Effectiveness of Topical Ganciclovir 2% Monotherapy Versus Combined Steroid Therapy in Cytomegalovirus Endotheliitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. CMV PCR Test
2.3. Treatment
2.4. Outcome Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyazaki, D.; Shimizu, D.; Shimizu, Y.; Inoue, Y.; Inoue, T.; Higaki, S.; Ueta, M.; Sugita, S. Diagnostic efficacy of real-time PCR for ocular cytomegalovirus infections. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Miyazaki, D.; Inoue, T.; Ohtani, F.; Kandori-Inoue, M.; Inatomi, T.; Sotozono, C.; Nakagawa, H.; Horikiri, T.; Ueta, M.; et al. The effect of topical application of 0.15% ganciclovir gel on cytomegalovirus corneal endotheliitis. Br. J. Ophthalmol. 2017, 101, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Yamasaki, K.; Kawasaki, S.; Sotozono, C.; Inatomi, T.; Mochida, C.; Kinoshita, S. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am. J. Ophthalmol. 2006, 141, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Shiraishi, A.; Ohashi, Y.; Kandori, M.; Miyazaki, D.; Inoue, Y.; Soma, T.; Nishida, K.; et al. Clinical features and management of cytomegalovirus corneal endotheliitis: Analysis of 106 cases from the Japan corneal endotheliitis study. Br. J. Ophthalmol. 2015, 99, 54–58. [Google Scholar] [CrossRef]
- Su, C.C.; Wang, I.J.; Chen, W.L.; Lin, C.P.; His, B.; Hu, F.R. Topical ganciclovir treatment in patients with cytomegalovirus endotheliitis receiving penetrating keratoplasty. Clin. Exp. Ophthalmol. 2013, 41, 339–347. [Google Scholar] [CrossRef]
- Chee, S.P.; Jap, A. Treatment outcome and risk factors for visual loss in Cytomegalovirus endotheliitis. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 383–389. [Google Scholar] [CrossRef]
