Comparative Study of Helicobacter pylori-Infected Gastritis in Okinawa and Tokyo Based on the Kyoto Classification of Gastritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Patients Studied
3.2. Endoscopic Findings of H. pylori Gastritis Based on the Kyoto Classification of Gastritis
3.3. Age Analysis
3.4. Age-Adjusted Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar]
- NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA 1994, 272, 65–69. [CrossRef]
- Marshall, B.J.; Goodwin, C.S.; Warren, J.R.; Murray, R.; Blincow, E.D.; Blackbourn, S.J.; Phillips, M.; Waters, T.E.; Sanderson, C.R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet 1988, 2, 1437–1442. [Google Scholar] [CrossRef]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Azuma, T.; Murakita, H.; Hirai, M.; Miyaji, H.; Ito, Y.; Ohtaki, Y.; Yamazaki, Y.; Kuriyama, M.; Keida, Y.; et al. Profile of Helicobacter pylori cytotoxin derived from two areas of Japan with different prevalence of atrophic gastritis. Gut 1996, 39, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Joo, Y.M.; Jang, S.; Yoo, Y.J.; Lee, H.S.; Chung, I.S.; Olsen, C.H.; Whitmire, J.M.; Merrell, D.S.; Cha, J.H. Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. J. Clin. Microbiol. 2009, 47, 959–968. [Google Scholar] [CrossRef]
- Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare). 2018. Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html (accessed on 8 April 2022).
- Azuma, T.; Yamakawa, A.; Yamazaki, S.; Ohtani, M.; Ito, Y.; Muramatsu, A.; Suto, H.; Yamazaki, Y.; Keida, Y.; Higashi, H.; et al. Distinct diversity of the cag pathogenicity island among Helicobacter pylori strains in Japan. J. Clin. Microbiol. 2004, 42, 2508–2517. [Google Scholar] [CrossRef]
- Satomi, S.; Yamakawa, A.; Matsunaga, S.; Masaki, R.; Inagaki, T.; Okuda, T.; Suto, H.; Ito, Y.; Yamazaki, Y.; Kuriyama, M.; et al. Relationship between the diversity of the cagA gene of Helicobacter pylori and gastric cancer in Okinawa, Japan. J. Gastroenterol. 2006, 41, 668–673. [Google Scholar] [CrossRef]
