Recruitment Maneuver to Reduce Postoperative Pulmonary Complications after Laparoscopic Abdominal Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
- The subjects were adult patients subjected to laparoscopic abdominal surgery requiring general anesthesia and mechanical ventilation.
- The included studies were required to compare RM groups with non-RM groups (or control groups).
- The included studies had to plainly state the mechanical ventilation strategies, and inclusion and exclusion criteria. Postoperative pulmonary complications had to be reported.
- Studies containing patients who were minors or had previous lung disease were excluded.
2.3. Data Extraction
2.4. Statistical Analysis
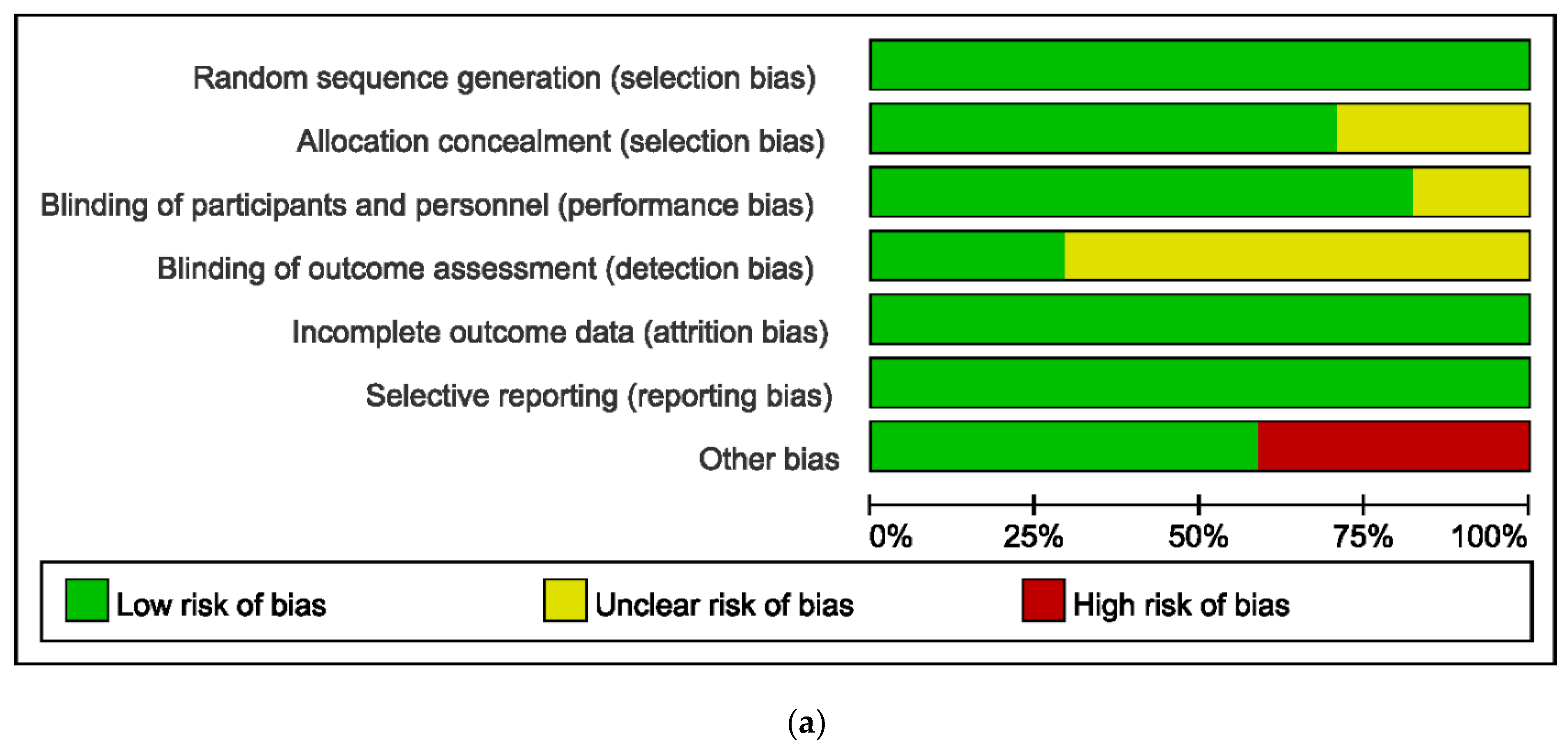
2.5. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Grading Evidence Quality
3.3. Primary Outcomes
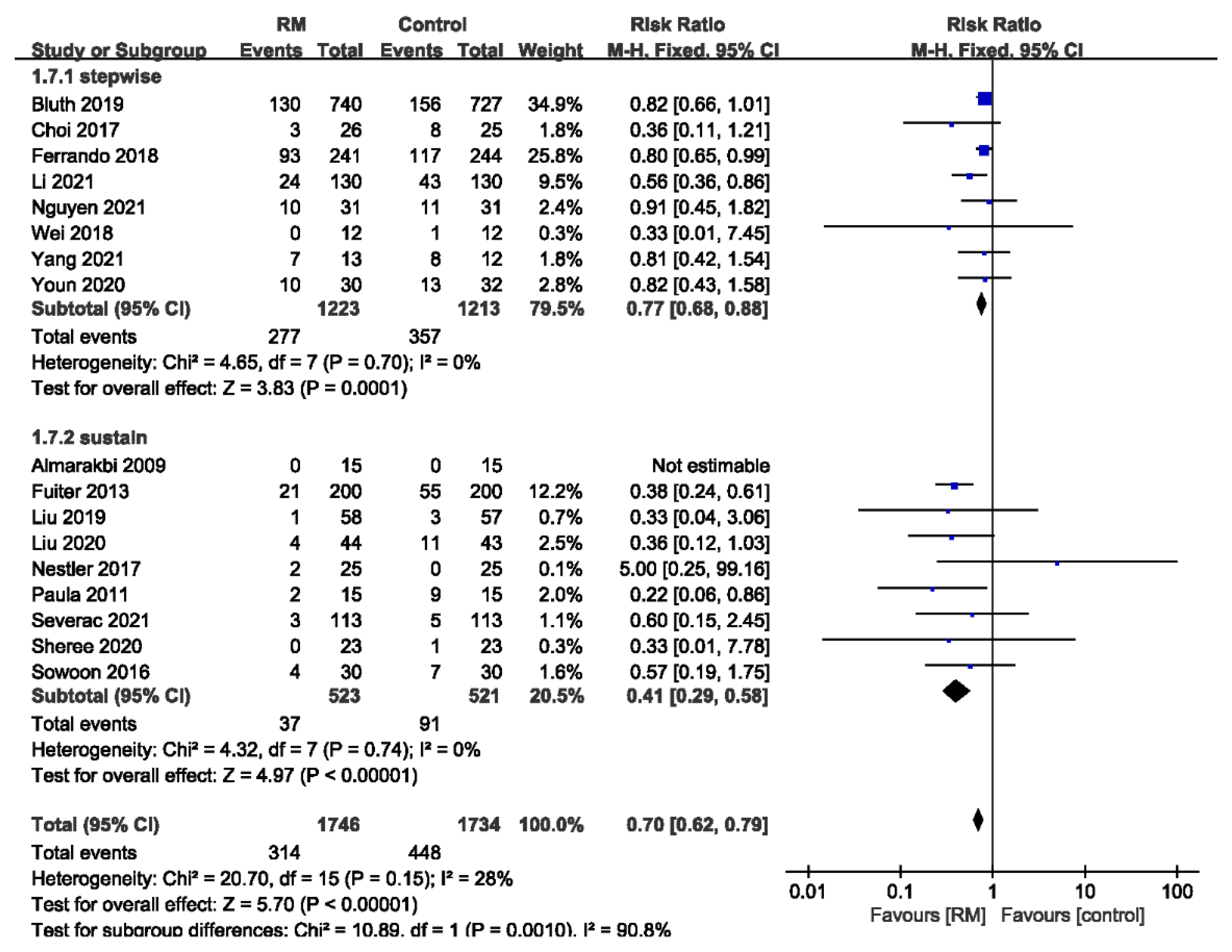
3.3.1. Incidence of PPC
3.3.2. Subgroup Analysis of PPC by BMI
3.3.3. Subgroup Analysis of PPCs by Age
3.3.4. Subgroup Analysis of PPCs by the Number of RMs
3.3.5. Subgroup Analysis of PPCs by the Type of RM
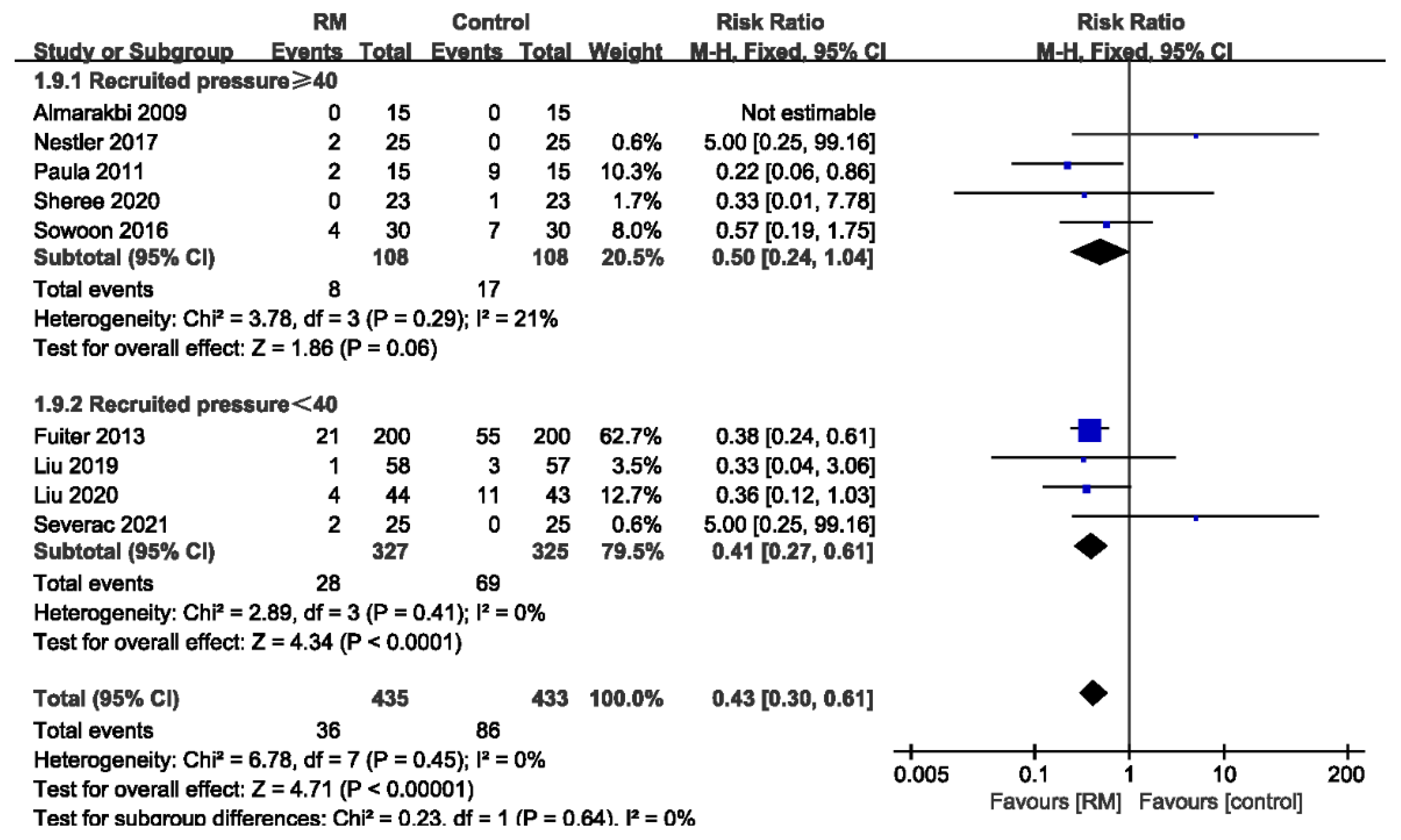
3.3.6. Subgroup Analysis of PPCs by Recruited Pressure
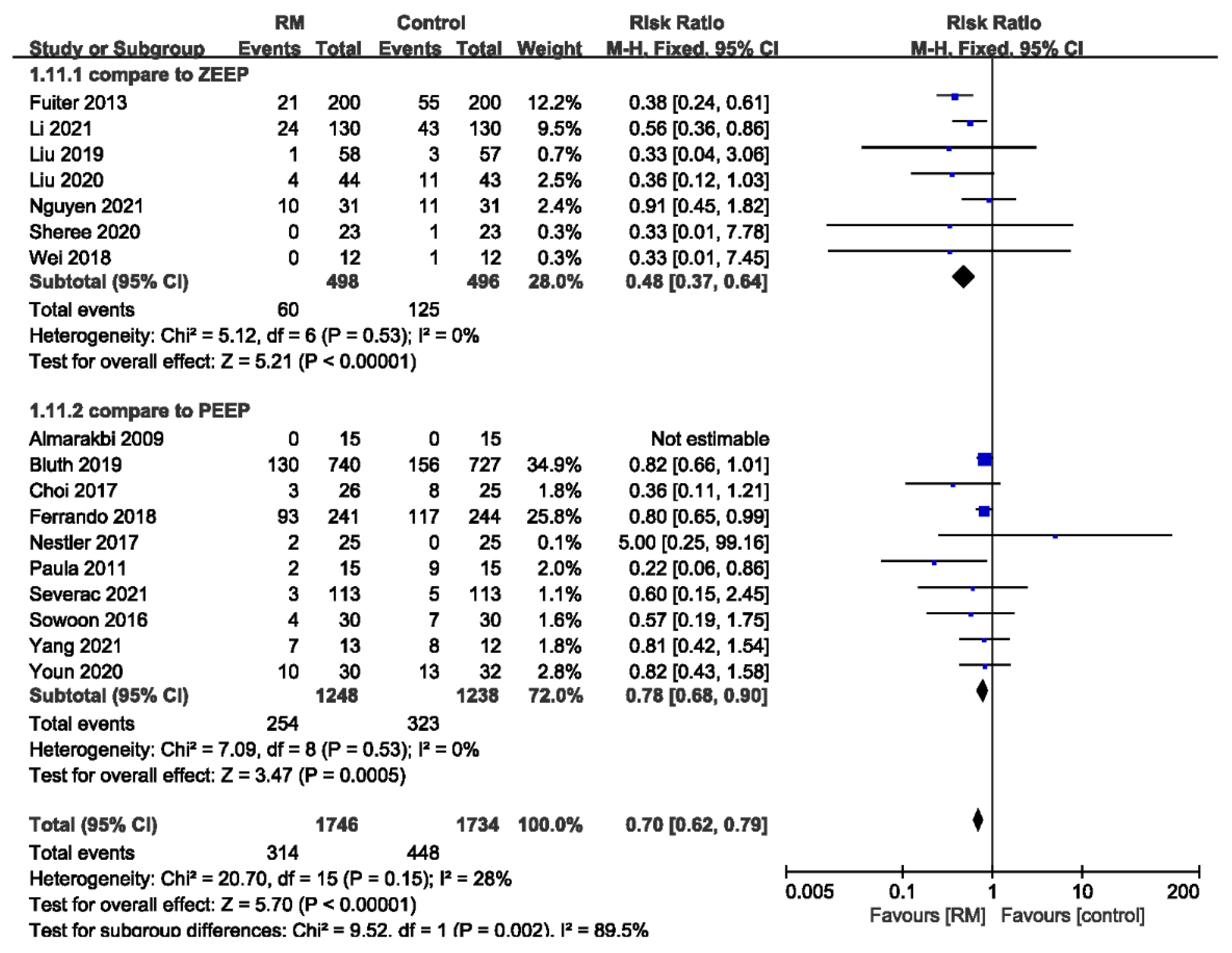
3.3.7. Subgroup Analysis of PPCs by ZEEP or PEEP Used in Control Group
3.4. Secondary Outcomes
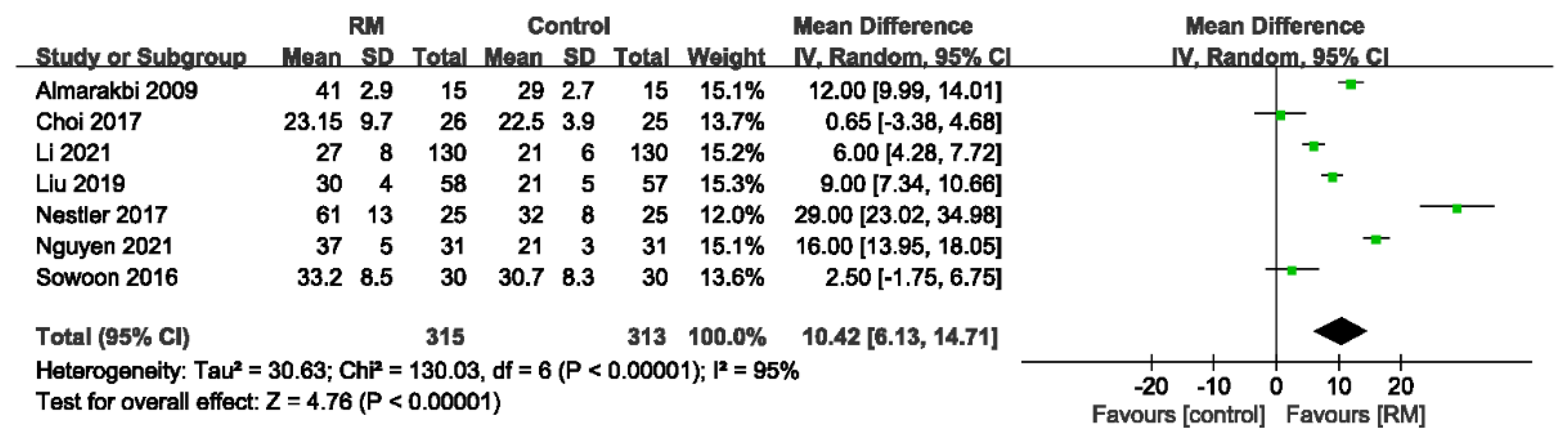
3.4.1. Static Lung Compliance
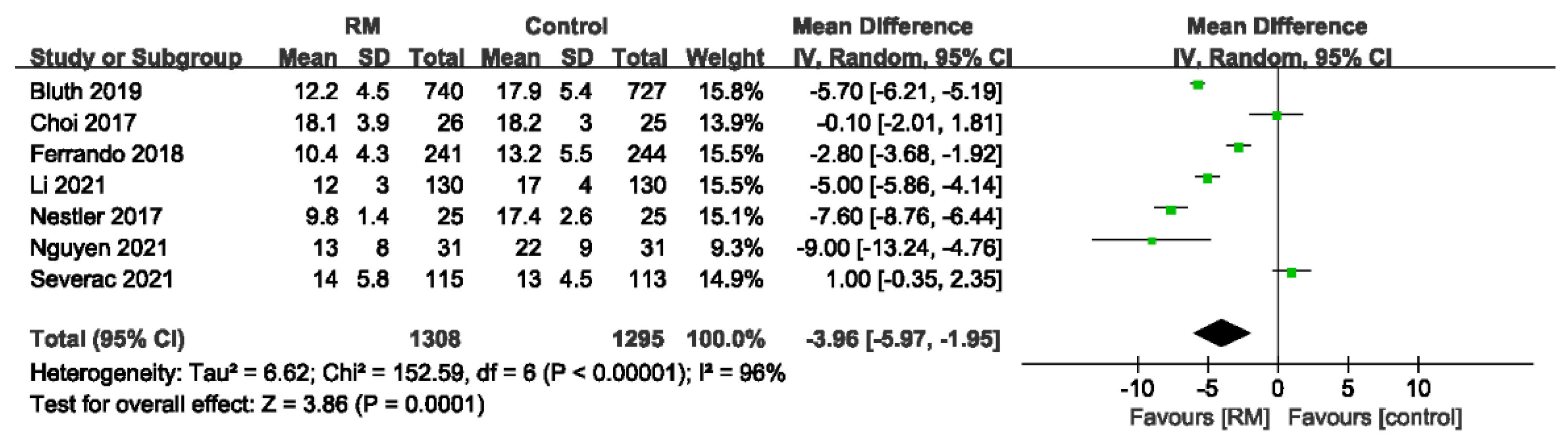
3.4.2. Driving Pressure
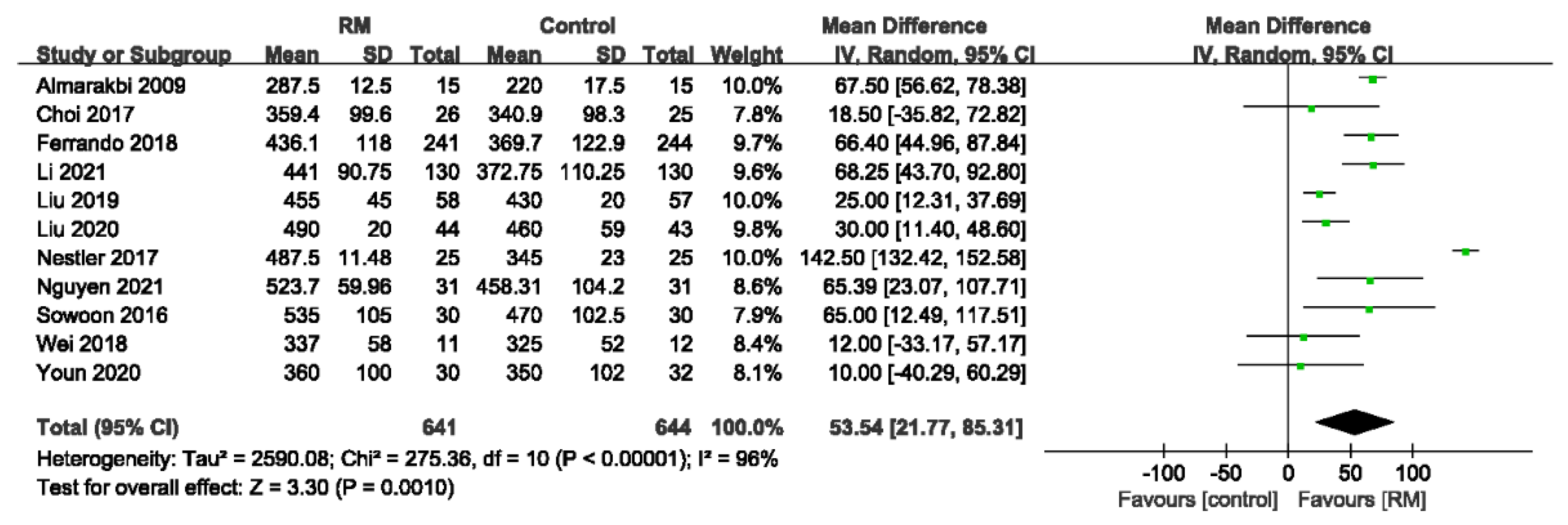
3.4.3. Intraoperative Oxygenation Index
3.4.4. Oxygenation Index in Post-Anesthesia Care Unit
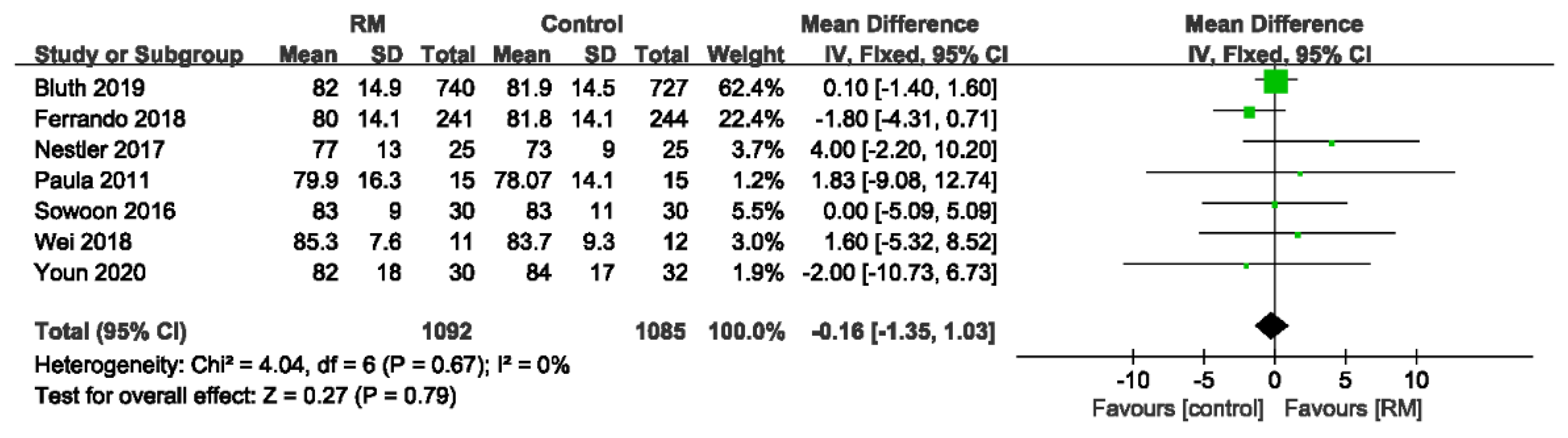
3.4.5. Mean Arterial Pressure
3.4.6. Heart Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caglià, P.; Tracia, A.; Buffone, A.; Amodeo, L.; Tracia, L.; Amodeo, C.; Veroux, M. Physiopathology and clinical considerations of laparoscopic surgery in the elderly. Int. J. Surg. 2016, 33 (Suppl. S1), S97–S102. [Google Scholar] [CrossRef] [PubMed]
- Barkun, J.S.; Barkun, A.N.; Sampalis, J.S.; Fried, G.; Taylor, B.; Wexler, M.J.; Goresky, C.A.; Meakins, J.L. Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment Group. Lancet 1992, 340, 1116–1119. [Google Scholar] [CrossRef]
- Awad, H.; Walker, C.M.; Shaikh, M.; Dimitrova, G.T.; Abaza, R.; O’Hara, J. Anesthetic considerations for robotic prostatectomy: A review of the literature. J. Clin. Anesth. 2012, 24, 494–504. [Google Scholar] [CrossRef]
- Fernandez-Bustamante, A.; Frendl, G.; Sprung, J.; Kor, D.J.; Subramaniam, B.; Martinez Ruiz, R.; Lee, J.W.; Henderson, W.G.; Moss, A.; Mehdiratta, N.; et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017, 152, 157–166. [Google Scholar] [CrossRef]
- Shander, A.; Fleisher, L.A.; Barie, P.S.; Bigatello, L.M.; Sladen, R.N.; Watson, C.B. Clinical and economic burden of postoperative pulmonary complications: Patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit. Care Med. 2011, 39, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Young, C.C.; Harris, E.M.; Vacchiano, C.; Bodnar, S.; Bukowy, B.; Elliott, R.R.D.; Migliarese, J.; Ragains, C.; Trethewey, B.; Woodward, A.; et al. Lung-protective ventilation for the surgical patient: International expert panel-based consensus recommendations. Br. J. Anaesth. 2019, 123, 898–913. [Google Scholar] [CrossRef] [PubMed]