- Kandori, M.; Inoue, T.; Takamatsu, F.; Kojima, Y.; Hori, Y.; Maeda, N.; Tano, Y. Prevalence and features of keratitis with quantitative polymerase chain reaction positive for cytomegalovirus. Ophthalmology 2010, 117, 216–222. [Google Scholar] [CrossRef]
- Hwang, J.H.; Ha, M.; Park, Y.; Chung, S.H. The Effect of Topical Ganciclovir and Corticosteroid on Cytomegalovirus Corneal Endotheliitis in Korean Patients. Ocul. Immunol. Inflamm. 2019, 27, 338–344. [Google Scholar] [CrossRef]
- Fan, N.W.; Chung, Y.C.; Liu, Y.C.; Liu, C.J.; Kuo, Y.S.; Lin, P.Y. Long-Term Topical Ganciclovir and Corticosteroids Preserve Corneal Endothelial Function in Cytomegalovirus Corneal Endotheliitis. Cornea 2016, 35, 596–601. [Google Scholar] [CrossRef]
- Su, C.C.; Hu, F.R.; Wang, T.H.; Huang, J.Y.; Yeh, P.T.; Lin, C.P.; Wang, I.J. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am. J. Ophthalmol. 2014, 158, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.P.; Bacsal, K.; Jap, A.; Se-Thoe, S.Y.; Cheng, C.L.; Tan, B.H. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am. J. Ophthalmol. 2008, 145, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Suzuki, T.; Uno, T.; Chihara, H.; Shiraishi, A.; Hara, Y.; Inatomi, T.; Sotozono, C.; Kawasaki, S.; Yamasaki, K.; et al. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology 2008, 115, 292–297.e3. [Google Scholar] [CrossRef] [PubMed]
- Faith, S.C.; Durrani, A.F.; Jhanji, V. Cytomegalovirus keratitis. Curr. Opin. Ophthalmol. 2018, 29, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Lin, K.K.; Lee, J.S.; Chang, S.H.; Chen, K.J.; Lai, C.C.; Huang, J.C.; Kuo, Y.H.; Hsiao, C.H. Intravitreal loading injection of ganciclovir with or without adjunctive oral valganciclovir for cytomegalovirus anterior uveitis. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 263–269. [Google Scholar] [CrossRef] [PubMed]
