Clinical Criteria for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome: An Exploratory Analysis of Optimal Cut-Off Values for Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Study Samples
- Patients treated in the ICU, including postoperative patients
- No long-term care insurance or home health care before admission.
- Hospital stays >14 days.
- BI recorded on the day of hospital discharge.
2.3. Measurements
2.4. Outcome Measurements
3. Statistical Analysis
3.1. Derivation Cohort
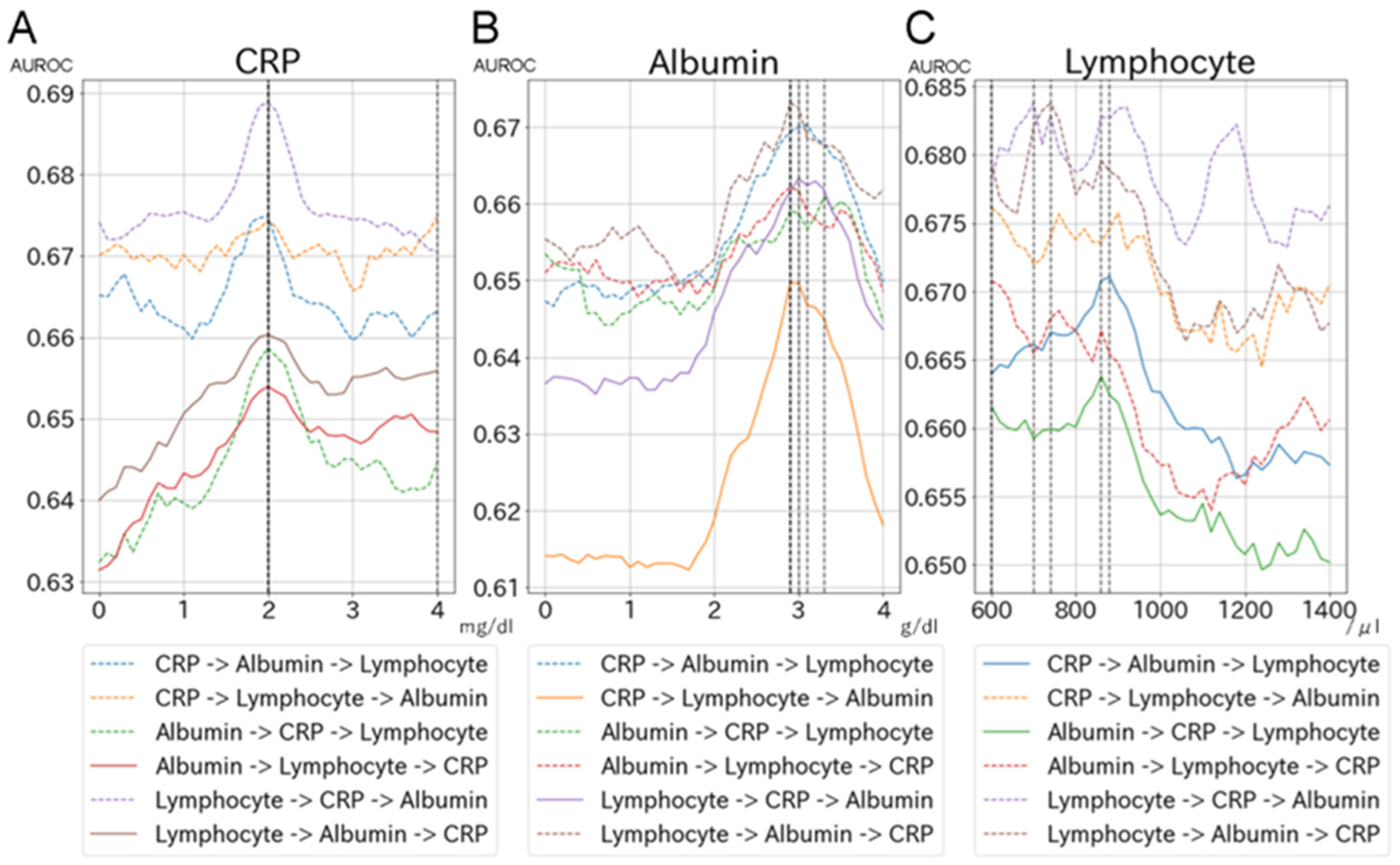
3.2. Evaluation of Optimal Cut-Off Values for CRP, Albumin, and Lymphocyte Count Using Machine-Learning Approaches
- We initially selected the search range and intervals for each biomarker based on knowledge: CRP levels from a range of 0 to 4.0 mg/dL with an interval of 0.1 mg/dL, albumin levels from a range of 0 to 4.0 g/dL with an interval of 0.1 g/dL, and lymphocyte counts from 600 to 1400/μL with an interval of 20.