- Yamazaki, S.; Yamakawa, A.; Okuda, T.; Ohtani, M.; Suto, H.; Ito, Y.; Yamazaki, Y.; Keida, Y.; Higashi, H.; Hatakeyama, M.; et al. Distinct diversity of vacA, cagA, and cagE genes of Helicobacter pylori associated with peptic ulcer in Japan. J. Clin. Microbiol. 2005, 43, 3906–3916. [Google Scholar] [CrossRef]
- Matsunari, O.; Shiota, S.; Suzuki, R.; Watada, M.; Kinjo, N.; Murakami, K.; Fujioka, T.; Kinjo, F.; Yamaoka, Y. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J. Clin. Microbiol. 2012, 50, 876–883. [Google Scholar] [CrossRef][Green Version]
- Ekström, A.M.; Held, M.; Hansson, L.E.; Engstrand, L.; Nyrén, O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology 2001, 121, 784–791. [Google Scholar] [CrossRef]
- Kato, M.; Inoue, K.; Murakami, K.; Kamada, T.; Haruma, K. Kyoto Classification of Gastritis; Nihon Medical Center: Singapore, 2014. (In Japanese) [Google Scholar]
- Kato, M.; Inoue, K.; Murakami, K.; Kamada, T.; Haruma, K. Kyoto Classification of Gastritis; Nihon Medical Center: Singapore, 2017. (In English) [Google Scholar]
- Kato, M.; Inoue, K.; Murakami, K.; Kamada, T.; Haruma, K. Kyoto Classification of Gastritis, 2nd ed.; Nihon Medical Center: Singapore, 2018. (In Japanese) [Google Scholar]
- Ebigbo, A.; Marienhagen, J.; Messmann, H. Regular arrangement of collecting venules and the Kimura-Takemoto classification for the endoscopic diagnosis of Helicobacter pylori infection: Evaluation in a Western setting. Dig. Endosc. 2021, 33, 587–591. [Google Scholar] [CrossRef]
- Glover, B.; Teare, J.; Ashrafian, H.; Patel, N. The endoscopic predictors of Helicobacter pylori status: A meta-analysis of diagnostic performance. Ther. Adv. Gastrointest. Endosc. 2020, 13, 2631774520950840. [Google Scholar] [CrossRef]
- Nomura, S.; Terao, S.; Adachi, K.; Kato, T.; Ida, K.; Watanabe, H.; Shimbo, T. Endoscopic diagnosis of gastric mucosal activity and inflammation. Dig. Endosc. 2013, 25, 136–146. [Google Scholar] [CrossRef]
- Kato, T.; Yagi, N.; Kamada, T.; Shimbo, T.; Watanabe, H.; Ida, K. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: A multicenter prospective study. Dig. Endosc. 2013, 25, 508–518. [Google Scholar] [CrossRef]
