Opportunities and Limits of Conventional IVF versus ICSI: It Is Time to Come off the Fence
Abstract
1. Introduction
2. Conventional IVF: Technical Details
2.1. Timing of Insemination from Oocyte Retrieval
2.2. Timing of Sperm-Cumulus Co-Incubation
2.3. Oxygen Tension
3. Conventional IVF, ART Indications and Clinical Situations
3.1. Should We Use Conventional IVF for an Indication of Advanced Maternal Age?
3.2. Should We Use Conventional IVF for an Indication of Decreased Ovarian Reserve?
3.3. Should We Use Conventional IVF for an Indication of Endometriosis?
3.4. Should We Use Conventional IVF in Couples with Autoimmunity?
3.5. Should We Use Conventional IVF in PGT Cycles?
3.6. Should We Use Conventional IVF in Single Oocyte Retrievals?
4. Conventional IVF and Male Gametes
4.1. What Characteristics of Spermatozoa in the Ejaculate to Consider Conventional IVF?
4.2. Which Features Should Spermatozoa Possess to Ensure Successful Conventional IVF?
4.3. How to Prepare the Semen?
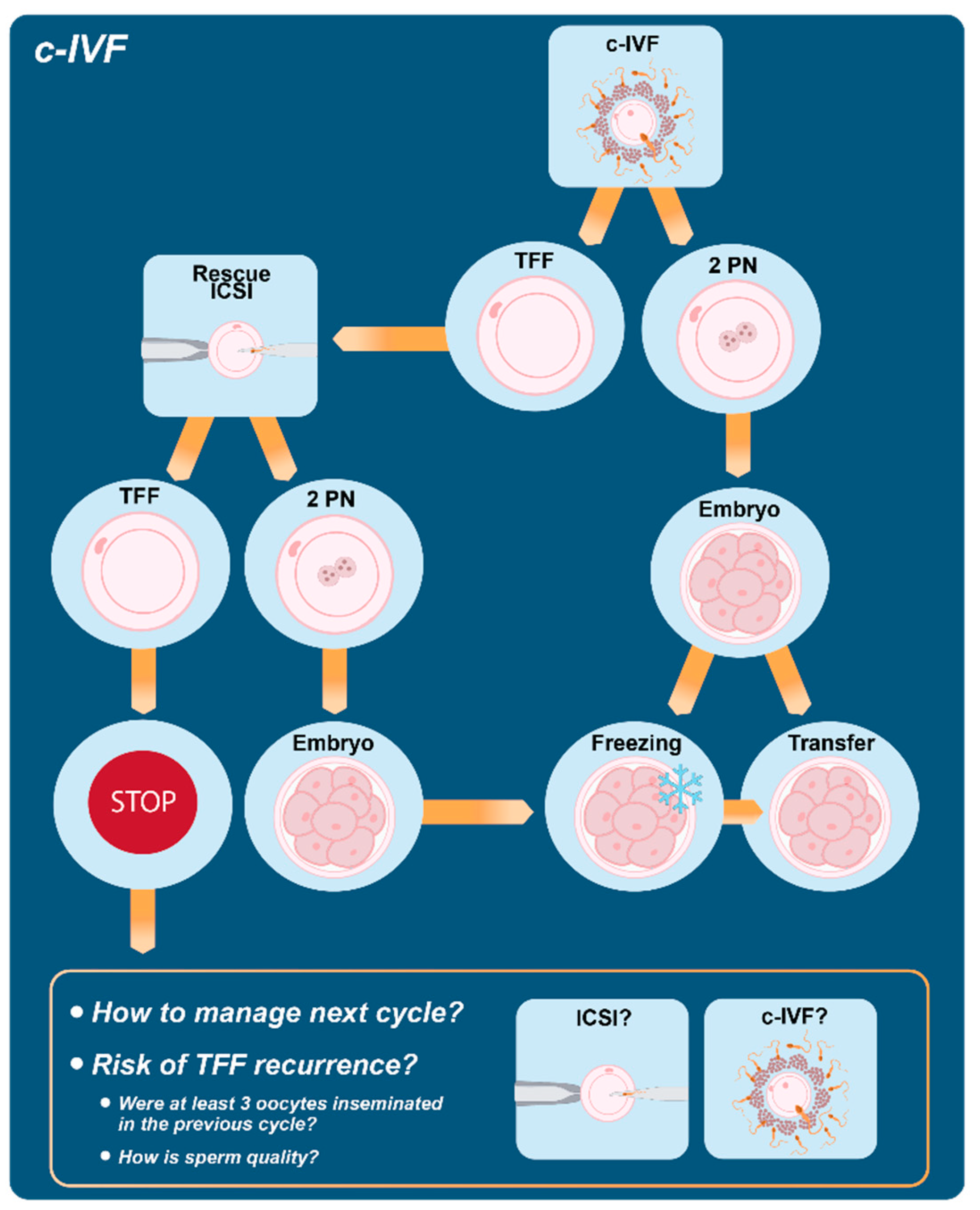
5. Conventional IVF and Fertilization Failure
5.1. The Role of the Rescue ICSI
5.2. How to Manage the Next Cycle?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Malgund, A.; Hamada, A.; Chyatte, M.R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- European Society of Human Reproduction and Embryology. Available online: https://www.sciencedaily.com/releases/2018/07/180703084127.htm (accessed on 25 May 2022).
- Niederberger, C.; Pellicer, A.; Cohen, J.; Gardner, D.K. Forty years of IVF. Fertil. Steril. 2018, 110, 2. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Boulet, S.L.; Mehta, A.; Kissin, D.M.; Warner, L.; Kawwass, J.F.; Jamieson, D.J. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA 2015, 313, 255–263. [Google Scholar] [CrossRef]
- Zheng, D.; Nguyen, Q.N.; Li, R.; Dang, V.Q. Is Intracytoplasmic Sperm Injection the Solution for all in Unexplained Infertility? Semin. Reprod. Med. 2020, 38, 36–47. [Google Scholar] [CrossRef]
- Fertility: Assessment and Treatment for People with Fertility Problems. Available online: https://www.nice.org.uk/guidance/cg156/evidence/full-guideline-pdf-188539453 (accessed on 13 April 2022).