- To search for the optimal cut-off values for biomarkers, we developed a machine-learning model by sequentially adding biomarkers to the model evaluated by area under the receiver operating characteristic (AUROC), which was calculated by five-fold cross-validation. We initially developed a CRP-only model and searched for the best AUROC by dichotomizing CRP from 0 to 4.0 mg/dL with an interval of 0.1 mg/dL. Once the best AUROC was identified, the corresponding CRP level was selected as the optimal CRP level. Using the optimal CRP level as a fixed value, we added albumin to the CRP-only model and searched for the best AUROC by dichotomizing albumin levels from 0 to 4.0 g/dL with an interval of 0.1 g/dL; the optimal albumin level was selected based on AUROC. Using the fixed optimal CRP and albumin levels, we then added lymphocyte count to the model and repeated the search process.
- We repeated step #2 for each machine-learning model by changing the order in which we added biomarkers to the model (i.e., six patterns for each machine-learning model based on combinations of the orders of CRP, albumin, and lymphocyte count). Therefore, we developed 36 models to search for the optimal cut-off values for biomarkers.
3.3. Development of Criteria and Performance Evaluation
3.4. External Validation
3.5. Sensitivity Analysis
4. Results
4.1. Study Flow and Patient Characteristics of the Derivation Cohort
4.2. Evaluation of Optimal Cut-Off Values and Development of Clinical Criteria
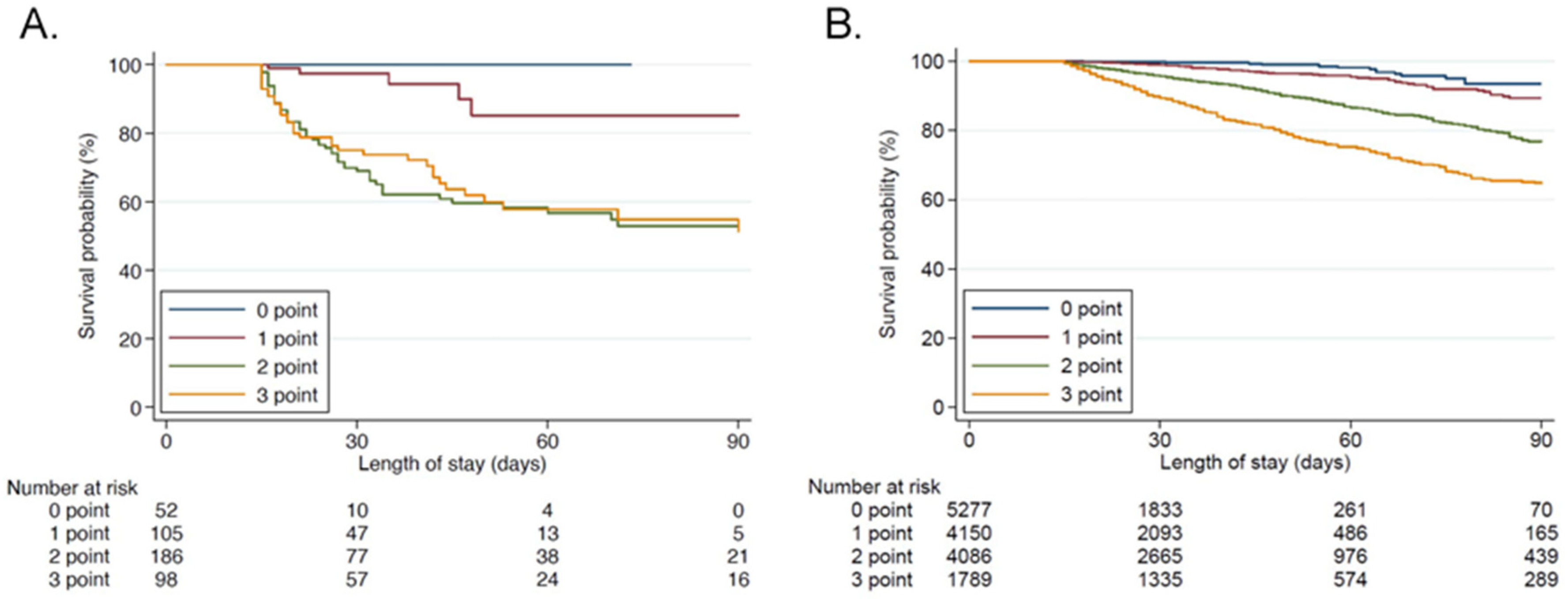
4.3. Criteria Validation
4.4. Sensitivity Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ confer-ence. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.M.; Le, T.; Angus, D.C.; Cox, C.E.; Hough, C.L.; White, D.B.; Yende, S.; Carson, S.S.; Investigators, P.S.G. The epidemiology of chron-ic critical illness in the United States*. Crit. Care Med. 2015, 43, 282–287. [Google Scholar] [CrossRef]
- Mira, J.C.; Cuschieri, J.; Ozrazgat-Baslanti, T.; Wang, Z.; Ghita, G.L.; Loftus, T.J.; Stortz, J.A.; Raymond, S.L.; Lanz, J.D.; Hennessy, L.V.; et al. The Epidemiology of Chronic Critical Illness After Severe Traumatic Injury at Two Level–One Trauma Centers*. Crit. Care Med. 2017, 45, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Ohbe, H.; Matsui, H.; Fushimi, K.; Yasunaga, H. Epidemiology of Chronic Critical Illness in Japan: A Nationwide Inpatient Da-tabase Study. Crit. Care Med. 2021, 49, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: What we do and do not know about cytokine regulation. Crit. Care Med. 1996, 24, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Casserly, B.; Ayala, A. The Compensatory Anti-inflammatory Response Syndrome (CARS) in Critically Ill Patients. Clin. Chest Med. 2008, 29, 617–625. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Rosenthal, M.; A Moore, F. Persistent inflammatory, immunosuppressed, catabolic syndrome (PICS): A new phenotype of multiple organ failure. J. Adv. Nutr. Hum. Metab. 2015, 1, e784. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Horiguchi, H.; Loftus, T.J.; Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Hollen, M.K.; Weiss, B.P.; Miller, E.S.; Bihorac, A.; Larson, S.D.; et al. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Ther-apy. Front. Immunol. 2018, 9, 595. [Google Scholar] [PubMed]
- Nakamura, K.; Ogura, K.; Nakano, H.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. C-reactive protein cluster-ing to clarify persistent inflammation, immunosuppression and catabolism syndrome. Intensive Care Med. 2020, 46, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, B.; Ye, B.; Ke, L.; Chen, F.; Lu, G.; Jiang, F.; Tong, Z.; Li, J.; Li, W. The long-term quality of life in patients with persistent in-flammation-immunosuppression and catabolism syndrome after severe acute pancreatitis: A retrospective cohort study. J. Crit. Care 2017, 42, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Uyttenboogaart, M.; Stewart, R.E.; Vroomen, P.C.A.J.; De Keyser, J.; Luijckx, G.-J. Optimizing Cutoff Scores for the Barthel Index and the Modified Rankin Scale for Defining Outcome in Acute Stroke Trials. Stroke 2005, 36, 1984–1987. [Google Scholar] [CrossRef]
- Liu, F.; Tsang, R.C.; Zhou, J.; Zhou, M.; Zha, F.; Long, J.; Wang, Y. Relationship of Barthel Index and its Short Form with the Modi-fied Rankin Scale in acute stroke patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 105033. [Google Scholar] [CrossRef]
- Scheffenbichler, F.T.; Teja, B.; Wongtangman, K.; Mazwi, N.; Waak, K.; Schaller, S.J.; Xu, X.; Barbieri, S.; Fagoni, N.; Cassavaugh, J.; et al. Effects of the Level and Duration of Mobilization Therapy in the Surgical ICU on the Loss of the Ability to Live Independently: An International Prospective Cohort Study. Crit. Care Med. 2021, 49, e247–e257. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Chen, T.; Carlos, G. XGBoost: A Scalable Tree Boosting System. In Proceedings of the KDD ’16: The 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Moore, F.A. Persistent Inflammation, Immunosuppression, and Catabolism: Evolution of Multiple Organ Dysfunction. Surg. Infect. 2016, 17, 167–172. [Google Scholar] [CrossRef]