- McGavin, J.K.; Goa, K.L. Ganciclovir: An update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 2001, 61, 1153–1183. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.P.; Jap, A. Immune ring formation associated with cytomegalovirus endotheliitis. Am. J. Ophthalmol. 2011, 152, 449–453.e1. [Google Scholar] [CrossRef]
- Espy, M.J.; Smith, T.F.; Persing, D.H. Dependence of polymerase chain reaction product inactivation protocols on amplicon length and sequence composition. J. Clin. Microbiol. 1993, 31, 2361–2365. [Google Scholar] [CrossRef]
- Pacsa, A.S.; Essa, S.; Voevodin, A.; el-Shazly, A.; Kazak, H.; Nampoory, M.R.; Johny, K.V.; Said, T.; Al-Nakib, W. Correlation between CMV genotypes, multiple infections with herpesviruses (HHV-6, 7) and development of CMV disease in kidney recipients in Kuwait. FEMS Immunol. Med. Microbiol. 2003, 20, 125–130. [Google Scholar] [CrossRef]
- Yoshida, J.; Heflin, T.; Zambrano, A.; Pan, Q.; Meng, H.; Wang, J.; Stark, W.J.; Daoud, Y.J. Gamma-Irradiated Sterile Cornea for Use in Corneal Transplants in a Rabbit Model. Middle East Afr. J. Ophthalmol. 2015, 22, 346–351. [Google Scholar] [CrossRef]
- Chee, S.P.; Jap, A. Presumed fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: Comparison of cytomegalovirus-positive and negative eyes. Am. J. Ophthalmol. 2008, 146, 883–889.e1. [Google Scholar] [CrossRef] [PubMed]
- Castela, N.; Vermerie, N.; Chast, F.; Sauvageon-Martre, H.; Denis, J.; Godard, V.; Goldschmidt, P.; Pouliquen, Y. Ganciclovir ophthalmic gel in herpes simplex virus rabbit keratitis: Intraocular penetration and efficacy. J. Ocul. Pharmacol. 1994, 10, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.; Hoh, H.B.; Easty, D.L.; Herbort, C.P.; Resnikoff, S.; Rigal, D.; Romdane, K. Ganciclovir ophthalmic gel (Virgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea 1997, 16, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.H.; Kimbrough, R.L. Intraocular pressure response to topically administered fluorometholone. Arch. Ophthalmol. 1979, 97, 2139–2140. [Google Scholar] [CrossRef]