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non-male factor indications: A committee opinion. Fertil. Steril. 2020, 114, 239–245. [Google Scholar] [CrossRef]
- Sciorio, R.; Esteves, S.C. Contemporary Use of ICSI and Epigenetic Risks to Future Generations. J. Clin. Med. 2022, 11, 2135. [Google Scholar] [CrossRef]
- Trounson, A.O.; Mohr, L.R.; Wood, C.; Leeton, J.F. Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J. Reprod. Fertil. 1982, 64, 285–294. [Google Scholar] [CrossRef]
- Harrison, K.L.; Wilson, L.M.; Breen, T.M.; Pope, A.K.; Cummins, J.M.; Hennessey, J.F. Fertilization of human oocytes in relation to varying delay before insemination. Fertil. Steril. 1988, 50, 294–297. [Google Scholar] [CrossRef]
- Khan, I.; Staessen, C.; Van den Abbeel, E.; Camus, M.; Wisanto, A.; Smitz, J.; Devroey, P.; Van Steirteghem, A.C. Time of insemination and its effect on in-vitro fertilization, cleavage and pregnancy rates in GnRH agonist/HMG-stimulated cycles. Hum. Reprod. 1989, 4, 921–926. [Google Scholar] [CrossRef]
- Fisch, B.; Kaplan-Kraicer, R.; Amit, S.; Ovadia, J.; Tadir, Y. The effect of preinsemination interval upon fertilization of human oocytes in vitro. Hum. Reprod. 1989, 4, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Y.; Chen, M.J.; Yi, Y.C.; Guu, H.F.; Ho, E.S. The effect of preincubation period of oocytes on nuclear maturity, fertilization rate, embryo quality, and pregnancy outcome in IVF and ICSI. J. Assist. Reprod. Genet. 2003, 20, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.; Stolwijk, A.M.; Wetzels, A.M. The effect of insemination/injection time on the results of IVF and ICSI. Hum. Reprod. 2001, 16, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Esiso, F.M.; Cunningham, D.; Lai, F.; Garcia, D.; Barrett, C.B.; Thornton, K.; Sakkas, D. The effect of rapid and delayed insemination on reproductive outcome in conventional insemination and intracytoplasmic sperm injection in vitro fertilization cycles. J. Assist. Reprod. Genet. 2021, 38, 2697–2706. [Google Scholar] [CrossRef]
- Barrie, A.; Smith, R.; Campbell, A.; Fishel, S. Optimisation of the timing of fertilisation assessment for oocytes cultured in standard incubation: Lessons learnt from time-lapse imaging of 78 348 embryos. Hum. Reprod. 2021, 36, 2840–2847. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Li, R.Q.; Ouyang, N.Y.; Ou, S.B.; Ni, R.M.; Mai, M.Q.; Zhang, Q.X.; Yang, D.Z.; Wang, W.J. Does reducing gamete co-incubation time improve clinical outcomes: A retrospective study. J. Assist. Reprod. Genet. 2016, 33, 33–38. [Google Scholar] [CrossRef]
- Anzalone, D.A.; Palazzese, L.; Czernik, M.; Sabatucci, A.; Valbonetti, L.; Capra, E.; Loi, P. Controlled spermatozoa-oocyte interaction improves embryo quality in sheep. Sci. Rep. 2021, 11, 22629. [Google Scholar] [CrossRef]
- Dirnfeld, M.; Shiloh, H.; Bider, D.; Harari, E.; Koifman, M.; Lahav-Baratz, S.; Abramovici, H. A prospective randomized controlled study of the effect of short coincubation of gametes during insemination on zona pellucida thickness. Gynecol. Endocrinol. 2003, 17, 397–403. [Google Scholar] [CrossRef]
- Gianaroli, L.; Fiorentino, A.; Magli, M.C.; Ferraretti, A.P.; Montanaro, N. Prolonged sperm-oocyte exposure and high sperm concentration affect human embryo viability and pregnancy rate. Hum. Reprod. 1996, 11, 2507–2511. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Wang, Y.; Ng, E.H.Y.; Zhao, M.; Pan, J.P.; Wu, H.X.; Teng, X.M. A randomized triple blind controlled trial comparing the live birth rate of IVF following brief incubation versus standard incubation of gametes. Hum. Reprod. 2019, 34, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Yin, M.; Tang, C.; Zhu, X.; Bukulmez, O.; Chen, M.; Teng, X. Effects of Early Cumulus Cell Removal on Treatment Outcomes in Patients Undergoing In Vitro Fertilization: A Retrospective Cohort Study. Front. Endocrinol. 2021, 12, 669507. [Google Scholar] [CrossRef] [PubMed]
- De Munck, N.; Janssens, R.; Segers, I.; Tournaye, H.; Van de Velde, H.; Verheyen, G. Influence of ultra-low oxygen (2%) tension on in-vitro human embryo development. Hum. Reprod. 2019, 34, 228–234. [Google Scholar] [CrossRef]
- Xiong, S.; Han, W.; Liu, J.X.; Zhang, X.D.; Liu, W.W.; Liu, H.; Huang, G.N. Effects of cumulus cells removal after 6 h co-incubation of gametes on the outcomes of human IVF. J. Assist. Reprod. Genet. 2011, 28, 1205–1211. [Google Scholar] [CrossRef]
- Le Bras, A.; Hesters, L.; Gallot, V.; Tallet, C.; Tachdjian, G.; Frydman, N. Shortening gametes co-incubation time improves live birth rate for couples with a history of fragmented embryos. Syst. Biol. Reprod. Med. 2017, 63, 331–337. [Google Scholar] [CrossRef]
- Fathollahipour, S.; Patil, P.S.; Leipzig, N.D. Oxygen Regulation in Development: Lessons from Embryogenesis towards Tissue Engineering. Cells Tissues Organs 2018, 205, 350–371. [Google Scholar] [CrossRef]
- Quinn, P.; Lydic, M.L.; Ho, M.; Bastuba, M.; Hendee, F.; Brody, S.A. Confirmation of the beneficial effects of brief coincubation of gametes in human in vitro fertilization. Fertil. Steril. 1998, 69, 399–402. [Google Scholar] [CrossRef]
- Coskun, S.; Roca, G.L.; Elnour, A.M.; al Mayman, H.; Hollanders, J.M.; Jaroudi, K.A. Effects of reducing insemination time in human in vitro fertilization and embryo development by using sibling oocytes. J. Assist. Reprod. Genet. 1998, 15, 605–608. [Google Scholar] [CrossRef]
- Dirnfeld, M.; Bider, D.; Koifman, M.; Calderon, I.; Abramovici, H. Shortened exposure of oocytes to spermatozoa improves in-vitro fertilization outcome: A prospective, randomized, controlled study. Hum. Reprod. 1999, 14, 2562–2564. [Google Scholar] [CrossRef]
- Lin, S.P.; Lee, R.K.; Su, J.T.; Lin, M.H.; Hwu, Y.M. The effects of brief gamete co-incubation in human in vitro fertilization. J Assist Reprod Genet. 2000, 17, 344–348. [Google Scholar] [CrossRef]
- Swenson, K.; Check, J.H.; Summers-Chase, D.; Choe, J.K.; Check, M.L. A randomized study comparing the effect of standard versus short incubation of sperm and oocyte on subsequent pregnancy and implantation rates following in vitro fertilization embryo transfer. Arch. Androl. 2000, 45, 73–76. [Google Scholar] [CrossRef]
- Boone, W.R.; Johnson, J.E. Extending the coincubation time of gametes improves in vitro fertilization. J. Assist. Reprod. Genet. 2001, 18, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Johansson, U.; Lundkvist, O.; Milton, K.; Westin, C.; Simberg, N. Reducing the time of co-incubation of gametes in human in-vitro fertilization has no beneficial effects. Reprod. Biomed. Online. 2001, 3, 21–24. [Google Scholar] [CrossRef]
