p-Value a | p-Value a | p-Value b | p-Value c |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | within TAU Groups | within CBT-I Groups | between Groups | between Groups | |
| n = 19 | n = 22 | Baseline vs. Follow Up | Baseline vs. Follow Up | Baseline and Follow Up | Difference (Ancova) | |
| HAM-D6 baseline | 9.8 (2.5) | 9.5 (1.7) | 0.61 (df 39) | |||
| HAMD-6 follow up | 9.8 (4.2) | 7.4 (4.2) | 0.71 (df 17) | 0.05 (df 15) | 0.10 (df 32) | |
| Change score | 0.3 (3.7) | −2.3 (4.3) | 0.16 (df 1) | |||
| HAM-D17 baseline | 21.4 (2.9) | 21.2 (2.1) | 0.76 (df 39) | |||
| HAM-D17 follow up | 19.9 (9.4) | 13.8 (7.0) | 0.58 (df 17) | 0.001(df 15) | 0.04 (df 32) | |
| Change score | −1.2 (1.9) | −7.7 (2.0) | 0.003 (df 1) | |||
| WHO-5 baseline | 21.8 (10.2) | 25.3 (11.6) | 0.21 (df 37) | |||
| WHO-5 follow up | 30.3 (23.1) | 39.2 (22.4) | 0.1 (df 17) | 0.013 (df 15) | 0.27 (df 32) | |
| Change score | 9.1 | −22.3 | 15.1 (21.5) | 0.003 (df 1) |
| Measures | TAU | CBT-I | p-Value a | p-Value a | p-Value b | p-Value c |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | within TAU Groups | within CBT-I Groups | between Groups | between Groups | |
| n = 19 | n = 22 | Baseline vs. Follow Up | Baseline vs. Follow Up | Baseline and Follow Up | Difference (Ancova) | |
| Consequences | ||||||
| Baseline | 36.0 (9.5) | 34.2 (8.4) | 0.55 | |||
| Follow up | 32.6 (10.1) | 27.4 (10.2) | 0.047 (df17) | 0.040 (df14) | 0.16 | |
| Change score | −2.9 (5.8) | −5.6 (9.6) | 0.004 | |||
| Worry/Helplessness | ||||||
| Baseline | 40.2 (9.5) | 42.8 (9.5) | 0.37 | |||
| Follow up | 36.3 10.0) | 30.4 (9.3) | 0.137 (df17) | 0.003 (df14) | 0.09 | |
| Change score | −3.2 (8.8) | −10.7 (11.3) | 0.001 | |||
| Expectations | ||||||
| Baseline | 12.7 (5.1) | 12.8 (5.06) | 0.94 | |||
| Follow up | 11.6 (5.4) | 7.6 (6.2) | 0.362 (df17) | 0.005 (df14) | 0.06 | |
| Change score | −0.78 (3.5) | −3.6 (4.2) | 0.003 | |||
| Medication | ||||||
| Baseline | 13.5 (7.3) | 11.6 (9.2) | 0.46 | |||
| Follow up | 12.6 (7.3) | 7.3 (6.9) | 0.309 (df17) | 0.232 (df14) | 0.04 | |
| Change score | −1.2 (4.9) | −1.9 (5.8) | 0.108 | |||
| Entire DBAS | ||||||
| Baseline | 102.3 (18.0) | 101.4 (26.3) | 0.9 | |||
| Follow up | 93.1 (21.8) | 72.8 (27.0) | 0.036 (df17) | 0.002 (df14) | 0.02 | |
| Change score | −8.2 (15.2) | −21.7 (21.9) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyrberg, H.; Bjorvatn, B.; Larsen, E.R. Cognitive Behavioral Therapy for Chronic Insomnia in Outpatients with Major Depression—A Randomised Controlled Trial. J. Clin. Med. 2022, 11, 5845. https://doi.org/10.3390/jcm11195845
Dyrberg H, Bjorvatn B, Larsen ER. Cognitive Behavioral Therapy for Chronic Insomnia in Outpatients with Major Depression—A Randomised Controlled Trial. Journal of Clinical Medicine. 2022; 11(19):5845. https://doi.org/10.3390/jcm11195845
Chicago/Turabian StyleDyrberg, Henny, Bjørn Bjorvatn, and Erik Roj Larsen. 2022. "Cognitive Behavioral Therapy for Chronic Insomnia in Outpatients with Major Depression—A Randomised Controlled Trial" Journal of Clinical Medicine 11, no. 19: 5845. https://doi.org/10.3390/jcm11195845
APA StyleDyrberg, H., Bjorvatn, B., & Larsen, E. R. (2022). Cognitive Behavioral Therapy for Chronic Insomnia in Outpatients with Major Depression—A Randomised Controlled Trial. Journal of Clinical Medicine, 11(19), 5845. https://doi.org/10.3390/jcm11195845