- Kato, M.; Terao, S.; Adachi, K.; Nakajima, S.; Ando, T.; Yoshida, N.; Uedo, N.; Murakami, K.; Ohara, S.; Ito, M.; et al. Changes in endoscopic findings of gastritis after cure of H. pylori infection: Multicenter prospective trial. Dig. Endosc. 2013, 25, 264–273. [Google Scholar] [CrossRef]
- Nagahara, A.; Shiotani, A.; Iijima, K.; Kamada, T.; Fujiwara, Y.; Kasugai, K.; Kato, M.; Higuchi, K. The role of advanced endoscopy in the management of inflammatory digestive diseases (upper gastrointestinal tract). Dig. Endosc. 2022, 34, 63–72. [Google Scholar] [CrossRef]
- Kimura, K.; Takemoto, T. An endoscopic recognition of atrophic border and its significance in chronic gastritis. Endocopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Ohno, A.; Miyoshi, J.; Kato, A.; Miyamoto, N.; Yatagai, T.; Hada, Y.; Kusuhara, M.; Jimbo, Y.; Ida, Y.; Tokunaga, K.; et al. Endoscopic severe mucosal atrophy indicates the presence of gastric cancer after Helicobacter pylori eradication -analysis based on the Kyoto classification. BMC Gastroenterol. 2020, 20, 232. [Google Scholar] [CrossRef]
- Sakitani, K.; Nishizawa, T.; Toyoshima, A.; Yoshida, S.; Matsuno, T.; Yamada, T.; Irokawa, M.; Takahashi, Y.; Nakai, Y.; Toyoshima, O.; et al. Kyoto classification in patients who developed multiple gastric carcinomas after Helicobacter pylori eradication. World J. Gastrointest. Endosc. 2020, 12, 276–284. [Google Scholar] [CrossRef]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Koike, K. Association between gastric cancer and the Kyoto classification of gastritis. J. Gastroenterol. Hepatol. 2017, 32, 1581–1586. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ban, H.; Ichikawa, H.; Sahara, S.; Otsuka, T.; Inatomi, O.; Bamba, S.; Furuta, T.; Andoh, A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern. Med. 2017, 56, 579–586. [Google Scholar] [CrossRef]
- Majima, A.; Dohi, O.; Takayama, S.; Hirose, R.; Inoue, K.; Yoshida, N.; Kamada, K.; Uchiyama, K.; Ishikawa, T.; Takagi, T.; et al. Linked color imaging identifies important risk factors associated with gastric cancer after successful eradication of Helicobacter pylori. Gastrointest. Endosc. 2019, 90, 763–769. [Google Scholar] [CrossRef]