- Shokoohi-Rad, S.; Daneshvar, R.; Jafarian-Shahri, M.; Rajaee, P. Comparison between Betamethasone, Fluorometholone and Loteprednol Etabonate on intraocular pressure in patients after keratorefractive surgery. J. Curr. Ophthalmol. 2017, 30, 130–135. [Google Scholar] [CrossRef]
- Akingbehin, A.O. Comparative study of the intraocular pressure effects of fluorometholone 0.1% versus dexamethasone 0.1%. Br. J. Ophthalmol. 1983, 67, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Yang, H.K.; Hwang, J.M. Efficacy and Safety of Loteprednol 0.5% and Fluorometholone 0.1% After Strabismus Surgery in Children. J. Ocul. Pharmacol. Ther. 2018, 34, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Arici, M.K.; Arici, D.S.; Topalkara, A.; Güler, C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin. Exp. Ophthalmol. 2000, 28, 113–117. [Google Scholar] [CrossRef]
- Keorochana, N.; Choontanom, R. Efficacy and safety of an extemporaneous preparation of 2% ganciclovir eye drops in CMV anterior uveitis. BMJ Open Ophthalmol. 2017, 2, e000061. [Google Scholar] [CrossRef]
- Touhami, S.; Qu, L.; Angi, M.; Bojanova, M.; Touitou, V.; Lehoang, P.; Rozenberg, F.; Bodaghi, B. Cytomegalovirus Anterior Uveitis: Clinical Characteristics and Long-term Outcomes in a French Series. Am. J. Ophthalmol. 2018, 194, 134–142. [Google Scholar] [CrossRef]
- Chee, S.P.; Jap, A. Cytomegalovirus anterior uveitis: Outcome of treatment. Br. J. Ophthalmol. 2010, 94, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.M.; Guo, Y.X.; Xiao, G.G.; Li, C.D.; Hong, J. Characteristics of Corneal Endotheliitis among Different Viruses by in Vivo Confocal Microscopy. Ocul. Immunol. Inflamm. 2021, 17, 324–332. [Google Scholar] [CrossRef] [PubMed]
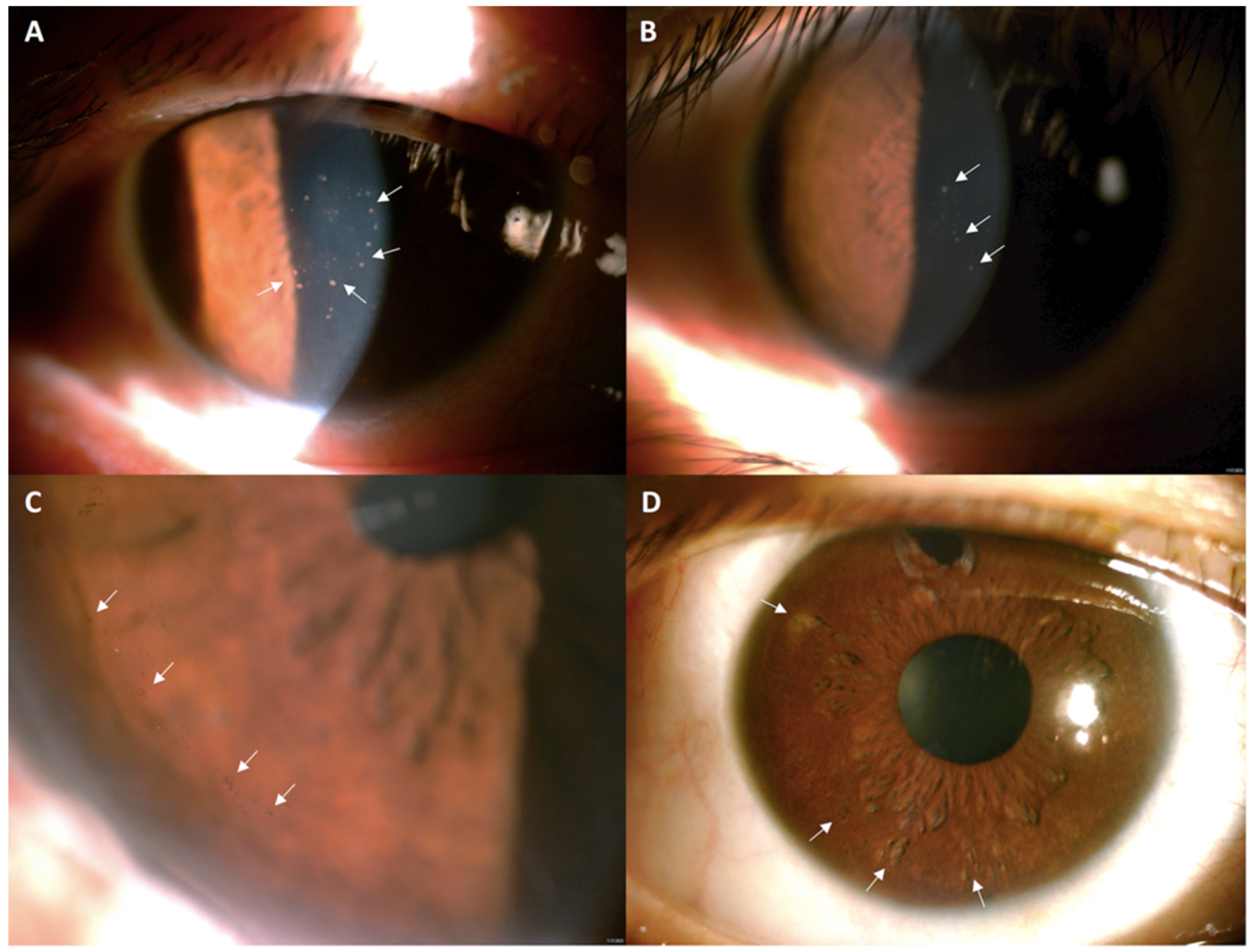