- Kattera, S.; Chen, C. Short coincubation of gametes in in vitro fertilization improves implantation and pregnancy rates: A prospective, randomized, controlled study. Fertil. Steril. 2003, 80, 1017–1021. [Google Scholar] [CrossRef]
- Barraud-Lange, V.; Sifer, C.; Pocaté, K.; Ziyyat, A.; Martin-Pont, B.; Porcher, R.; Hugues, J.N.; Wolf, J.P. Short gamete co-incubation during in vitro fertilization decreases the fertilization rate and does not improve embryo quality: A prospective auto controlled study. J. Assist. Reprod. Genet. 2008, 25, 305–310. [Google Scholar] [CrossRef]
- Dai, S.J.; Qiao, Y.H.; Jin, H.X.; Xin, Z.M.; Su, Y.C.; Sun, Y.P.; Chian, R.C. Effect of coincubation time of sperm-oocytes on fertilization, embryonic development, and subsequent pregnancy outcome. Syst. Biol. Reprod. Med. 2012, 58, 348–353. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Wang, L.; Yan, J.; Shi, Y.; Li, S. Brief co-incubation of sperm and oocytes for in vitro fertilization techniques. Cochrane Database Syst. Rev. 2013, CD009391. [Google Scholar] [CrossRef]
- Zhang, X.D.; Liu, J.X.; Liu, W.W.; Gao, Y.; Han, W.; Xiong, S.; Wu, L.H.; Huang, G.N. Time of insemination culture and outcomes of in vitro fertilization: A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 685–695. [Google Scholar] [CrossRef]
- He, Y.; Liu, H.; Zheng, H.; Li, L.; Fu, X.; Liu, J. Effect of early cumulus cells removal and early rescue ICSI on pregnancy outcomes in high-risk patients of fertilization failure. Gynecol. Endocrinol. 2018, 34, 689–693. [Google Scholar] [CrossRef]
- Steptoe, P.C.; Edwards, R.G.; Purdy, J.M. Human blastocysts grown in culture. Nature 1971, 229, 132–133. [Google Scholar] [CrossRef]
- Waldenström, U.; Engström, A.B.; Hellberg, D.; Nilsson, S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil. Steril. 2009, 91, 2461–2465. [Google Scholar] [CrossRef] [PubMed]
- Kasterstein, E.; Strassburger, D.; Komarovsky, D.; Bern, O.; Komsky, A.; Raziel, A.; Friedler, S.; Ron-El, R. The effect of two distinct levels of oxygen concentration on embryo development in a sibling oocyte study. J. Assist. Reprod. Genet. 2013, 30, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Smith, G.D. Embryo culture at a reduced oxygen concentration of 5%: A mini review. Zygote 2019, 27, 355–361. [Google Scholar] [CrossRef]
- Kea, B.; Gebhardt, J.; Watt, J.; Westphal, L.M.; Lathi, R.B.; Milki, A.A.; Behr, B. Effect of reduced oxygen concentrations on the outcome of in vitro fertilization. Fertil. Steril. 2007, 87, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, K.; Hindkjaer, J.J.; Ingerslev, H.J. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil. Steril. 2013, 99, 738–744. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boguenet, M.; Hachem, H.E.; Bouet, P.E.; Reynier, P. Embryo and Its Mitochondria. Antioxidants 2021, 10, 139. [Google Scholar] [CrossRef]
- Bontekoe, S.; Mantikou, E.; van Wely, M.; Seshadri, S.; Repping, S.; Mastenbroek, S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- ESHRE Guideline Group on Good Practice in IVF Labs; De los Santos, M.J.; Apter, S.; Coticchio, G.; Debrock, S.; Lundin, K.; Plancha, C.E.; Prados, F.; Rienzi, L.; Verheyen, G.; et al. Revised guidelines for good practice in IVF laboratories (2015). Hum. Reprod. 2016, 31, 685–686. [Google Scholar]
- Christianson, M.S.; Zhao, Y.; Shoham, G.; Granot, I.; Safran, A.; Khafagy, A.; Leong, M.; Shoham, Z. Embryo catheter loading and embryo culture techniques: Results of a worldwide Web-based survey. J. Assist. Reprod. Genet. 2014, 31, 1029–1036. [Google Scholar] [CrossRef]
- Guo, N.; Li, Y.; Ai, J.; Gu, L.; Chen, W.; Liu, Q. Two different concentrations of oxygen for culturing precompaction stage embryos on human embryo development competence: A prospective randomized sibling-oocyte study. Int. J. Clin. Exp. Pathol. 2014, 7, 6191–6198. [Google Scholar]
- Guarneri, C.; Restelli, L.; Mangiarini, A.; Ferrari, S.; Somigliana, E.; Paffoni, A. Can we use incubators with atmospheric oxygen tension in the first phase of in vitro fertilization? A retrospective analysis. J. Assist. Reprod. Genet. 2015, 32, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mantikou, E.; Bontekoe, S.; van Wely, M.; Seshadri, S.; Repping, S.; Mastenbroek, S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Hum. Reprod. Update 2013, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Archieved ART Reports and Spreadsheets. Available online: https://www.cdc.gov/art/reports/archive.html (accessed on 17 April 2022).
- Sunderam, S.; Boulet, S.L.; Kawwass, J.F.; Kissin, D.M. Comparing fertilization rates from intracytoplasmic sperm injection to conventional in vitro fertilization among women of advanced age with non-male factor infertility: A meta-analysis. Fertil. Steril. 2020, 113, 354–363. [Google Scholar] [CrossRef]
- Haas, J.; Miller, T.E.; Nahum, R.; Aizer, A.; Kirshenbaum, M.; Zilberberg, E.; Lebovitz, O.; Orvieto, R. The role of ICSI vs. conventional IVF for patients with advanced maternal age-a randomized controlled trial. J. Assist. Reprod. Genet. 2021, 38, 95–100. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Yu, G.; Li, M.; Ma, S.; Zhang, H.; Wu, K. Conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI): Which is preferred for advanced age patients with five or fewer oocytes retrieved? Arch. Gynecol. Obstet. 2018, 297, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Tannus, S.; Son, W.Y.; Gilman, A.; Younes, G.; Shavit, T.; Dahan, M.H. The role of intracytoplasmic sperm injection in non-male factor infertility in advanced maternal age. Hum. Reprod. 2017, 32, 119–124. [Google Scholar] [CrossRef]
- Ferraretti, A.P.; Gianaroli, L. The Bologna criteria for the definition of poor ovarian responders: Is there a need for revision? Hum. Reprod. 2014, 29, 1842–1845. [Google Scholar] [CrossRef]
- Supramaniam, P.R.; Granne, I.; Ohuma, E.O.; Lim, L.N.; McVeigh, E.; Venkatakrishnan, R.; Becker, C.M.; Mittal, M. ICSI does not improve reproductive outcomes in autologous ovarian response cycles with non-male factor subfertility. Hum. Reprod. 2020, 35, 583–594. [Google Scholar] [CrossRef]
- Isikoglu, M.; Ceviren, A.K.; Cetin, T.; Avci, A.; Aydinuraz, B.; Akgul, O.K.; Karaca, M. Comparison of ICSI and conventional IVF in non-male factor patients with less than four oocytes. Arch. Gynecol. Obstet. 2022, 306, 493–499. [Google Scholar] [CrossRef]
- Drakopoulos, P.; Garcia-Velasco, J.; Bosch, E.; Blockeel, C.; de Vos, M.; Santos-Ribeiro, S.; Makrigiannakis, A.; Tournaye, H.; Polyzos, N.P. ICSI does not offer any benefit over conventional IVF across different ovarian response categories in non-male factor infertility: A European multicenter analysis. J. Assist. Reprod. Genet. 2019, 36, 2067–2076. [Google Scholar] [CrossRef]