- Güldner, A.; Kiss, T.; Serpa Neto, A.; Hemmes, S.N.; Canet, J.; Spieth, P.M.; Rocco, P.R.; Schultz, M.J.; Pelosi, P.; Gama de Abreu, M. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: A comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 2015, 123, 692–713. [Google Scholar] [CrossRef]
- Hartland, B.L.; Newell, T.J.; Damico, N. Alveolar recruitment maneuvers under general anesthesia: A systematic review of the literature. Respir. Care 2015, 60, 609–620. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Pensier, J.; de Jong, A.; Hajjej, Z.; Molinari, N.; Carr, J.; Belafia, F.; Chanques, G.; Futier, E.; Azoulay, E.; Jaber, S. Effect of lung recruitment maneuver on oxygenation, physiological parameters and mortality in acute respiratory distress syndrome patients: A systematic review and meta-analysis. Intensive Care Med. 2019, 45, 1691–1702. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, R.; Wang, Y.; Li, G. Lung Recruitment Maneuvers for ARDS Patients: A Systematic Review and Meta-Analysis. Respir. Int. Rev. Thorac. Dis. 2020, 99, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W., Jr.; Kunz, R.; Craig, J.; Montori, V.M.; et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336, 1106–1110. [Google Scholar] [CrossRef]
- Almarakbi, W.A.; Fawzi, H.M.; Alhashemi, J.A. Effects of four intraoperative ventilatory strategies on respiratory compliance and gas exchange during laparoscopic gastric banding in obese patients. Br. J. Anaesth. 2009, 102, 862–868. [Google Scholar] [CrossRef]
- Bluth, T.; Serpa Neto, A.; Schultz, M.J.; Pelosi, P.; Gama de Abreu, M.; Bluth, T.; Bobek, I.; Canet, J.C.; Cinnella, G.; de Baerdemaeker, L.; et al. Effect of Intraoperative High Positive End-Expiratory Pressure (PEEP) With Recruitment Maneuvers vs Low PEEP on Postoperative Pulmonary Complications in Obese Patients: A Randomized Clinical Trial. JAMA 2019, 321, 2292–2305. [Google Scholar] [CrossRef]
- Choi, E.S.; Oh, A.Y.; In, C.B.; Ryu, J.H.; Jeon, Y.T.; Kim, H.G. Effects of recruitment manoeuvre on perioperative pulmonary complications in patients undergoing robotic assisted radical prostatectomy: A randomised single-blinded trial. PLoS ONE 2017, 12, e0183311. [Google Scholar] [CrossRef]
- Ferrando, C.; Soro, M.; Unzueta, C.; Suarez-Sipmann, F.; Canet, J.; Librero, J.; Pozo, N.; Peiró, S.; Llombart, A.; León, I.; et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): A randomised controlled trial. Lancet Respir. Med. 2018, 6, 193–203. [Google Scholar] [CrossRef]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. New Engl. J. Med. 2013, 369, 428–437. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Z.N.; Zhang, N.R.; Guo, J.; Wang, K.; Wang, W.; Li, L.G.; Jin, J.; Tang, J.; Liao, Y.J.; et al. Intra-operative open-lung ventilatory strategy reduces postoperative complications after laparoscopic colorectal cancer resection: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, Z.; Lv, R.; Zhang, Y.; Wang, G.; Xie, J. Effect of intraoperative lung-protective mechanical ventilation on pulmonary oxygenation function and postoperative pulmonary complications after laparoscopic radical gastrectomy. Braz. J. Med. Biol. Res. 2019, 52, e8523. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.; Hu, S.; Meng, Z.; He, H. Individualized lung protective ventilation vs. conventional ventilation during general anesthesia in laparoscopic total hysterectomy. Exp. Ther. Med. 2020, 19, 3051–3059. [Google Scholar] [CrossRef] [PubMed]