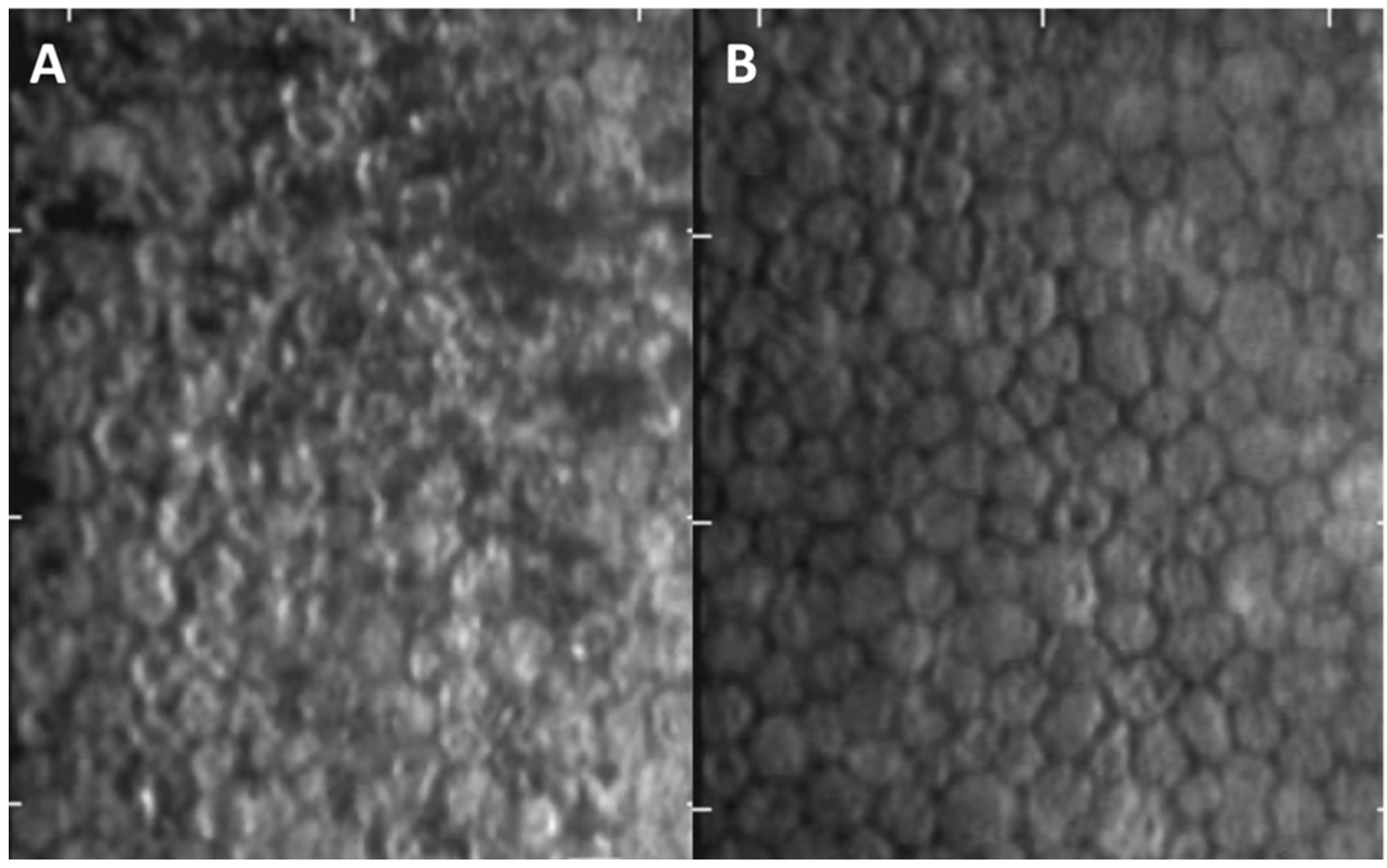
| Parameter | Pre-Treatment (n = 15) | Post-Treatment (n = 15) | p-Value |
|---|---|---|---|
| IOP (mmHg, median (IQR)) | 42.00 (28.00–48.00) | 12.00 (10.00–16.00) | <0.001 * |
| BCVA (LogMAR, median (IQR)) | 0.52 (0.30–0.52) | 0.22 (0.00–0.52) | 0.001 * |
| Corneal edema grading | 0.024 * | ||
| 0 | 6 | 13 | |
| 1 | 8 | 2 | |
| 2 | 1 | 0 | |
| KP | 15 | 6 | <0.001 * |
| Iritis 0 Trace 1+ ≥2+ | 6 3 5 1 | 11 3 1 0 | 0.006 * |
| ECD (mm2, median (IQR)) | 1976 (1647–2331) | 1900 (1600–2500) | 0.302 |
| CV (%, median (IQR)) | 49 (42–54) | 45 (41–67) | 0.712 |
| HEX (%, median (IQR)) | 33 (27–49) | 38 (34–50) | 0.035 * |
| CCT (mm, median (IQR)) | 597 (571–629) | 597 (563–632) | 0.607 |
| Number of anti-glaucoma drug 0 1 2 3 4 | 2 2 5 4 2 | 5 7 3 0 0 | 0.039 * |
| Clinical Manifestation | Steroid Group (n = 7) | Non-Steroid Group (n = 8) | p-Value |
|---|---|---|---|
| IOP (mmHg, median (IQR)) | 29.00 (25.00–51.00) | 44.50 (33.00–48.00) | 0.245 |
| BCVA (LogMAR, median (IQR)) | 0.52 (0.30–0.52) | 0.46 (0.13–0.88) | 0.632 |
| Corneal edema grading 0 1 | 3 4 | 3 4 | 0.626 |
| 2 | 0 | 1 | |
| KP | 7 | 8 | 1.000 |
| Iritis 0 Trace 1+ | 1 2 3 | 5 1 2 | 0.246 |
| 3+ | 1 | 0 | |
| ECD (mm2, median (IQR)) | 1969 (1621–2233) | 1976 (1647–2558) | 0.685 |
| CV (%, median (IQR)) | 47 (42–59) | 49 (41–54) | 0.870 |
| HEX (%, median (IQR)) | 34 (29–41) | 33 (27–50) | 0.871 |
| CCT (mm, median (IQR)) | 615 (597–629) | 591 (569–622) | 0.364 |
| Number of anti-glaucoma drug 0 1 2 | 1 1 3 | 1 1 2 | 0.710 |
| 3 | 2 | 2 | |
| 4 | 0 | 2 |
| Outcome | Steroid Group (n = 7) | Non-Steroid Group (n = 8) | p-Value |
|---|---|---|---|
| IOP (mmHg, median (IQR)) | 12.00 (10.00–17.00) | 12.00 (9.00–15.75) | 0.613 |
| BCVA (LogMAR, median (IQR)) | 0.15 (0.00–0.52) | 0.26 (0.02–0.62) | 0.867 |
| Corneal edema grading 0 1 | 7 0 | 6 2 | 0.467 |