- Guo, N.; Hua, X.; Li, Y.F.; Jin, L. Role of ICSI in Non-male Factor Cycles as the Number of Oocytes Retrieved Decreases from Four to One. Curr. Med. Sci. 2018, 38, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Bigelow, C.; Duke, M.; Ruman, J.; Sandler, B.; Grunfeld, L.; Copperman, A.B. Should ICSI be recommended routinely in patients with four or fewer oocytes retrieved? J. Assist. Reprod. Genet. 2011, 28, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Li, Z.; Niu, J.; Tang, R. Obstetric and perinatal outcomes of intracytoplasmic sperm injection versus conventional in vitro fertilization in couples with nonsevere male infertility. Fertil. Steril. 2020, 114, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Ruiz, A.; Simón, C.; Pellicer, A.; Remohí, J. Intracytoplasmic sperm injection as a routine indication in low responder patients. Hum. Reprod. 1998, 13, 2126–2129. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Vanni, V.S.; Bartiromo, L.; Papaleo, E.; Zilberberg, E.; Candiani, M.; Orvieto, R.; Viganò, P. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 2017, 10, 43. [Google Scholar] [CrossRef]
- Komsky-Elbaz, A.; Raziel, A.; Friedler, S.; Strassburger, D.; Kasterstein, E.; Komarovsky, D.; Ron-El, R.; Ben-Ami, I. Conventional IVF versus ICSI in sibling oocytes from couples with endometriosis and normozoospermic semen. J. Assist. Reprod. Genet. 2013, 30, 251–257. [Google Scholar] [CrossRef]
- Vanni, V.S.; De Lorenzo, R.; Privitera, L.; Canti, V.; Viganò, P.; Rovere-Querini, P. Safety of fertility treatments in women with systemic autoimmune diseases (SADs). Expert. Opin. Drug Saf. 2019, 18, 841–852. [Google Scholar] [CrossRef]
- Berestoviy, V.O.; Mahmood, A.A.; Berestoviy, O.O.; Ginzburg, V.G.; Govsieiev, D.O. An overview of autoimmunity in implantation failure: A literature review. Wiad. Lek. 2021, 74, 777–783. [Google Scholar] [CrossRef]
- Vickram, A.S.; Dhama, K.; Chakraborty, S.; Samad, H.A.; Latheef, S.K.; Sharun, K.; Khurana, S.K.; Archana, K.; Tiwari, R.; Bhatt, P.; et al. Role of Antisperm Antibodies in Infertility, Pregnancy, and Potential for Contraceptive and Antifertility Vaccine Designs: Research Progress and Pioneering Vision. Vaccines 2019, 7, 116. [Google Scholar]
- Acosta, A.A.; Van der Merwe, J.P.; Doncel, G.; Kruger, T.F.; Sayilgan, A.; Franken, D.R.; Kolm, P. Fertilization efficiency of morphologically abnormal spermatozoa in assisted reproduction is further impaired by antisperm antibodies on the male partner’s sperm. Fertil. Steril. 1994, 62, 826–833. [Google Scholar] [CrossRef]
- Junk, S.M.; Matson, P.L.; Yovich, J.M.; Bootsma, B.; Yovich, J.L. The fertilization of human oocytes by spermatozoa from men with antispermatozoal antibodies in semen. J. In Vitro Fert. Embryo Transf. 1986, 3, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Culligan, P.J.; Crane, M.M.; Boone, W.R.; Allen, T.C.; Price, T.M.; Blauer, K.L. Validity and cost-effectiveness of antisperm antibody testing before in vitro fertilization. Fertil. Steril. 1998, 69, 894–898. [Google Scholar] [CrossRef]
- Vujisić, S.; Lepej, S.Z.; Jerković, L.; Emedi, I.; Sokolić, B. Antisperm antibodies in semen, sera and follicular fluids of infertile patients: Relation to reproductive outcome after in vitro fertilization. Am. J. Reprod. Immunol. 2005, 54, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Li, X.; Wang, S.L.; Yang, X.L.; Xu, Y.Z.; Huang, L.L.; Liu, J.L.; Cai, F.F.; Chen, Z.J. Success rates of in vitro fertilization versus intracytoplasmic sperm injection in men with serum anti-sperm antibodies: A consecutive cohort study. Asian J. Androl. 2019, 21, 473–477. [Google Scholar]
- Nagy, Z.P.; Verheyen, G.; Liu, J.; Joris, H.; Janssenswillen, C.; Wisanto, A.; Devroey, P.; Van Steirteghem, A.C. Results of 55 intracytoplasmic sperm injection cycles in the treatment of male-immunological infertility. Hum. Reprod. 1995, 10, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki, A.; Reima, I.; Hovatta, O. Treatment of severe male immunological infertility by intracytoplasmic sperm injection. Hum. Reprod. 1995, 10, 2824–2828. [Google Scholar] [CrossRef]
- Poppe, K.; Velkeniers, B. Thyroid and infertility. Verh. K. Acad. Geneeskd. Belg. 2002, 64, 389–399. [Google Scholar]
- Monteleone, P.; Parrini, D.; Faviana, P.; Carletti, E.; Casarosa, E.; Uccelli, A.; Cela, V.; Genazzani, A.R.; Artini, P.G. Female infertility related to thyroid autoimmunity: The ovarian follicle hypothesis. Am. J. Reprod Immunol. 2011, 66, 108–114. [Google Scholar] [CrossRef]
- Karacan, M.; Alwaeely, F.; Cebi, Z.; Berberoglugil, M.; Batukan, M.; Ulug, M.; Arvas, A.; Camlıbel, T. Effect of antithyroid antibodies on ICSI outcome in antiphospholipid antibody-negative euthyroid women. Reprod. Biomed. Online 2013, 27, 376–380. [Google Scholar] [CrossRef][Green Version]
- Litwicka, K.; Arrivi, C.; Varricchio, M.T.; Mencacci, C.; Greco, E. In women with thyroid autoimmunity, does low-dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J. Obstet. Gynaecol. Res. 2015, 41, 722–728. [Google Scholar] [CrossRef]
- Łukaszuk, K.; Kunicki, M.; Kulwikowska, P.; Liss, J.; Pastuszek, E.; Jaszczołt, M.; Męczekalski, B.; Skowroński, K. The impact of the presence of antithyroid antibodies on pregnancy outcome following intracytoplasmatic sperm injection-ICSI and embryo transfer in women with normal thyreotropine levels. J. Endocrinol. Investig. 2015, 38, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, A.; Paffoni, A.; Fedele, L.; Somigliana, E. The impact of thyroid autoimmunity on IVF/ICSI outcome: A systematic review and meta-analysis. Hum. Reprod. Update 2016, 22, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo, D.; Rienzi, L.; Capalbo, A.; Rubio, C.; Innocenti, F.; García-Pascual, C.M.; Ubaldi, F.M.; Handyside, A. The dawn of the future: 30 years from the first biopsy of a human embryo. The detailed history of an ongoing revolution. Hum. Reprod. Update 2020, 26, 453–473. [Google Scholar] [CrossRef]
- Parikh, F.R.; Athalye, A.S.; Naik, N.J.; Naik, D.J.; Sanap, R.R.; Madon, P.F. Preimplantation Genetic Testing: Its Evolution, Where Are We Today? J. Hum. Reprod. Sci. 2018, 11, 306–314. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, M.; Berckmoes, V.; De Vos, A.; Van De Voorde, S.; Verdyck, P.; Verpoest, W.; Keymolen, K. PREIMPLANTATION GENETIC TESTING: Clinical experience of preimplantation genetic testing. Reprod 2020, 160, A45–A58. [Google Scholar] [CrossRef]