- Take, S.; Mizuno, M.; Ishiki, K.; Yoshida, T.; Ohara, N.; Yokota, K.; Oguma, K.; Okada, H.; Yamamoto, K. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J. Gastroenterol. 2011, 46, 318–324. [Google Scholar] [CrossRef]
- Hatta, W.; Iijima, K.; Koike, T.; Kondo, Y.; Ara, N.; Asanuma, K.; Uno, K.; Asano, N.; Imatani, A.; Shimosegawa, T. Endoscopic findings for predicting gastric acid secretion status. Dig. Endosc. 2015, 27, 582–589. [Google Scholar]
- Haruma, K.; Kamada, T.; Kawaguchi, H.; Okamoto, S.; Yoshihara, M.; Sumii, K.; Inoue, M.; Kishimoto, S.; Kajiyama, G.; Miyoshi, A. Effect of age and Helicobacter pylori infection on gastric acid secretion. J. Gastroenterol. Hepatol. 2000, 15, 277–283. [Google Scholar] [CrossRef]
- Haruma, K.; Mihara, M.; Okamoto, E.; Kusunoki, H.; Hananoki, M.; Tanaka, S.; Yoshihara, M.; Sumii, K.; Kajiyama, G. Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus-evaluation of 24-h pH monitoring. Aliment Pharm. Ther. 1999, 13, 155–162. [Google Scholar] [CrossRef]
- Koike, T.; Ohara, S.; Sekine, H.; Iijima, K.; Kato, K.; Toyota, T.; Shimosegawa, T. Increased gastric acid secretion after Helicobacter pylori eradication may be a factor for developing reflux oesophagitis. Aliment. Pharm. Ther. 2001, 15, 813–820. [Google Scholar] [CrossRef]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Suzuki, M. The Okinawan diet: Health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J. Am. Coll. Nutr. 2009, 28, 500s–516s. [Google Scholar] [CrossRef]
- Parsonnet, J.; Friedman, G.D.; Orentreich, N.; Vogelman, H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 1997, 40, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kikuchi, S.; el-Zimaity, H.M.; Gutierrez, O.; Osato, M.S.; Graham, D.Y. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology 2002, 123, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Falush, D.; Wirth, T.; Linz, B.; Pritchard, J.K.; Stephens, M.; Kidd, M.; Blaser, M.J.; Graham, D.Y.; Vacher, S.; Perez-Perez, G.I.; et al. Traces of human migrations in Helicobacter pylori populations. Science 2003, 299, 1582–1585. [Google Scholar] [CrossRef]