- Efron, P.A.; Mohr, A.M.; Bihorac, A.; Horiguchi, H.; Hollen, M.K.; Segal, M.S.; Baker, H.V.; Leeuwenburgh, C.; Moldawer, L.L.; Moore, F.A.; et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after sur-gery. Surgery 2018, 164, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakano, H.; Naraba, H.; Mochizuki, M.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. High protein versus medium protein delivery under equal total energy delivery in critical care: A randomized controlled trial. Clin. Nutr. 2020, 40, 796–803. [Google Scholar] [CrossRef]
- Wiedermann, C. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Cull, W.; Henderson, W.; Daley, J.; Hur, K.; Khuri, S.F. Preoperative serum albumin level as a predictor of operative mortality and morbidity: Results from the National VA Surgical Risk Study. Arch. Surg. 1999, 134, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Cilloniz, C.; Peroni, H.J.; Gabarrús, A.; García-Vidal, C.; Pericàs, J.M.; Bermejo-Martin, J.; Torres, A. Lymphopenia Is Associated With Poor Outcomes of Patients With Community-Acquired Pneumonia and Sepsis. Open Forum Infect. Dis. 2021, 8, ofab169. [Google Scholar] [CrossRef] [PubMed]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.; Compton, S.M.; Hotchkiss, R.S. Persistent Lymphopenia After Diagnosis of Sepsis Predicts Mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef]
- Pourhassan, M.; Cederholm, T.; Trampisch, U.; Volkert, D.; Wirth, R. Inflammation as a diagnostic criterion in the GLIM defini-tion of malnutrition-what CRP-threshold relates to reduced food intake in older patients with acute disease? Eur. J. Clin. Nutr. 2021, 76, 397–400. [Google Scholar] [CrossRef]
- Bona, D.; Micheletto, G.; Bonitta, G.; Panizzo, V.; Cavalli, M.; Rausa, E.; Cirri, S.; Aiolfi, A. Does C-reactive Protein Have a Predictive Role in the Early Diagnosis of Postoperative Complications After Bariatric Surgery? Systematic Review and Bayesian Me-ta-analysis. Obes. Surg. 2019, 29, 3448–3456. [Google Scholar] [CrossRef]
- Iwashita, Y.; Yamashita, K.; Ikai, H.; Sanui, M.; Imanaka, Y. Epidemiology of mechanically ventilated patients treated in ICU and non-ICU settings in Japan: A retrospective database study. Crit. Care 2018, 22, 329. [Google Scholar] [CrossRef]


| Overall | Non-PICS (Barthel Index ≥ 70 at Discharge) | PICS (Barthel Index < 70 or In-Hospital Death) | ||
|---|---|---|---|---|
| Variables | n = 441 | n = 150 | n = 291 | p Value |
| Age | 71.1 ± 15.5 | 64.4 ± 16.6 | 74.6 ± 13.7 | <0.001 |
| Age ≥ 75, n (%) | 235 (53.4) | 52 (34.7) | 183 (63.1) | <0.001 |
| Male, n (%) | 282 (64.0) | 103 (68.7) | 179 (61.5) | 0.14 |