- ESHRE PGT Consortium and SIG-Embryology Biopsy Working Group; Kokkali, G.; Coticchio, G.; Bronet, F.; Celebi, C.; Cimadomo, D.; Goossens, V.; Liss, J.; Nunes, S.; Sfontouris, I.; et al. ESHRE PGT Consortium and SIG Embryology good practice recommendations for polar body and embryo biopsy for PGT. Hum. Reprod. Open 2020, 2020, hoaa020. [Google Scholar] [CrossRef]
- ESHRE Capri Workshop Group. Intracytoplasmic sperm injection (ICSI) in 2006: Evidence and evolution. Hum. Reprod. Update 2007, 13, 515–526. [Google Scholar] [CrossRef]
- Practice Committees of the American Society for Reproductive Medicine and Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: A committee opinion. Fertil. Steril. 2012, 98, 1395–1399. [Google Scholar] [CrossRef]
- De Munck, N.; El Khatib, I.; Abdala, A.; El-Damen, A.; Bayram, A.; Arnanz, A.; Melado, L.; Lawrenz, B.; Fatemi, H.M. Intracytoplasmic sperm injection is not superior to conventional IVF in couples with non-male factor infertility and preimplantation genetic testing for aneuploidies (PGT-A). Hum. Reprod. 2020, 35, 317–327. [Google Scholar] [CrossRef]
- De Rycke, M.; Belva, F.; Goossens, V.; Moutou, C.; SenGupta, S.B.; Traeger-Synodinos, J.; Coonen, E. ESHRE PGD Consortium data collection XIII: Cycles from January to December 2010 with pregnancy follow-up to October 2011. Hum. Reprod. 2015, 30, 1763–1789. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.; Aizer, A.; Brengauz, M.; Dotan, K.; Levron, J.; Schiff, E.; Orvieto, R. Pre-implantation genetic diagnosis-should we use ICSI for all? J. Assist. Reprod. Genet. 2017, 34, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Sahin, L.; Bozkurt, M.; Şahin, H.; Gürel, A.; Calıskan, E. To compare aneuploidy rates between ICSI and IVF Cases. Niger. J. Clin. Pract. 2017, 20, 652–658. [Google Scholar] [PubMed]
- Palmerola, K.L.; Vitez, S.F.; Amrane, S.; Fischer, C.P.; Forman, E.J. Minimizing mosaicism: Assessing the impact of fertilization method on rate of mosaicism after next-generation sequencing (NGS) preimplantation genetic testing for aneuploidy (PGT-A). J. Assist. Reprod. Genet. 2019, 36, 153–157. [Google Scholar] [CrossRef]
- Deng, J.; Kuyoro, O.; Zhao, Q.; Behr, B.; Lathi, R.B. Comparison of aneuploidy rates between conventional in vitro fertilization and intracytoplasmic sperm injection in in vitro fertilization-intracytoplasmic sperm injection split insemination cycles. F S Rep. 2020, 1, 277–281. [Google Scholar] [CrossRef]
- Gozlan, I.; Dor, A.; Farber, B.; Meirow, D.; Feinstein, S.; Levron, J. Comparing intracytoplasmic sperm injection and in vitro fertilization in patients with single oocyte retrieval. Fertil. Steril. 2007, 87, 515–518. [Google Scholar] [CrossRef]
- Sfontouris, I.A.; Kolibianakis, E.M.; Lainas, G.T.; Navaratnarajah, R.; Tarlatzis, B.C.; Lainas, T.G. Live birth rates using conventional in vitro fertilization compared to intracytoplasmic sperm injection in Bologna poor responders with a single oocyte retrieved. J. Assist. Reprod. Genet. 2015, 32, 691–697. [Google Scholar] [CrossRef]
- Verheyen, G.; Tournaye, H.; Staessen, C.; De Vos, A.; Vandervorst, M.; Van Steirteghem, A. Controlled comparison of conventional in-vitro fertilization and intracytoplasmic sperm injection in patients with asthenozoospermia. Hum. Reprod. 1999, 14, 2313–2319. [Google Scholar] [CrossRef]
- Villani, M.T.; Morini, D.; Spaggiari, G.; Falbo, A.I.; Melli, B.; La Sala, G.B.; Romeo, M.; Simoni, M.; Aguzzoli, L.; Santi, D. Are sperm parameters able to predict the success of assisted reproductive technology? A retrospective analysis of over 22,000 assisted reproductive technology cycles. Andrology 2022, 10, 310–321. [Google Scholar] [CrossRef]
- Larsen, L.; Scheike, T.; Jensen, T.K.; Bonde, J.P.; Ernst, E.; Hjollund, N.H.; Zhou, Y.; Skakkebaek, N.E.; Giwercman, A. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. The Danish First Pregnancy Planner Study Team. Hum. Reprod. 2000, 15, 1562–1567. [Google Scholar] [CrossRef]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.Q.; Vuong, L.N.; Luu, T.M.; Pham, T.D.; Ho, T.M.; Ha, A.N.; Truong, B.T.; Phan, A.K.; Nguyen, D.P.; Pham, T.N.; et al. Intracytoplasmic sperm injection versus conventional in-vitro fertilisation in couples with infertility in whom the male partner has normal total sperm count and motility: An open-label, randomised controlled trial. Lancet 2021, 397, 1554–1563. [Google Scholar] [CrossRef]
- Xie, B.G.; Huang, Y.H.; Zhu, W.J.; Jin, S. Comparison of the outcome of conventional in vitro fertilization and intracytoplasmic sperm injection in moderate male infertility from ejaculate. Urol. Int. 2015, 94, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.L.; Ye, Q.; Huang, Y.H.; Xie, B.G. Comparison of conventional in vitro fertilisation and intracytoplasmic sperm injection outcomes in patients with moderate oligoasthenozoospermia. Andrologia 2015, 47, 499–504. [Google Scholar] [CrossRef]
- van der Westerlaken, L.; Naaktgeboren, N.; Verburg, H.; Dieben, S.; Helmerhorst, F.M. Conventional in vitro fertilization versus intracytoplasmic sperm injection in patients with borderline semen: A randomized study using sibling oocytes. Fertil. Steril. 2006, 85, 395–400. [Google Scholar] [CrossRef]
- Zhu, D.L.; Zhang, H.G.; Wang, R.X.; Jiang, Y.T.; Liu, R.Z. Re-evaluation of the value of sperm morphology in classical in vitro fertilization in a Northeastern Chinese population. J. Int. Med. Res. 2019, 47, 4134–4142. [Google Scholar] [CrossRef]
- Kihaile, P.E.; Misumi, J.; Hirotsuru, K.; Kumasako, Y.; Kisanga, R.E.; Utsunomiya, T. Comparison of sibling oocyte outcomes after intracytoplasmic sperm injection and in vitro fertilization in severe teratozoospermic patients in the first cycle. Int. J. Androl. 2003, 26, 57–62. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Cheng, H.; Sun, X.X.; Jiang, F. Modified strict sperm morphology threshold aids in the clinical selection of conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). Asian J. Androl. 2022, 24, 62–66. [Google Scholar]
- Keegan, B.R.; Barton, S.; Sanchez, X.; Berkeley, A.S.; Krey, L.C.; Grifo, J. Isolated teratozoospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertil. Steril. 2007, 88, 1583–1588. [Google Scholar] [CrossRef]
- Fan, W.; Li, S.W.; Li, L.; Huang, Z.; Ma, Q.; Wang, Y.; Xiao, Z. Outcome of conventional IVF and ICSI on sibling oocytes in the case of isolated teratozoospermia. J. Assist. Reprod. Genet. 2012, 29, 905–910. [Google Scholar] [CrossRef]
- Stimpfel, M.; Jancar, N.; Vrtacnik-Bokal, E.; Virant-Klun, I. Conventional IVF improves blastocyst rate and quality compared to ICSI when used in patients with mild or moderate teratozoospermia. Syst. Biol. Reprod. Med. 2019, 65, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Glezerman, M.; Bernstein, D.; Zakut, C.; Misgav, N.; Insler, V. Polyzoospermia: A definite pathologic entity. Fertil. Steril. 1982, 38, 605–608. [Google Scholar] [CrossRef]