- Linz, B.; Balloux, F.; Moodley, Y.; Manica, A.; Liu, H.; Roumagnac, P.; Falush, D.; Stamer, C.; Prugnolle, F.; van der Merwe, S.W.; et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007, 445, 915–918. [Google Scholar] [CrossRef]
- Moodley, Y.; Linz, B.; Yamaoka, Y.; Windsor, H.M.; Breurec, S.; Wu, J.Y.; Maady, A.; Bernhöft, S.; Thiberge, J.M.; Phuanukoonnon, S.; et al. The peopling of the Pacific from a bacterial perspective. Science 2009, 323, 527–530. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Orito, E.; Mizokami, M.; Gutierrez, O.; Saitou, N.; Kodama, T.; Osato, M.S.; Kim, J.G.; Ramirez, F.C.; Mahachai, V.; et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002, 517, 180–184. [Google Scholar] [CrossRef]
- Kersulyte, D.; Mukhopadhyay, A.K.; Velapatiño, B.; Su, W.; Pan, Z.; Garcia, C.; Hernandez, V.; Valdez, Y.; Mistry, R.S.; Gilman, R.H.; et al. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 2000, 182, 3210–3218. [Google Scholar] [CrossRef]


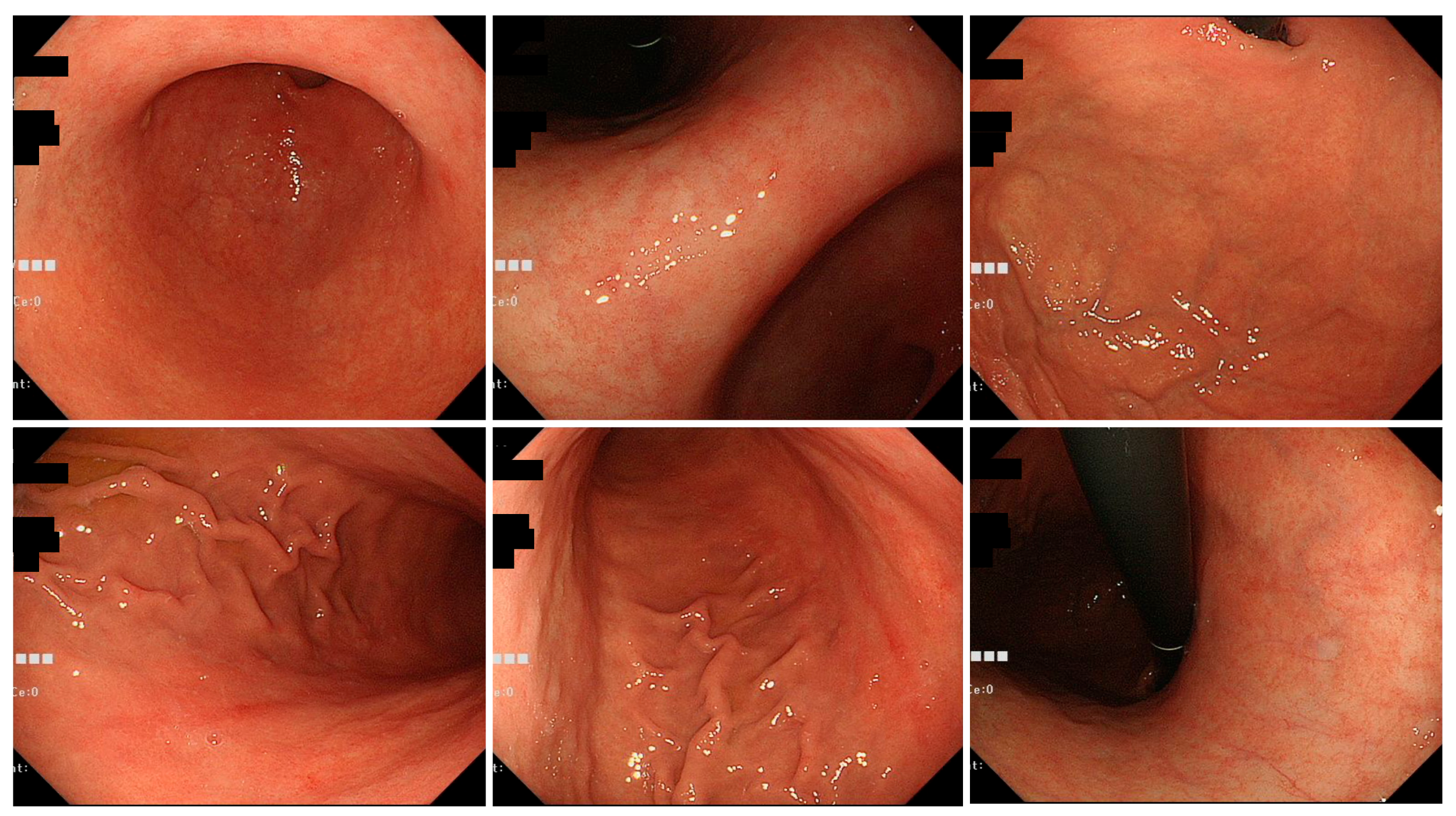
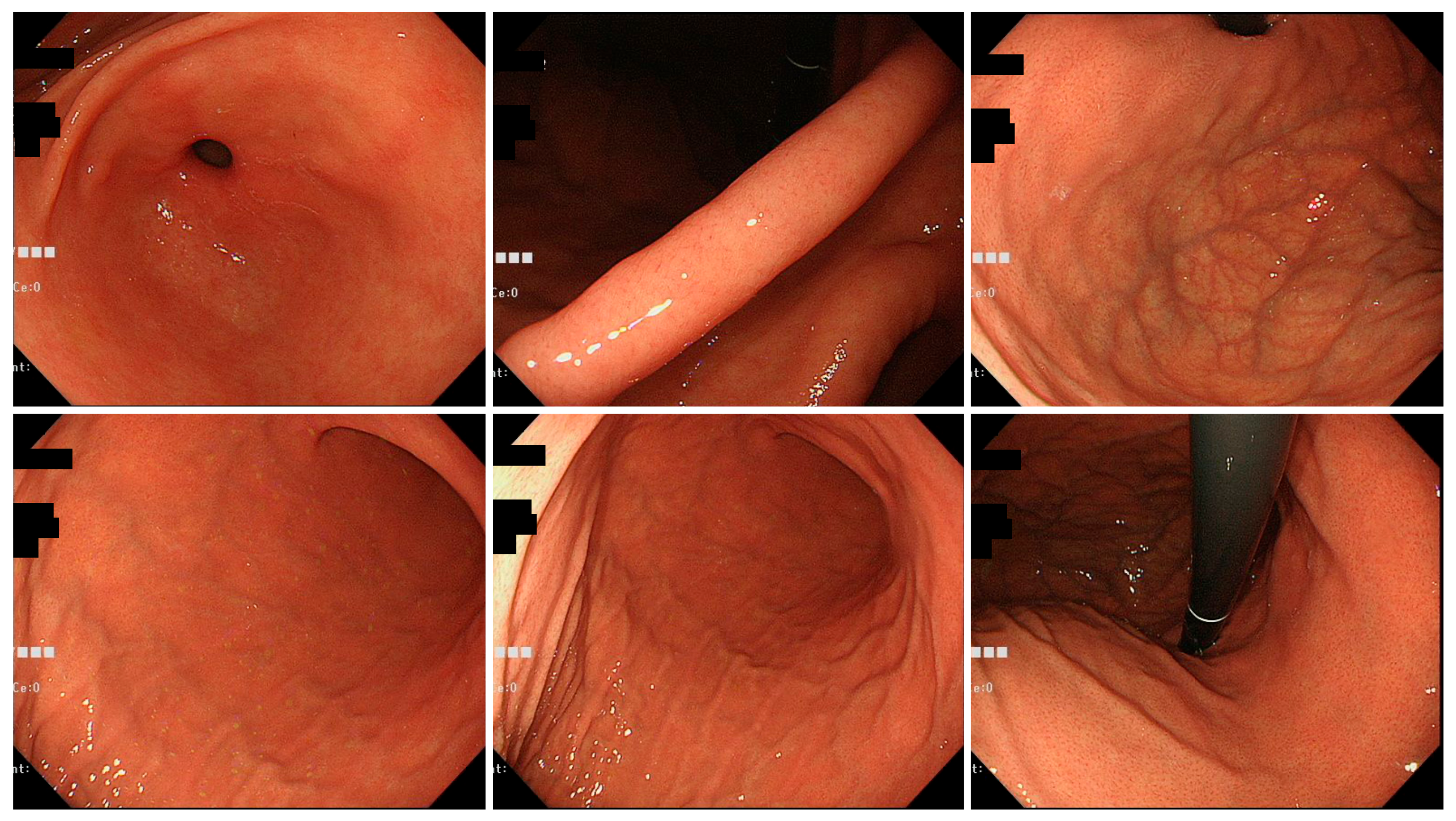
| Tokyo Group | Okinawa Group | p-Value | ||
|---|---|---|---|---|
| Number of patients | 352 | 435 | ||
| Age (yr; mean ± SD) | 64.6 ± 14.3 | 53.3 ± 14.7 | <0.001 | |
| Sex | male | 181 | 247 | 0.133 |
| female | 171 | 188 | ||
| Tokyo Group | Okinawa Group | p-Value | ||
|---|---|---|---|---|
| Group of patients under 65 years old | Number of patients | 156 | 327 | |
| Age (yr; mean ± SD) | 52.0 ± 11.3 | 46.9 ± 10.5 | <0.001 | |
| Male/female | 72/84 | 181/146 | 0.058 | |
| Group of patients 65 years old or older | Number of patients | 196 | 108 | |
| Age (yr; mean ± SD) | 74.6 ± 6.1 | 72.7 ± 5.6 | 0.006 | |
| Male/female | 109/87 | 66/42 | 0.353 | |
| Kyoto Classification of Gastritis | Tokyo Group (%) | Okinawa Group (%) | p-Value | |
|---|---|---|---|---|
| n = 352 | n = 435 | |||
| Atrophy 1 | closed | 131 (37.2) 2 | 318 (73.1) | <0.001 |
| open | 221 (62.8) | 117 (26.9) | ||
| diffuse redness | 298 (84.7) | 351 (80.7) | 0.145 | |
| mucous swelling | 165 (46.9) | 212 (48.7) | 0.696 | |