- Nestler, C.; Simon, P.; Petroff, D.; Hammermüller, S.; Kamrath, D.; Wolf, S.; Dietrich, A.; Camilo, L.M.; Beda, A.; Carvalho, A.R.; et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: A randomized controlled clinical trial using electrical impedance tomography. Br. J. Anaesth. 2017, 119, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Nguyen, V.L.; Nguyen, T.G.; Mai, D.H.; Nguyen, N.Q.; Vu, T.A.; Le, A.N.; Nguyen, Q.H.; Nguyen, C.T.; Nguyen, D.T. Lung-protective mechanical ventilation for patients undergoing abdominal laparoscopic surgeries: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 95. [Google Scholar] [CrossRef]
- Remístico, P.P.; Araújo, S.; de Figueiredo, L.C.; Aquim, E.E.; Gomes, L.M.; Sombrio, M.L.; Ambiel, S.D. Impact of alveolar recruitment maneuver in the postoperative period of videolaparoscopic bariatric surgery. Rev. Bras. Anestesiol. 2011, 61, 163–168, 169–176, 188–194. [Google Scholar] [CrossRef]
- Severac, M.; Chiali, W.; Severac, F.; Perus, O.; Orban, J.C.; Iannelli, A.; Debs, T.; Gugenheim, J.; Raucoules-Aimé, M. Alveolar recruitment manoeuvre results in improved pulmonary function in obese patients undergoing bariatric surgery: A randomised trial. Anaesth. Crit. Care Pain Med. 2021, 40, 100775. [Google Scholar] [CrossRef]
- Abd Ellatif, S.E.; Mowafy, S.M.S. Ultrasonographic evaluation of the effect of recruitment maneuvers and positive end-expiratory pressure on diaphragmatic functions in obese patients undergoing laparoscopic sleeve gastrectomy: A randomized controlled study. Egypt. J. Anaesth. 2020, 36, 69–77. [Google Scholar] [CrossRef]
- Ahn, S.; Byun, S.H.; Chang, H.; Koo, Y.B.; Kim, J.C. Effect of recruitment maneuver on arterial oxygenation in patients undergoing robot-assisted laparoscopic prostatectomy with intraoperative 15 cmH(2)O positive end expiratory pressure. Korean J. Anesthesiol. 2016, 69, 592–598. [Google Scholar] [CrossRef]
- Wei, K.; Min, S.; Cao, J.; Hao, X.; Deng, J. Repeated alveolar recruitment maneuvers with and without positive end-expiratory pressure during bariatric surgery: A randomized trial. Minerva Anestesiol. 2018, 84, 463–472. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, Y.; Zhang, D.; Wan, Y.; Wang, R. Effect of Lung Recruitment Maneuvers on Reduction of Atelectasis Determined by Lung Ultrasound in Patients More Than 60 Years Old Undergoing Laparoscopic Surgery for Colorectal Carcinoma: A Prospective Study at a Single Center. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e926748. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.Y.; Lee, K.C.; Chang, Y.J.; Jung, W.S.; Park, J.; Kwak, H.J. Effects of an Alveolar Recruitment Maneuver During Lung Protective Ventilation on Postoperative Pulmonary Complications in Elderly Patients Undergoing Laparoscopy. Clin. Interv. Aging 2020, 15, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Rothen, H.U.; Sporre, B.; Engberg, G.; Wegenius, G.; Hedenstierna, G. Re-expansion of atelectasis during general anaesthesia: A computed tomography study. Br. J. Anaesth. 1993, 71, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Bluth, T.; Pelosi, P.; de Abreu, M.G. The obese patient undergoing nonbariatric surgery. Curr. Opin. Anaesthesiol. 2016, 29, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Altermatt, F.R.; Muñoz, H.R.; Delfino, A.E.; Cortínez, L.I. Pre-oxygenation in the obese patient: Effects of position on tolerance to apnoea. Br. J. Anaesth. 2005, 95, 706–709. [Google Scholar] [CrossRef]