- Magdanz, V.; Boryshpolets, S.; Ridzewski, C.; Eckel, B.; Reinhardt, K. The motility-based swim-up technique separates bull sperm based on differences in metabolic rates and tail length. PLoS ONE 2019, 14, e0223576. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zeng, L.; Yang, R.; Lian, Y.; Zhu, Y.M.; Liang, X.; Tang, L.; Wang, H.; Cao, Y.; Hao, G.; et al. Intracytoplasmic sperm injection (ICSI) versus conventional in vitro fertilisation (IVF) in couples with non-severe male infertility (NSMI-ICSI): Protocol for a multicentre randomised controlled trial. BMJ Open 2019, 9, e030366. [Google Scholar] [CrossRef]
- Colaco, S.; Sakkas, D. Paternal factors contributing to embryo quality. J. Assist. Reprod. Genet. 2018, 35, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni-Tealdi, E.; Barad, D.H.; Albertini, D.F.; Yu, Y.; Kushnir, V.A.; Russell, H.; Wu, Y.G.; Gleicher, N. Oocyte Scoring Enhances Embryo-Scoring in Predicting Pregnancy Chances with IVF Where It Counts Most. PLoS ONE 2015, 10, e0143632. [Google Scholar] [CrossRef]
- Parinaud, J.; Mieusset, R.; Vieitez, G.; Labal, B.; Richoilley, G. Influence of sperm parameters on embryo quality. Fertil. Steril. 1993, 60, 888–892. [Google Scholar] [CrossRef]
- Dcunha, R.; Hussein, R.S.; Ananda, H.; Kumari, S.; Adiga, S.K.; Kannan, N.; Zhao, Y.; Kalthur, G. Current Insights and Latest Updates in Sperm Motility and Associated Applications in Assisted Reproduction. Reprod. Sci. 2022, 29, 7–25. [Google Scholar] [CrossRef]
- Aghajanova, L.; Kao, C.N.; Cedars, M.; Tran, N. Assessing the impact of semen quality on embryo development in an egg donation model. F S Rep. 2020, 2, 22–29. [Google Scholar] [CrossRef]
- Fiorentino, A.; Magli, M.C.; Fortini, D.; Feliciani, E.; Ferraretti, A.P.; Dale, B.; Gianaroli, L. Sperm:oocyte ratios in an in vitro fertilization (IVF) program. J. Assist. Reprod. Genet. 1994, 11, 97–103. [Google Scholar] [CrossRef]
- Chamayou, S.; Ragolia, C.; Alecci, C.; Storaci, G.; Romano, S.; Sapienza, R.; Maglia, E.; Liprino, A.; Cardea, C.; Fichera, M.; et al. More blastocysts are produced from fewer oocytes in ICSI compared to IVF—Results from a sibling oocytes study and definition of a new key performance indicator. Reprod. Biol. Endocrinol. 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.M.; Arnett, D.M.; Meacham, R.B. The use of in vitro fertilization in the management of male infertility: What the urologist needs to know. Rev. Urol. 2013, 15, 154–160. [Google Scholar] [PubMed]
- Aghazarian, A.; Huf, W.; Pflüger, H.; Klatte, T. Standard Semen Parameters vs. Sperm Kinematics to Predict Sperm DNA Damage. World J. Mens. Health 2021, 39, 116–122. [Google Scholar] [CrossRef]
- Vasan, S.S. Semen analysis and sperm function tests: How much to test? Indian J. Urol. 2011, 27, 41–48. [Google Scholar] [CrossRef]
- Çil, N.; Kabukçu, C.; Çabuş, Ü.; Turan, T.; Mete, G.A. Retrospective comparison of the semen preparation techniques for intrauterine insemination: Swim-up versus density gradient method. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102321. [Google Scholar] [CrossRef]
- Rappa, K.L.; Rodriguez, H.F.; Hakkarainen, G.C.; Anchan, R.M.; Mutter, G.L.; Asghar, W. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol. Adv. 2016, 34, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Butt, F.; Chohan, M.A. Comparative efficacy of density gradient and swim-up methods of semen preparation in intrauterine insemination cycles. J. Pak. Med. Assoc. 2016, 66, 932–937. [Google Scholar]
- Ricci, G.; Perticarari, S.; Boscolo, R.; Montico, M.; Guaschino, S.; Presani, G. Semen preparation methods and sperm apoptosis: Swim-up versus gradient-density centrifugation technique. Fertil. Steril. 2009, 91, 632–638. [Google Scholar] [CrossRef]
- Colleu, D.; Lescoat, D.; Gouranton, J. Nuclear maturity of human spermatozoa selected by swim-up or by Percoll gradient centrifugation procedures. Fertil. Steril. 1996, 65, 160–164. [Google Scholar] [CrossRef]
- Tomlinson, M.J.; Moffatt, O.; Manicardi, G.C.; Bizzaro, D.; Afnan, M.; Sakkas, D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: Implications for assisted conception. Hum. Reprod. 2001, 16, 2160–2165. [Google Scholar] [CrossRef]
- Mortimer, D. Sperm preparation techniques and iatrogenic failures of in-vitro fertilization. Hum. Reprod. 1991, 6, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Sakkas, D.; Gardner, D.K. Laboratory procedures for human in vitro fertilization. Semin. Reprod. Med. 2014, 32, 272–282. [Google Scholar] [PubMed]
- Aitken, R.J.; Clarkson, J.S. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J. Androl. 1988, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Mak, V.; Phang, D.; Jarvi, K. Potential adverse effect of semen processing on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 1999, 72, 496–499. [Google Scholar] [CrossRef]
- Donnelly, E.T.; O’Connell, M.; McClure, N.; Lewis, S.E. Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum. Reprod. 2000, 15, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef]
- Henkel, R.R.; Franken, D.R.; Lombard, C.J.; Schill, W.B. Selective capacity of glass-wool filtration for the separation of human spermatozoa with condensed chromatin: A possible therapeutic modality for male-factor cases? J. Assist. Reprod. Genet. 1994, 11, 395–400. [Google Scholar] [CrossRef]
- Spanò, M.; Cordelli, E.; Leter, G.; Lombardo, F.; Lenzi, A.; Gandini, L. Nuclear chromatin variations in human spermatozoa undergoing swim-up and cryopreservation evaluated by the flow cytometric sperm chromatin structure assay. Mol. Hum. Reprod. 1999, 5, 29–37. [Google Scholar] [CrossRef]
- Younglai, E.V.; Holt, D.; Brown, P.; Jurisicova, A.; Casper, R.F. Sperm swim-up techniques and DNA fragmentation. Hum. Reprod. 2001, 16, 1950–1953. [Google Scholar] [CrossRef]
- Sakkas, D.; Manicardi, G.C.; Tomlinson, M.; Mandrioli, M.; Bizzaro, D.; Bianchi, P.G.; Bianchi, U. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum. Reprod. 2000, 15, 1112–1126. [Google Scholar] [CrossRef]
- Zini, A.; Finelli, A.; Phang, D.; Jarvi, K. Influence of semen processing technique on human sperm DNA integrity. Urology 2000, 56, 1081–1084. [Google Scholar] [CrossRef]
- Jayaraman, V.; Upadhya, D.; Narayan, P.K.; Adiga, S.K. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J. Assist. Reprod. Genet. 2012, 29, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.R.; Schill, W.B. Sperm preparation for ART. Reprod. Biol. Endocrinol. 2003, 1, 108. [Google Scholar] [CrossRef] [PubMed]