| KP | 3 | 3 | 0.622 |
| Iritis 0 Trace 1+ | 3 3 1 | 8 0 0 | 0.044 * |
| ECD (mm2, median (IQR)) | 1900 (1669–2747) | 1658 (1540–2464) | 0.281 |
| CV (%, median (IQR)) | 54 (45–58) | 43 (41–69) | 0.536 |
| HEX (%, median (IQR)) | 38 (37–60) | 39 (34–45) | 0.336 |
| CCT (mm, median (IQR)) | 610 (535–642) | 586 (566–599) | 0.397 |
| Number of anti-glaucoma drug 0 1 2 | 3 1 3 | 2 6 0 | 0.034 * |
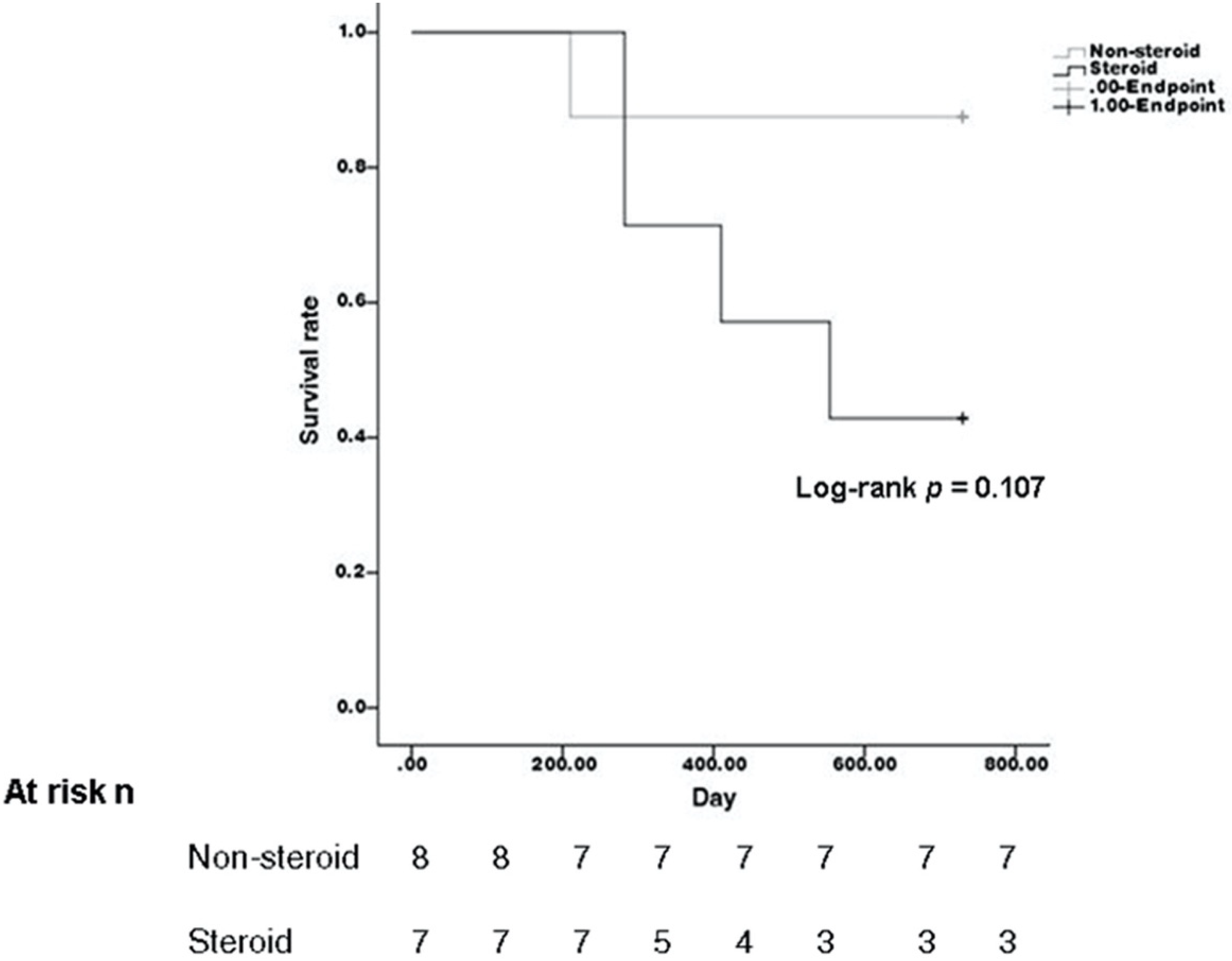
| Recurrence | 4 | 1 | 0.100 |
| Duration of GCV use (month, median (IQR)) | 24.5 (13.5–40) | 11 (9–13.5) | 0.002 * |
| Duration of steroid use (month, median (IQR)) | 19.5 (13.5–30) | 0.5 (0–1) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-W.; Chang, E.-C.; Lee, C.-Y.; Lee, S.-H.; Liang, I.-C.; Chen, Y.-C.; Hou, Y.-C. Effectiveness of Topical Ganciclovir 2% Monotherapy Versus Combined Steroid Therapy in Cytomegalovirus Endotheliitis. J. Clin. Med. 2022, 11, 5811. https://doi.org/10.3390/jcm11195811
Kuo Y-W, Chang E-C, Lee C-Y, Lee S-H, Liang I-C, Chen Y-C, Hou Y-C. Effectiveness of Topical Ganciclovir 2% Monotherapy Versus Combined Steroid Therapy in Cytomegalovirus Endotheliitis. Journal of Clinical Medicine. 2022; 11(19):5811. https://doi.org/10.3390/jcm11195811
Chicago/Turabian StyleKuo, Yu-Wei, En-Che Chang, Chia-Yi Lee, Shwu-Huey Lee, I-Chia Liang, Yi-Chun Chen, and Yu-Chih Hou. 2022. "Effectiveness of Topical Ganciclovir 2% Monotherapy Versus Combined Steroid Therapy in Cytomegalovirus Endotheliitis" Journal of Clinical Medicine 11, no. 19: 5811. https://doi.org/10.3390/jcm11195811
APA StyleKuo, Y.-W., Chang, E.-C., Lee, C.-Y., Lee, S.-H., Liang, I.-C., Chen, Y.-C., & Hou, Y.-C. (2022). Effectiveness of Topical Ganciclovir 2% Monotherapy Versus Combined Steroid Therapy in Cytomegalovirus Endotheliitis. Journal of Clinical Medicine, 11(19), 5811. https://doi.org/10.3390/jcm11195811