- Eichenberger, A.; Proietti, S.; Wicky, S.; Frascarolo, P.; Suter, M.; Spahn, D.R.; Magnusson, L. Morbid obesity and postoperative pulmonary atelectasis: An underestimated problem. Anesth. Analg. 2002, 95, 1788–1792. [Google Scholar] [CrossRef]
- Reinius, H.; Jonsson, L.; Gustafsson, S.; Sundbom, M.; Duvernoy, O.; Pelosi, P.; Hedenstierna, G.; Fredén, F. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: A computerized tomography study. Anesthesiology 2009, 111, 979–987. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, R.; Li, G.; Gong, T.; Ou, Y.; Huang, J. The effect of lung recruitment maneuvers on post-operative pulmonary complications for patients undergoing general anesthesia: A meta-analysis. PLoS ONE 2019, 14, e0217405. [Google Scholar] [CrossRef]
- Karsten, J.; Luepschen, H.; Grossherr, M.; Bruch, H.P.; Leonhardt, S.; Gehring, H.; Meier, T. Effect of PEEP on regional ventilation during laparoscopic surgery monitored by electrical impedance tomography. Acta Anaesthesiol. Scand. 2011, 55, 878–886. [Google Scholar] [CrossRef]
- De Jong, M.A.C.; Ladha, K.S.; Vidal Melo, M.F.; Staehr-Rye, A.K.; Bittner, E.A.; Kurth, T.; Eikermann, M. Differential Effects of Intraoperative Positive End-expiratory Pressure (PEEP) on Respiratory Outcome in Major Abdominal Surgery Versus Craniotomy. Ann. Surg. 2016, 264, 362–369. [Google Scholar] [CrossRef]
- Celebi, S.; Köner, O.; Menda, F.; Korkut, K.; Suzer, K.; Cakar, N. The pulmonary and hemodynamic effects of two different recruitment maneuvers after cardiac surgery. Anesth. Analg. 2007, 104, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Reis Miranda, D.; Gommers, D.; Struijs, A.; Meeder, H.; Schepp, R.; Hop, W.; Bogers, A.; Klein, J.; Lachmann, B. The open lung concept: Effects on right ventricular afterload after cardiac surgery. Br. J. Anaesth. 2004, 93, 327–332. [Google Scholar] [CrossRef] [PubMed][Green Version]







| String | Condition | Search |
|---|---|---|
| #1 | - | ((“Abdomen”[Mesh]) OR “Laparoscopy”[Mesh]) OR “Hand-Assisted Laparoscopy”[Mesh] |
| #2 | OR | ((((((((((((((((((((((((((((((((((Laparoscop*[Title/Abstract]) OR (Celioscop*[Title/Abstract])) OR (Peritoneoscop*[Title/Abstract])) OR (Surgical Procedures, Laparoscopic[Title/Abstract])) OR (Laparoscopic Surgical Procedure[Title/Abstract])) OR (Procedure, Laparoscopic Surgical[Title/Abstract])) OR (Procedures, Laparoscopic Surgical[Title/Abstract])) OR (Surgery, Laparoscopic[Title/Abstract])) OR (Laparoscopic Surgical Procedures[Title/Abstract])) OR (Laparoscopic Surgery[Title/Abstract])) OR (Laparoscopic Surgeries[Title/Abstract])) OR (Surgeries, Laparoscopic[Title/Abstract])) OR (Laparoscopic Assisted Surgery[Title/Abstract])) OR (Laparoscopic Assisted Surgeries[Title/Abstract])) OR (Surgeries, Laparoscopic Assisted[Title/Abstract])) OR (Surgery, Laparoscopic Assisted[Title/Abstract])) OR (Surgical Procedure, Laparoscopic[Title/Abstract])) OR (Hand Assisted Laparoscop*[Title/Abstract])) OR (Laparoscopies, Hand-Assisted[Title/Abstract])) OR (Laparoscopy, Hand-Assisted[Title/Abstract])) OR (Hand-Assisted Laparoscopic Surgery[Title/Abstract])) OR (Hand