| SOFA score on admission | 6 (3, 9) | 5 (2, 8) | 6 (4, 9) | 0.008 |
| APACHEII score on admission | 17 (12, 22) | 14 (11, 21) | 18 (12, 23) | 0.002 |
| Mechanical ventilation, n (%) | 206 (46.7) | 57 (38.0) | 149 (51.2) | 0.008 |
| Duration (days) | 9 (4, 18) | 6 (3, 10.5) | 10 (5, 25.5) | <0.001 |
| Blood purification, n (%) | 98 (22.2) | 19 (12.7) | 79 (27.2) | <0.001 |
| Duration (days) | 5 (2, 15) | 3 (2, 8) | 5 (3, 15) | 0.31 |
| Extracorporeal membrane oxygenation | 10 (2.3) | 5 (3.3) | 5 (1.7) | 0.29 |
| Duration (days) | 4.5 (2.8, 15) | 4 (1.5, 5.5) | 14 (3.5, 18.5) | 0.17 |
| Basic diseases on admission | ||||
| Sepsis n (%) | 151 (34.2) | 45 (30.0) | 106 (36.5) | 0.18 |
| Cardiac failure, n (%) | 67 (15.2) | 26 (17.3) | 41 (14.1) | 0.37 |
| Renal failure, n (%) | 73 (16.6) | 20 (13.3) | 53 (18.2) | 0.19 |
| Respiratory failure, n (%) | 58 (13.2) | 16 (10.7) | 42 (14.4) | 0.26 |
| Stroke, n (%) | 42 (9.5) | 5 (3.3) | 37 (12.7) | 0.0015 |
| Endocrine and metabolic disorder, n (%) | 69 (15.6) | 25 (16.7) | 44 (15.1) | 0.67 |
| Trauma, n (%) | 67 (15.2) | 25 (16.7) | 42 (14.4) | 0.54 |
| Post-scheduled operation, n (%) | 24 (5.4) | 8 (5.3) | 16 (5.5) | 0.94 |
| Mortality, n (%) | 100 (22.7) | 0 (0) | 100 (34.4) | <0.001 |
| Day on which patients died, days | 21 (17, 33.8) | 21 (17, 33.8) | ||
| Length of ICU stay, days | 9 (5, 14) | 8 (5, 11) | 10 (6, 16) | 0.001 |
| Length of hospital stay, days | 26 (18, 45.5) | 26 (17, 43.5) | 26 (18, 47) | 0.97 |
| Barthel index at hospital discharge | 55 (10, 100) | 100 (90, 100) | 15 (0, 40) | <0.001 |
| Laboratory findings on day 1 | ||||
| CRP (mg/dL) | 2.9 (0.4, 12) | 2.0 (0.2, 16.6) | 3.7 (0.6, 10.5) | 0.73 |
| Albumin (g/dL) | 3.2 ± 0.8 | 3.3 ± 0.9 | 3.1 ± 0.8 | 0.015 |
| Lymphocytes (/μL) | 1071 (574, 1852) | 1092 (614, 2102) | 1056 (544, 1764) | 0.23 |
| Laboratory findings on day 14 | ||||
| CRP (mg/dL) | 3.3 (1.1, 7.3) | 1.8 (0.6, 5.7) | 4.0 (1.5, 7.8) | <0.001 |
| Albumin (g/dL) | 2.5 ± 0.6 | 2.7 ± 0.6 | 2.4 ± 0.5 | <0.001 |
| Lymphocytes (/μL) | 1080 (729, 1441) | 1180 (908, 1547) | 1008 (660, 1330) | <0.001 |
| Derivation Cohort | Validation Cohort | |||||
|---|---|---|---|---|---|---|
| AUROC | Sensitivity | Specificity | AUROC | Sensitivity | Specificity | |
| Discrimination ability | 0.67 | - | - | 0.71 | - | - |
| Sum of points in criteria | ||||||
| 1 | - | 0.94 | 0.23 | - | 0.85 | 0.43 |
| 2 | - | 0.74 | 0.54 | - | 0.62 | 0.71 |
| 3 | - | 0.27 | 0.88 | - | 0.24 | 0.93 |
| Sum of Points in PICS Criteria (One Point Is Given When Any of the Following Items Are Positive: CRP > 2.0 mg/dL, Albumin < 3.0 g/dL, or a Lymphocyte Count < 800/μL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | ≥1 | <2 | ≥2 | <3 | 3 | ||||