| enlarged fold | 114 (32.4) | 113 (26.0) | 0.048 | |
| spotty redness | 239 (67.9) | 338 (77.7) | 0.002 | |
| sticky mucus | 125 (35.5) | 73 (16.8) | <0.001 | |
| foveolar-hyperplastic polyp | 50 (14.2) | 39 (8.97) | 0.021 | |
| patchy redness | 29 (8.24) | 59 (13.6) | 0.018 | |
| intestinal metaplasia | 147 (41.8) | 142 (32.6) | 0.007 | |
| nodularity | 23 (6.53) | 37 (8.51) | 0.300 | |
| xanthoma | 33 (9.38) | 20 (4.60) | 0.007 | |
| hematin | 6 (1.70) | 28 (6.44) | 0.001 | |
| (a) The under-65-years age group | ||||
| Kyoto Classification of Gastritis | Tokyo Group (%) | Okinawa Group (%) | p-Value | |
| n = 156 | n = 327 | |||
| Atrophy | closed | 82 (52.6) | 270 (82.6) | <0.001 |
| open | 74 (47.4) | 57 (17.4) | ||
| diffuse redness | 132 (84.6) | 260 (79.6) | 0.180 | |
| mucosal swelling | 71 (45.5) | 149 (45.6) | 0.991 | |
| enlarged fold | 51 (32.7) | 76 (23.2) | 0.027 | |
| spotty redness | 100 (64.1) | 248 (75.8) | 0.007 | |
| sticky mucus | 58 (37.2) | 45 (13.7) | <0.001 | |
| foveolar-hyperplastic polyp | 21 (13.5) | 23 (7.03) | 0.021 | |
| patchy redness | 7 (4.48) | 39 (11.9) | 0.009 | |
| intestinal metaplasia | 48 (30.8) | 89 (27.2) | 0.340 | |
| nodularity | 19 (12.2) | 36 (11.0) | 0.705 | |
| xanthoma | 9 (5.77) | 7 (2.14) | 0.037 | |
| hematin | 2 (1.28) | 23 (7.03) | 0.008 | |
| (b) The 65-years-and-older age group | ||||
| Kyoto Classification of Gastritis | Tokyo Group (%) | Okinawa Group (%) | p-Value | |
| n = 196 | n = 108 | |||
| Atrophy | closed | 49 (25.0) | 48 (44.4) | <0.001 |
| open | 147 (75.0) | 60 (55.6) | ||
| diffuse redness | 166 (84.7) | 91 (84.3) | 0.920 | |
| mucosal swelling | 94 (48.0) | 63 (58.3) | 0.083 | |
| enlarged fold | 63 (32.1) | 37 (34.3) | 0.707 | |
| spotty redness | 139 (70.9) | 90 (83.3) | 0.016 | |
| sticky mucus | 67 (34.2) | 28 (26.0) | 0.137 | |
| foveolar-hyperplastic polyp | 29 (14.8) | 16 (14.8) | 0.966 | |
| patchy redness | 22 (11.2) | 20 (18.5) | 0.078 | |
| intestinal metaplasia | 99 (50.5) | 53 (49.1) | 0.811 | |
| nodularity | 4 (2.04) | 1 (0.93) | 0.659 | |
| xanthoma | 24 (12.2) | 13 (12.0) | 0.958 | |
| hematin | 4 (2.04) | 5 (4.63) | 0.288 | |