- Viswambharan, N.; Murugan, M. Effect of wash and swim-up and density gradient sperm preparation on sperm DNA fragmentation. Mater. Today Proc. 2021, 45, 2002–2005. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Yamanaka, M.; Tomita, K.; Hashimoto, S.; Matsumoto, H.; Satoh, M.; Kato, H.; Hosoi, Y.; Inoue, M.; Nakaoka, Y.; Morimoto, Y. Combination of density gradient centrifugation and swim-up methods effectively decreases morphologically abnormal sperms. J. Reprod. Dev. 2016, 62, 599–606. [Google Scholar] [CrossRef]
- Van der Zwalmen, P.; Bertin-Segal, G.; Geerts, L.; Debauche, C.; Schoysman, R. Sperm morphology and IVF pregnancy rate: Comparison between Percoll gradient centrifugation and swim-up procedures. Hum. Reprod. 1991, 6, 581–588. [Google Scholar] [CrossRef]
- Li, Z.; Wang, A.Y.; Bowman, M.; Hammarberg, K.; Farquhar, C.; Johnson, L.; Safi, N.; Sullivan, E.A. ICSI does not increase the cumulative live birth rate in non-male factor infertility. Hum. Reprod. 2018, 33, 1322–1330. [Google Scholar] [CrossRef]
- Combelles, C.M.H.; Morozumi, K.; Yanagimachi, R.; Zhu, L.; Fox, J.H.; Racowsky, C. Diagnosing cellular defects in an unexplained case of total fertilization failure. Hum. Reprod. 2010, 25, 1666–1671. [Google Scholar] [CrossRef]
- Abbas, A.M.; Hussein, R.S.; Elsenity, M.A.; Samaha, I.I.; El Etriby, K.A.; Abd El-Ghany, M.F.; Khalifa, M.A.; Abdelrheem, S.S.; Ahmed, A.A.; Khodry, M.M. Higher clinical pregnancy rate with in-vitro fertilization versus intracytoplasmic sperm injection in treatment of non-male factor infertility: Systematic review and meta-analysis. J. Gynecol. Obstet. Hum. Rep. 2020, 49, 101706. [Google Scholar] [CrossRef]
- Johnson, L.N.; Sasson, I.E.; Sammel, M.D.; Dokras, A. Does intracytoplasmic sperm injection improve the fertilization rate and decrease the total fertilization failure rate in couples with well-defined unexplained infertility? A systematic review and meta-analysis. Fertil. Steril. 2013, 100, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Ratna, M.B.; Bhattacharya, S.; Abdulrahim, B.; McLernon, D.J. A systematic review of the quality of clinical prediction models in in vitro fertilisation. Hum. Reprod. 2020, 35, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Chen, L.; Yang, R.; Long, X.; Li, Q.; Hao, Y.; Kong, F.; Li, R.; Wang, Y.; Qiao, J. Prediction of Fertilization Disorders in the In Vitro Fertilization/Intracytoplasmic Sperm Injection: A Retrospective Study of 106,728 Treatment Cycles. Front. Endocrinol. 2022, 13, 870708. [Google Scholar] [CrossRef] [PubMed]
- Beck-Fruchter, R.; Lavee, M.; Weiss, A.; Geslevich, Y.; Shalev, E. Rescue intracytoplasmic sperm injection: A systematic review. Fertil. Steril. 2014, 101, 690–698. [Google Scholar]
- Ming, L.; Liu, P.; Qiao, J.; Lian, Y.; Zheng, X.; Ren, X.; Huang, J.; Wu, Y. Synchronization between embryo development and endometrium is a contributing factor for rescue ICSI outcome. Reprod. Biomed. Online 2012, 24, 527–531. [Google Scholar]
- Sermondade, N.; Hugues, J.N.; Cedrin-Durnerin, I.; Poncelet, C.; Benzacken, B.; Lévy, R.; Sifer, C. Should all embryos from day 1 rescue intracytoplasmic sperm injection be transferred during frozen–thawed cycles? Fertil. Steril. 2010, 94, 1157–1158. [Google Scholar] [CrossRef]
- Paffoni, A.; Reschini, M.; Pisaturo, V.; Guarneri, C.; Palini, S.; Viganò, P. Should rescue ICSI be re-evaluated considering the deferred transfer of cryopreserved embryos in in-vitro fertilization cycles? A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 121. [Google Scholar] [CrossRef]
- Roest, J.; Van Heusden, A.M.; Zeilmaker, G.H.; Verhoeff, A. Treatment policy after poor fertilization in the first IVF cycle. J. Assist. Reprod. Genet. 1998, 15, 18–21. [Google Scholar] [CrossRef]
- Krog, M.; Prior, M.; Carlsen, E.; Loft, A.; Forman, J.; Pinborg, A.; Andersen, A.N. Fertilization failure after IVF in 304 couples—A case-control study on predictors and long-term prognosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 32–37. [Google Scholar] [CrossRef]
- Lipitz, S.; Rabinovici, J.; Ben-Shlomo, I.; Bider, D.; Ben-Rafael, Z.; Mashiach, S.; Dor, J. Complete failure of fertilization in couples with unexplained infertility: Implications for subsequent in vitro fertilization cycles. Fertil. Steril. 1993, 59, 348–352. [Google Scholar] [CrossRef]
- van der Westerlaken, L.; Helmerhorst, F.; Dieben, S.; Naaktgeboren, N. Intracytoplasmic sperm injection as a treatment for unexplained total fertilization failure or low fertilization after conventional in vitro fertilization. Fertil. Steril. 2005, 83, 612–617. [Google Scholar] [CrossRef]
- Kinzer, D.R.; Barrett, C.B.; Powers, R.D. Prognosis for clinical pregnancy and delivery after total fertilization failure during conventional in vitro fertilization or intracytoplasmic sperm injection. Fertil. Steril. 2008, 90, 284–288. [Google Scholar] [CrossRef]
- Miller, K.A.; Patton, G.W., Jr.; Queenan, J.T., Jr. The performance of subcutaneously injected Fertinex when used as the sole gonadotropin for in vitro fertilization stimulation. Fertil. Steril. 1998, 69, 658–664. [Google Scholar] [CrossRef]
- Tomás, C.; Orava, M.; Tuomivaara, L.; Martikainen, H. Low pregnancy rate is achieved in patients treated with intracytoplasmic sperm injection due to previous low or failed fertilization in in-vitro fertilization. Hum. Reprod. 1998, 13, 65–70. [Google Scholar] [CrossRef]
- Kekalainen, J. Genetic incompatibility of the reproductive partners: An evolutionary perspective on infertility. Hum. Reprod. 2021, 36, 3028–3035. [Google Scholar] [CrossRef]
- Vercellini, P.; Giudice, L.C.; Evers, J.L.; Abrao, M.S. Reducing low-value care in endometriosis between limited evidence and unresolved issues: A proposal. Hum. Reprod. 2015, 30, 1996–2004. [Google Scholar] [CrossRef]
| Authors, Year | Interval of Co-Incubation | Fertilization Rate (FR) in Short IVF | Clinical Pregnancy Rate (CPR) in Short IVF | Implantation Rate (IP) in Short IVF |
|---|---|---|---|---|
| Gianaroli et al., 1996 [22] | 1 h vs. overnight | Improved | Improved | Improved |
| Quinn et al., 1998 [29] | 1 h vs. overnight | Unchanged | Improved | Improved |
| Coskun et al., 1998 [30] | 1 h vs. overnight | Unchanged | Not assessed | Not assessed |
| Dirnfeld et al., 1999 [31] | 1 h vs. overnight | Unchanged | Improved | Improved |
| Lin et al., 2000 [32] | 1–3 h vs. overnight | Unchanged | Not assessed | Not assessed |
| Swenson et al., 2000 [33] | 2 h vs. overnight | Not assessed | Worsened | Unchanged |