| Variables | n = 5277 | n = 10,025 | p Value | n = 9427 | n = 5875 | p Value | n = 13,513 | n = 1789 | p Value |
| Age | 68.0 (13.4) | 72.0 (12.4) | <0.001 | 69.3 (13.2) | 72.8 (12.1) | <0.001 | 70.2 (12.9) | 74.0 (11.7) | <0.001 |
| Age ≥ 75, n (%) | 1803 (34.2) | 4817 (48.0) | <0.001 | 3659 (38.8) | 2961 (50.4) | <0.001 | 5645 (41.8) | 975 (54.5) | <0.001 |
| Male, n (%) | 3235 (61.3) | 6416 (64.0) | 0.001 | 5796 (61.5) | 3855 (65.6) | <0.001 | 8511 (63.0) | 1140 (63.7) | 0.54 |
| SOFA on admission | 3 (1–4) | 4 (1–6) | <0.001 | 3 (1–5) | 4 (2–7) | <0.001 | 3 (1–5) | 5 (2–7) | <0.001 |
| Mechanical ventilation, n (%) | 1086 (20.6) | 4169 (41.6) | <0.001 | 2414 (25.6) | 2841 (48.4) | <0.001 | 4248 (31.4) | 1007 (56.3) | <0.001 |
| Duration (days) | 1 (1–4) | 4 (1–15) | <0.001 | 2 (1–5) | 7 (2–21) | <0.001 | 3 (1–8) | 12 (3–28) | <0.001 |
| Blood purification, n (%) | 154 (2.9) | 1470 (14.7) | <0.001 | 498 (5.3) | 1126 (19.2) | <0.001 | 1138 (8.4) | 486 (27.2) | <0.001 |
| Duration (days) | 7 (3–19) | 9 (4–22) | 0.012 | 7 (3–22) | 10 (4–21) | 0.007 | 8 (4–21) | 11 (5–21) | 0.003 |
| Extracorporeal membrane oxygenation | 9 (0.2) | 127 (1.3) | <0.001 | 25 (0.3) | 111 (1.9) | <0.001 | 89 (0.7) | 47 (2.6) | <0.001 |
| Duration (days) | 1 (1–1) | 1 (1–4) | 0.090 | 1 (1–1) | 1 (1–4) | 0.047 | 1 (1–2) | 1 (1–7) | 0.003 |
| Basic diseases on admission | |||||||||
| Sepsis n (%) | 221 (4.2) | 1312 (13.1) | <0.001 | 585 (6.2) | 948 (16.1) | <0.001 | 1206 (8.9) | 327 (18.3) | <0.001 |
| Cardiac failure, n (%) | 1476 (28.0) | 2082 (20.8) | <0.001 | 2445 (25.9) | 1113 (18.9) | <0.001 | 3257 (24.1) | 301 (16.8) | <0.001 |
| Renal failure, n (%) | 31 (0.6) | 281 (2.8) | <0.001 | 125 (1.3) | 187 (3.2) | <0.001 | 240 (1.8) | 72 (4.0) | <0.001 |
| Respiratory failure, n (%) | 138 (2.6) | 800 (8.0) | <0.001 | 364 (3.9) | 574 (9.8) | <0.001 | 727 (5.4) | 211 (11.8) | <0.001 |
| Stroke, n (%) | 392 (7.4) | 488 (4.9) | <0.001 | 607 (6.4) | 273 (4.6) | <0.001 | 806 (6.0) | 74 (4.1) | 0.002 |
| Endocrine and metabolic disorder, n (%) | 70 (1.3) | 137 (1.4) | 0.84 | 142 (1.5) | 65 (1.1) | 0.037 | 189 (1.4) | 18 (1.0) | 0.18 |
| Trauma, n (%) | 256 (4.9) | 540 (5.4) | 0.16 | 446 (4.7) | 350 (6.0) | <0.001 | 696 (5.2) | 100 (5.6) | 0.43 |
| Post-scheduled operation, n (%) | 2871 (54.4) | 4078 (40.7) | <0.001 | 4867 (51.6) | 2082 (35.4) | <0.001 | 6369 (47.1) | 580 (32.4) | <0.001 |
| Mortality, n (%) | 27 (0.5) | 1034 (10.3) | <0.001 | 143 (1.5) | 918 (15.6) | <0.001 | 603 (4.5) | 458 (25.6) | <0.001 |