Assisted Laparoscopic Surgery[Title/Abstract])) OR (Hand-Assisted Laparoscopic Surgeries[Title/Abstract])) OR (Laparoscopic Surgeries, Hand-Assisted[Title/Abstract])) OR (Laparoscopic Surgery, Hand-Assisted[Title/Abstract])) OR (Surgeries, Hand-Assisted Laparoscopic[Title/Abstract])) OR (Surgery, Hand-Assisted Laparoscopic[Title/Abstract])) OR (Hand-Assisted Laparoscopic Surgical Procedures[Title/Abstract])) OR (Hand Assisted Laparoscopic Surgical Procedures[Title/Abstract])) OR (abdomen[Title/Abstract])) OR (abdominal[Title/Abstract])) OR (belly[Title/Abstract])) OR (stomach[Title/Abstract])) OR (tummy[Title/Abstract])) OR (midriff[Title/Abstract]) |
| #3 | AND | ((((((((recruitment maneuver [Title/Abstract]) OR (recruitment maneuver*[Title/Abstract])) OR (RM[Title/Abstract])) OR (open lung [Title/Abstract])) OR (open-lung [Title/Abstract])) OR (protected ventilation [Title/Abstract])) OR (protective ventilation [Title/Abstract])) OR (protected mechanical ventilation [Title/Abstract])) OR (protective mechanical ventilation [Title/Abstract]) |
| #4 | AND | (((randomized controlled trial [Publication Type]) OR (randomized [Title/Abstract])) OR (placebo [Title/Abstract])) OR (trial*[Title/Abstract]) |
| #5 | AND | (“1980/01/01”[Date—Publication]: “2021/12/31”[Date—Publication]) |
| #6 | - | [#1 OR #2] AND #3 AND #4 AND #5 |
| Study | Surgery | No. of Patients (Male/Female) | Age, Years Mean (SD) | ASA Class | BMI, kg/m2, Mean (SD) | Tidal Volume (ml/kg) | Recruitment Maneuver | PEEP (cmH2O) |
|---|---|---|---|---|---|---|---|---|
| Almarakbi, 2009 | laparoscopic gastric banding | C:15 (8/7) RM1: 15 (9/6) RM2:15 (7/8) RM3:15 (8/7) | C: 38 (3) RM1: 38 (3) RM2: 38 (3) RM3: 38 (4) | II | C: 33 (2) RM1: 33 (1) RM2: 34 (1) RM3: 33 (1) | C:10 RM1:10 RM2:10 RM3:10 | sustained inspiratory pressure of 40 cm H2O for 15 s; performed 10 min after pneumoperitoneum, before surgery | C:10 RM1:0 RM2:10 RM3:10 |
| Bluth, 2019 | general anesthesia surgery | RM:740 (221/519) C:727 (221/506) | RM:48.6 (13.8) C:48.9 (13.3) | I–IV | > 35 | RM:7 C:7 | The tidal volume was increased 4 mL/kg until Pplat reaches 40–50 cmH2O,3 breaths for 40–50 cmH2O; performed after induction of anesthesia, after any disconnection from the mechanical ventilator, every 1 h during surgery, and before extubation | RM:12 C:4 |
| Choi, 2017 | RARP | RM:26 (26/0) C:25 (25/0) | RM:67.6 (4.3) C:66.6 (4.3) | I–II | RM:24.5 (2) C:24.5 (2.1) | RM:6–8 C:6–8 | staircase PEEP(4–16 cmH2O), Ppeak < 35 cmH2O; performed after intubation | RM:5 C:5 |
| Ferrando, 2018 | abdominal surgery | RM1:241 (141/100) RM2:237 (157/80) C1:243 (163/80) C2:244 (154/90) | RM1:64.3 (13) RM2:64.7 (13.2) C1:66.5 (11.4) C2:64.8 (12.9) | I–IV | RM1:26 (4) RM2:26.2 (4) C1:25.8 (3.7) C2:26.1 (3.9) | RM1:8 RM2:8 C1:8 C2:8 | step-wise RM until Paw reached 40 cm H2O + PEEP titration trial; performed after intubation, every 40 min during surgery | RM1:iPEEP RM2:iPEEP C1:5 C2:5 |
| Fuiter, 2013 | abdominal surgery | RM:41 (200) C:44 (200) | RM:61.6 (11) C:63.4 (10) | N | RM:24.8 (3.8) C:24.7 (3.8) | RM:6.4 (0.8) C:11.1 (1.1) | CPAP 30 cm H2O for 30 s; performed after pneumoperitoneum, every 0.5 h during surgery | RM:6–8 C: 0 |