| Boone et al., 2001 [34] | 3 h vs. overnight | Worsened | Not assessed | Not assessed |
| Lundqvist et al., 2001 [35] | 2 h vs. overnight | Worsened | Unchanged | Unchanged |
| Dirnfeld et al., 2003 [21] | 1 h vs. overnight | Unchanged | Not assessed | Not assessed |
| Kattera et al., 2003 [36] | 2 h vs. overnight | Unchanged | Improved | Improved |
| Barraud-Lange et al., 2008 [37] | 1 h vs. overnight | Worsened | Not assessed | Not assessed |
| Xiong et al., 2009 [26] | 1–6 h vs. overnight | Unchanged | Unchanged | Not assessed |
| Dai et al., 2012 [38] | 1–4 h vs. overnight | Unchanged | Unchanged | Unchanged |
| Huang et al., 2013 [39] | 1–4 h vs. overnight | Not assessed | Improved | Not assessed |
| Zhang et al., 2013 [40] | 1–6 h vs. overnight | Unchanged | Improved | Improved |
| Li et al., 2016 [19] | 2 h vs. overnight | Unchanged | Improved | Improved |
| Le Bras et al., 2017 [27] | 2 h vs. overnight | Worsened | Improved | Improved |
| He et al., 2018 [41] | 4/6 h vs. overnight | Worsened | Unchanged | Unchanged |
| Chen et al., 2019 [23] | 3/4 h vs. overnight | Unchanged | Unchanged | Unchanged |
| Kong et al., 2021 [24] | 4 h vs. overnight | Unchanged | Unchanged | Not assessed |
| Author, Year | Design | Analysis Years | Number of Oocytes | Number of Patients/Cycles | Result |
|---|---|---|---|---|---|
| Moreno et al., 1998 | Prospective | 1996–1997 | ≤6, ≤3 | IVF = 52 ICSI = 52 |
|
| Luna et al., 2011 | Retrospective | 2002–2009 | ≤4 | IVF = 179 ICSI = 171 |
|
| Tannus et al., 2017 | Retrospective | 2012–2015 | ≤3 | IVF = 72 ICSI = 164 |
|
| Liu et al., 2018 | Retrospective | 2011–2016 | ≤5 | IVF = 534 ICSI = 110 |
|
| Guo et al., 2018 | Retrospective | 2012–2015 | 1, 2, 3 or 4 | IVF = 870 ICSI = 435 |
|
| Drakopoulos et al., 2019 | Retrospective Multicentre | 2009–2014 | 1–3 | IVF = 90 ICSI = 600 |
|
| Supramaniam et al., 2020 | Retrospective | 1998–2016 | ≤3 | IVF= 33,436 ICSI = 29,205 |
|
| Liu et al., 2020 | Retrospective | 2012–2016 | ≤6 | IVF = 5071 ICSI = 734 |
|
| Haas et al., 2020 | Prospective | 2018–2019 | mean 4.3 | IVF= 258 ICSI= 257 |
|
| Isikoglu et al., 2022 | Retrospective | 2017–2019 | ≤3 | IVF = 77ICSI =65 |
|
| Authors, Year | Design | Antibodies District | Fertilization Rate | Clinical Pregnancy Rate | Live Birth Rate |
|---|---|---|---|---|---|
| c-IVF | |||||
| Junk et al., 1986 | Retrospective | Semen | Reduced | Not assessed | Not assessed |
| Acosta et al., 1994 | Retrospective | Semen | Reduced | Reduced | Not assessed |
| Lähteenmäki et al., 1995 | Retrospective | Semen | Reduced | Unchanged | Not assessed |
| Culligan et al., 1998 | Retrospective | Semen | Unchanged | Not assessed | Not assessed |
| Vujisić et al., 2005 | Prospective | Semen | Unchanged | Unchanged | Not assessed |
| Lu et al., 2019 | Retrospective | Serum | Reduced | Reduced | Reduced |
| ICSI | |||||
| Nagy et al., 1995 | Retrospective | Semen | Increased | Unchanged | Not assessed |
| Lähteenmäki et al., 1995 | Retrospective | Semen | Unchanged | Unchanged | Not assessed |
| Lu et al., 2019 | Retrospective | Serum | Unchanged | Unchanged | Unchanged |
| Authors, Years | Design | Analysis Years | Insemination Technique | Fertilization Rate (%) | Embryos Analyzed (%) | Euploid Embryos (%) | p |
|---|---|---|---|---|---|---|---|
| PGT-M | |||||||
| Feldman et al., 2017 | Cohort-historical | 2006–2014 | c-IVF | 696% | 84.2% | 38.9% | n.s. |
| ICSI | 58.8% | 86.3% | 36.2% | n.s. | |||
| PGT-A | |||||||
| Palmerola et al., 2019 | Retrospective | 2015–2017 | c-IVF | 61.8% | 25.7% | 27.9% | n.s. |
| ICSI | 61.4% | 74.3% | 30.0% | n.s. | |||
| De Munck et al., 2020 | Single-center prospective | 2018–2019 | c-IVF | 64.0% | 67.4% | 49.8% | n.s. |
| ICSI | 65.4% | 60.6% | 44.1% | n.s. | |||
| Authors, years | Insemination techniques | No. of embryos analyzed | Aneuploid embryos (%) | p | |||
| FISH | |||||||
| Sahin et al., 2017 | Retrospective | NR | c-IVF | 57 | 65.0% | n.s. | |
| ICSI | 183 | 69.9% | n.s. | ||||
| Indication | Main Findings |
|---|---|
| Advanced maternal age | Most available data fail to demonstrate an advantage of ICSI over c-IVF in terms of fertilization rate, embryo development rate, pregnancy and live birth rates according to the insemination technique. |
| Decreased ovarian reserve | Fertilization rate, fertilization failure, implantation rate, clinical pregnancy rate and live birth rate are comparable after c-IVF and ICSI. |
| Endometriosis | A higher fertilization rate is reported using ICSI, without a significant advantage in terms of implantation rate, pregnancy rate, chemical pregnancy, clinical abortion and ongoing pregnancy rate compared to c-IVF. |
| Autoimmunity | Lower fertilization, clinical pregnancy and live birth rates are documented in partners of antisperm antibodies positive men treated with c-IVF. ICSI can overcome these issues. Superiority of ICSI over c-IVF in couples with thyroid autoimmunity has not been documented. |
| Preimplantation genetic testing | Comparable percentages of embryos with a complete diagnosis and comparable percentages of unaffected/transferable embryos are obtained with c-IVF and ICSI in cycles with genetic testing for aneuploidy. No significant differences in contamination rates of the washing medium samples after c-IVF or ICSI are reported. |
| Single oocyte retrievals | Fertilization, implantation and live birth rates per oocyte retrieval are comparable using c-IVF or ICSI. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balli, M.; Cecchele, A.; Pisaturo, V.; Makieva, S.; Carullo, G.; Somigliana, E.; Paffoni, A.; Vigano’, P. Opportunities and Limits of Conventional IVF versus ICSI: It Is Time to Come off the Fence. J. Clin. Med. 2022, 11, 5722. https://doi.org/10.3390/jcm11195722
Balli M, Cecchele A, Pisaturo V, Makieva S, Carullo G, Somigliana E, Paffoni A, Vigano’ P. Opportunities and Limits of Conventional IVF versus ICSI: It Is Time to Come off the Fence. Journal of Clinical Medicine. 2022; 11(19):5722. https://doi.org/10.3390/jcm11195722
Chicago/Turabian StyleBalli, Martina, Anna Cecchele, Valerio Pisaturo, Sofia Makieva, Giorgia Carullo, Edgardo Somigliana, Alessio Paffoni, and Paola Vigano’. 2022. "Opportunities and Limits of Conventional IVF versus ICSI: It Is Time to Come off the Fence" Journal of Clinical Medicine 11, no. 19: 5722. https://doi.org/10.3390/jcm11195722
APA StyleBalli, M., Cecchele, A., Pisaturo, V., Makieva, S., Carullo, G., Somigliana, E., Paffoni, A., & Vigano’, P. (2022). Opportunities and Limits of Conventional IVF versus ICSI: It Is Time to Come off the Fence. Journal of Clinical Medicine, 11(19), 5722. https://doi.org/10.3390/jcm11195722