| Kyoto Classification of Gastritis | OR * | 95%CI | p-Value |
|---|---|---|---|
| atrophy (closed 1; open 0) | 2.843 | 2.032–3.985 | <0.001 |
| diffuse redness | 0.787 | 0.524–1.174 | 0.244 |
| mucosal swelling | 1.221 | 0.900–1.658 | 0.200 |
| enlarged fold | 0.816 | 0.583–1.141 | 0.234 |
| spotty redness | 1.947 | 1.379–2.761 | <0.001 |
| sticky mucus | 0.439 | 0.306–0.626 | <0.001 |
| foveolar hyperplastic polyp | 0.816 | 0.504–1.317 | 0.406 |
| patchy redness | 2.336 | 1.421–3.917 | 0.001 |
| intestinal metaplasia | 0.975 | 0.708–1.346 | 0.876 |
| nodularity | 0.601 | 0.318–1.142 | 0.117 |
| xanthoma | 0.731 | 0.390–1.344 | 0.319 |
| hematin | 3.526 | 1.471–9.849 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oki, S.; Takeda, T.; Hojo, M.; Uchida, R.; Suzuki, N.; Abe, D.; Ikeda, A.; Akazawa, Y.; Ueyama, H.; Nojiri, S.; et al. Comparative Study of Helicobacter pylori-Infected Gastritis in Okinawa and Tokyo Based on the Kyoto Classification of Gastritis. J. Clin. Med. 2022, 11, 5739. https://doi.org/10.3390/jcm11195739
Oki S, Takeda T, Hojo M, Uchida R, Suzuki N, Abe D, Ikeda A, Akazawa Y, Ueyama H, Nojiri S, et al. Comparative Study of Helicobacter pylori-Infected Gastritis in Okinawa and Tokyo Based on the Kyoto Classification of Gastritis. Journal of Clinical Medicine. 2022; 11(19):5739. https://doi.org/10.3390/jcm11195739
Chicago/Turabian StyleOki, Shotaro, Tsutomu Takeda, Mariko Hojo, Ryota Uchida, Nobuyuki Suzuki, Daiki Abe, Atsushi Ikeda, Yoichi Akazawa, Hiroya Ueyama, Shuko Nojiri, and et al. 2022. "Comparative Study of Helicobacter pylori-Infected Gastritis in Okinawa and Tokyo Based on the Kyoto Classification of Gastritis" Journal of Clinical Medicine 11, no. 19: 5739. https://doi.org/10.3390/jcm11195739
APA StyleOki, S., Takeda, T., Hojo, M., Uchida, R., Suzuki, N., Abe, D., Ikeda, A., Akazawa, Y., Ueyama, H., Nojiri, S., Hoshino, S., Shokita, H., & Nagahara, A. (2022). Comparative Study of Helicobacter pylori-Infected Gastritis in Okinawa and Tokyo Based on the Kyoto Classification of Gastritis. Journal of Clinical Medicine, 11(19), 5739. https://doi.org/10.3390/jcm11195739