| Li, 2021 | laparoscopic colorectal cancer resection | RM:130 (102/28) C:130 (98/32) | RM:69.7 (5.8) C:70.8 (5.8) | II–III | RM:23 (2.7) C:22.3 (2.8) | RM:6–8 C:6–8 | PEEP= 12 cm H2O, tidal volumes are increased in steps of 4 mL/kg PBW until a plateau pressure of 30–35 cm H2O, 3 breaths for 30–35 cm H2O; performed after induction of anesthesia, every 0.5 h during surgery | RM:6–8 C:0 |
| Liu, 2019 | laparoscopic gastric cancer radical surgery | RM:58 (26/32) C:57 (26/31) | RM:63.2 (8.31) C:66.13 (9.12) | I–III | RM:22.45 (2.1) C:23.27 (2.95) | RM:7 C:10 | CPAP and applying 30 cm H2O PEEP for 30 s; followed by a decremental PEEP titration procedure | RM:iPEEP C:0 |
| Liu, 2020 | laparoscopic total hysterectomy | RM:44 (0/44) C:43 (0/43) | RM:51.08 (8.86) C:50.32 (9.83) | I–III | RM:23.31 (3.98) C:22.58 (3.05) | RM:7 C:9 | 30 cm H2O PEEP for 30 s followed by a decremental PEEP titration procedure; immediately after induction of anesthesia and orotracheal intubation | RM:iPEEP C:0 |
| Nestler, 2017 | elective laparoscopic surgery | RM:25 (8/17) C:25 (8/17) | RM:44.9 (11.14) C:46.2 (12.57) | N | RM:48.3 (7.1) C:53.8 (8.2) | RM:8 C:8 | Ppeak < 50 H2O, PEEP 30 cm H2O, RR 6 bpm, for 10 cycles; an RM followed by a decremental PEEP titration, additional RM was performed before extubating | RM:iPEEP [18(4.27)] C:5 |
| Nguyen, 2021 | laparoscopic abdominal surgery | RM:31 (9/22) C:31 (13/18) | RM:59 (9) C:55 (12) | II–III | RM:21 (2) C:21 (3) | RM:7 C:10 | staircase PEEP (10,15,20 cm H2O), PIP < 50 cm H2O; right after intubation, 30 min after CO2 insufflation, then every hour, and finally before extubating | RM:10 C:0 |
| Paula, 2011 | bariatric surgery by video-laparoscopy | RM:15 (4/11) C:15 (5/10) | RM:42.1 (14.5) C:37.2 (12.2) | N | RM:35.2 (5.5) C:35.4 (5.5) | RM:11.5 (2.36) C:10.8 (1.3) | PEEP of 30 cm H2O and inspiratory plateau pressure of 15 cm H2O above PEEP for 2 min | RM: 5.7 (0.9) C: 5.4 (0.91) |
| Mathilde, 2021 | laparoscopic bariatric surgery | RM:115 (19/96) C:113 (26/87) | RM:38.8 (15) C:39.4 (17.6) | I–III | 41 (4.5) | RM:6–8 C:6–8 | maintaining the airway pressure at 30 cm H2O for 30 s; performed after intubation and every 30 min for the all duration of anesthesia | RM:5–10 C:5–10 |
| Sheree, 2020 | LSG | RM:23 (11/12) C1:23 (10/13) C2:23 (12/11) | RM:29.8 (8.98) C1:29.7 (8.2) C2:29.7 (9.3) | II–III | RM:39.3 (1.9) C1:39.5 (2.5) C2:39 (2.68) | RM:6–8 C1:6–8 C2:6–8 | airway pressure 40 cm H2O for 40 s; performed post-induction of anesthesia, 2 min after completion of pneumoperitoneum, 2 min after placing the patient in Trendelenburg position, and finally 2 min after exsufflation of pneumoperitoneum | RM:5 C1:5 C2:0 |
| Sowoon, 2016 | RALP | RM:30 (30/0) C:30 (30/0) | RM:63 (6) C:62 (6) | N | N | RM:6 C:6 | CPAP of 40 cm H2O for 40 s; 15 min after Trendelenburg position | RM:15 C:15 |
| Wei, 2018 | LSG | RM1:12 (6/6) RM2:11 (5/6) C:12 (5/7) | RM1:39 (10.48) RM2:33 (10.49) C:37 (14) | II–III | RM1:43 (6) RM2:48 (8) C:45 (6) | RM1:8 RM2:8 C:8 | staircase PEEP (5–15 cm H2O), Ppeak < 40; performed immediately after the inflation of pneumoperitoneum, repeated every 30 min during the procedure, the last RM followed the deflation of pneumoperitoneum. | RM1:8 RM2:0 C:0 |
| Yang, 2021 | laparoscopic surgery for colorectal cancer | RM:20 (16/4) C:20 (14/6) | RM:66.4 (4.6) C:69.5 (6.2) | II–III | RM:22.3 (2.2) C:22.7 (2.3) | RM:6–8 C:6–8 | gradual rise in airway pressure under ultrasound guidance from 10 cm H2O by 5 cmH2O increments, until no collapsed lung areas were visible on the sonogram, the pressure was maintained for 40 s, Ppeak < 40 cm H2O; performed at the end of the surgery | RM:4 C:4 |