| Day on which patients died, days | 5 (1–13) | 13 (5–14) | 0.006 | 9 (3–14) | 13 (5–14) | 0.009 | 11 (4–14) | 14 (5–14) | 0.027 |
| Length of ICU stay, days | 2 (1–4) | 3 (1–8) | <0.001 | 2 (1–4) | 4 (2–12) | <0.001 | 2 (1–5) | 6 (2–14) | <0.001 |
| Length of hospital stay, days | 25 (19–34) | 34 (24–53) | <0.001 | 26 (20–37) | 39 (26–61) | <0.001 | 29 (21–43) | 43 (29–68) | <0.001 |
| Barthel index at hospital discharge | 100 (100–100) | 100 (55–100) | <0.001 | 100 (95–100) | 100 (30–100) | <0.001 | 100 (85–100) | 85 (15–100) | <0.001 |
| Laboratory findings on day 1 | |||||||||
| CRP (mg/dL) | 3.9 (1.6–7.1) | 6.4 (3.3–11.0) | <0.001 | 4.7 (2.1–8.0) | 7.0 (3.6–12.8) | <0.001 | 5.2 (2.5–8.9) | 7.8 (4.0–14.6) | <0.001 |
| Albumin (g/dL) | 3.1 (0.4) | 2.8 (0.6) | <0.001 | 3.0 (0.5) | 2.7 (0.6) | <0.001 | 2.9 (0.5) | 2.6 (0.6) | <0.001 |
| Lymphocytes (/μL) | 1069 (772–1429) | 801 (536–1162) | <0.001 | 993 (700–1356) | 736 (488–1082) | <0.001 | 945 (648–1307) | 583 (383–833) | <0.001 |
| Laboratory findings on day 14 | |||||||||
| CRP (mg/dL) | 0.5 (0.2–1.0) | 3.5 (1.7–7.1) | <0.001 | 0.8 (0.3–1.7) | 5.4 (3.1–9.5) | <0.001 | 1.4 (0.5–3.7) | 7.1 (4.3–12.3) | <0.001 |
| Albumin (g/dL) | 3.5 (0.3) | 2.7 (0.5) | <0.001 | 3.3 (0.5) | 2.4 (0.5) | <0.001 | 3.0 (0.6) | 2.2 (0.4) | <0.001 |
| Lymphocytes (/μL) | 1490 (1190–1884) | 1043 (739–1418) | <0.001 | 1385 (1088–1785) | 856 (624–1225) | <0.001 | 1287 (994–1682) | 578 (429–700) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Ogura, K.; Ohbe, H.; Goto, T. Clinical Criteria for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome: An Exploratory Analysis of Optimal Cut-Off Values for Biomarkers. J. Clin. Med. 2022, 11, 5790. https://doi.org/10.3390/jcm11195790
Nakamura K, Ogura K, Ohbe H, Goto T. Clinical Criteria for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome: An Exploratory Analysis of Optimal Cut-Off Values for Biomarkers. Journal of Clinical Medicine. 2022; 11(19):5790. https://doi.org/10.3390/jcm11195790
Chicago/Turabian StyleNakamura, Kensuke, Kentaro Ogura, Hiroyuki Ohbe, and Tadahiro Goto. 2022. "Clinical Criteria for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome: An Exploratory Analysis of Optimal Cut-Off Values for Biomarkers" Journal of Clinical Medicine 11, no. 19: 5790. https://doi.org/10.3390/jcm11195790
APA StyleNakamura, K., Ogura, K., Ohbe, H., & Goto, T. (2022). Clinical Criteria for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome: An Exploratory Analysis of Optimal Cut-Off Values for Biomarkers. Journal of Clinical Medicine, 11(19), 5790. https://doi.org/10.3390/jcm11195790