| Youn, 2020 | laparoscopic low anterior resection for colorectal cancer | RM:30 (15/15) C:32 (19/13) | RM:74 (5) C:76 (7) | I–II | N | RM:6 C:6 | staircase PEEP (10, 15, 20 cm H2O), 3 breaths for each PEEP, Ppeak < 40 cm H2O; immediately before and after CO2 pneumoperitoneum | RM:5 C:5 |
| Quality Assessment | No of Patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | RM | Control | Relative (95% CI) | Absolute | ||
| PPC | ||||||||||||
| 17 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 314/1746 (18%) | 448/1734 (25.8%) | RR 0.7 (0.62 to 0.79) | 78 fewer per 1000 (from 54 fewer to 98 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 25.6% | 77 fewer per 1000 (from 54 fewer to 97 fewer) | |||||||||||
| PPC-BMI ≥ 35 | ||||||||||||
| 6 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 137/928 (14.8%) | 172/915 (18.8%) | RR 0.79 (0.64 to 0.96) | 39 fewer per 1000 (from 8 fewer to 68 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 6.4% | 13 fewer per 1000 (from 3 fewer to 23 fewer) | |||||||||||
| PPC-BMI < 35 | ||||||||||||
| 8 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 163/743 (21.9%) | 256/742 (34.5%) | RR 0.64 (0.54 to 0.75) | 124 fewer per 1000 (from 86 fewer to 159 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 32.5% | 117 fewer per 1000 (from 81 fewer to 149 fewer) | |||||||||||
| PPC-Age < 65 | ||||||||||||
| 6 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 135/918 (14.7%) | 172/905 (19%) | RR 0.77 (0.63 to 0.95) | 44 fewer per 1000 (from 10 fewer to 70 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 6.4% | 15 fewer per 1000 (from 3 fewer to 24 fewer) | |||||||||||
| PPC- Single RM | ||||||||||||
| 5 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association 1 | 13/127 (10.2%) | 36/125 (28.8%) | RR 0.36 (0.21 to 0.64) | 184 fewer per 1000 (from 104 fewer to 228 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 25.6% | 164 fewer per 1000 (from 92 fewer to 202 fewer) | |||||||||||
| PPC-Repeated RM | ||||||||||||
| 12 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 301/1619 (18.6%) | 412/1609 (25.6%) | RR 0.73 (0.64 to 0.83) | 69 fewer per 1000 (from 44 fewer to 92 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 24.5% | 66 fewer per 1000 (from 42 fewer to 88 fewer) | |||||||||||
| PPC- Stepwise RM | ||||||||||||
| 8 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 277/1223 (22.6%) | 357/1213 (29.4%) | RR 0.77 (0.68 to 0.88) | 68 fewer per 1000 (from 35 fewer to 94 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 34.3% | 79 fewer per 1000 (from 41 fewer to 110 fewer) | |||||||||||
| PPC-Sustained RM | ||||||||||||
| 9 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association 1 | 37/523 (7.1%) | 91/521 (17.5%) | RR 0.41 (0.29 to 0.58) | 103 fewer per 1000 (from 73 fewer to 124 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 5.3% | 31 fewer per 1000 (from 22 fewer to 38 fewer) | |||||||||||
| PPC-Recruited pressure ≥ 40 | ||||||||||||
| 5 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 8/108 (7.4%) | 17/108 (15.7%) | RR 0.5 (0.24 to 1.04) | 79 fewer per 1000 (from 120 fewer to 6 more) | ÅÅÅÅ HIGH | CRITICAL |
| 4.4% | 22 fewer per 1000 (from 33 fewer to 2 more) | |||||||||||
| PPC-Recruited pressure < 40 | ||||||||||||
| 4 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association 1 | 28/327 (8.6%) | 69/325 (21.2%) | RR 0.41 (0.27 to 0.61) | 125 fewer per 1000 (from 83 fewer to 155 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 15.4% | 91 fewer per 1000 (from 60 fewer to 112 fewer) | |||||||||||
| PPC-Compare to ZEEP | ||||||||||||
| 7 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association 1 | 60/498 (12%) | 125/496 (25.2%) | RR 0.48 (0.37 to 0.64) | 131 fewer per 1000 (from 91 fewer to 159 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 25.6% | 133 fewer per 1000 (from 92 fewer to 161 fewer) | |||||||||||
| PPC-Compare to PEEP | ||||||||||||
| 10 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 254/1248 (20.4%) | 323/1238 (26.1%) | RR 0.78 (0.68 to 0.9) | 57 fewer per 1000 (from 26 fewer to 83 fewer) | ÅÅÅÅ HIGH | CRITICAL |
| 27.7% | 61 fewer per 1000 (from 28 fewer to 89 fewer) | |||||||||||
| Static lung compliance | ||||||||||||
| 7 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | serious 2 | none | 315 | 313 | - | MD 10.42 higher (6.13 to 14.71 higher) | ÅÅÅO MODERATE | CRITICAL |
| Driving pressure | ||||||||||||
| 7 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | serious 2 | none | 1308 | 1295 | - | MD 3.96 lower (5.97 to 1.95 lower) | ÅÅÅO MODERATE | CRITICAL |
| Intraoperative oxygenation index | ||||||||||||
| 11 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | serious 2 | none | 641 | 644 | - | MD 53.54 higher (21.77 to 85.31 higher) | ÅÅÅO MODERATE | CRITICAL |
| Postoperative oxygenation index | ||||||||||||
| 7 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | serious 2 | none | 934 | 919 | - | MD 59.4 higher (39.1 to 79.69 higher) | ÅÅÅO MODERATE | CRITICAL |
| Mean arterial pressure | ||||||||||||
| 7 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 1092 | 1085 | - | MD 0.16 lower (1.35 lower to 1.03 higher) | ÅÅÅÅ HIGH | CRITICAL |
| Heart rate | ||||||||||||
| 6 | randomized trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none | 851 | 841 | - | MD 1.1 lower (2.29 lower to 0.1 higher) | ÅÅÅÅ HIGH | CRITICAL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, S.; Wei, W.; Yang, K.; Yang, Y.; Pan, Y.; Wei, J.; Yao, S.; Xia, H. Recruitment Maneuver to Reduce Postoperative Pulmonary Complications after Laparoscopic Abdominal Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5841. https://doi.org/10.3390/jcm11195841
Pei S, Wei W, Yang K, Yang Y, Pan Y, Wei J, Yao S, Xia H. Recruitment Maneuver to Reduce Postoperative Pulmonary Complications after Laparoscopic Abdominal Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(19):5841. https://doi.org/10.3390/jcm11195841
Chicago/Turabian StylePei, Shuaijie, Wei Wei, Kai Yang, Yiyi Yang, Yu Pan, Jinrui Wei, Shanglong Yao, and Haifa Xia. 2022. "Recruitment Maneuver to Reduce Postoperative Pulmonary Complications after Laparoscopic Abdominal Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 19: 5841. https://doi.org/10.3390/jcm11195841
APA StylePei, S., Wei, W., Yang, K., Yang, Y., Pan, Y., Wei, J., Yao, S., & Xia, H. (2022). Recruitment Maneuver to Reduce Postoperative Pulmonary Complications after Laparoscopic Abdominal Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(19), 5841. https://doi.org/10.3390/jcm11195841