A Rare Case of Neurosarcoidosis Overlapped with Sjogren’s Syndrome
Abstract
1. Introduction
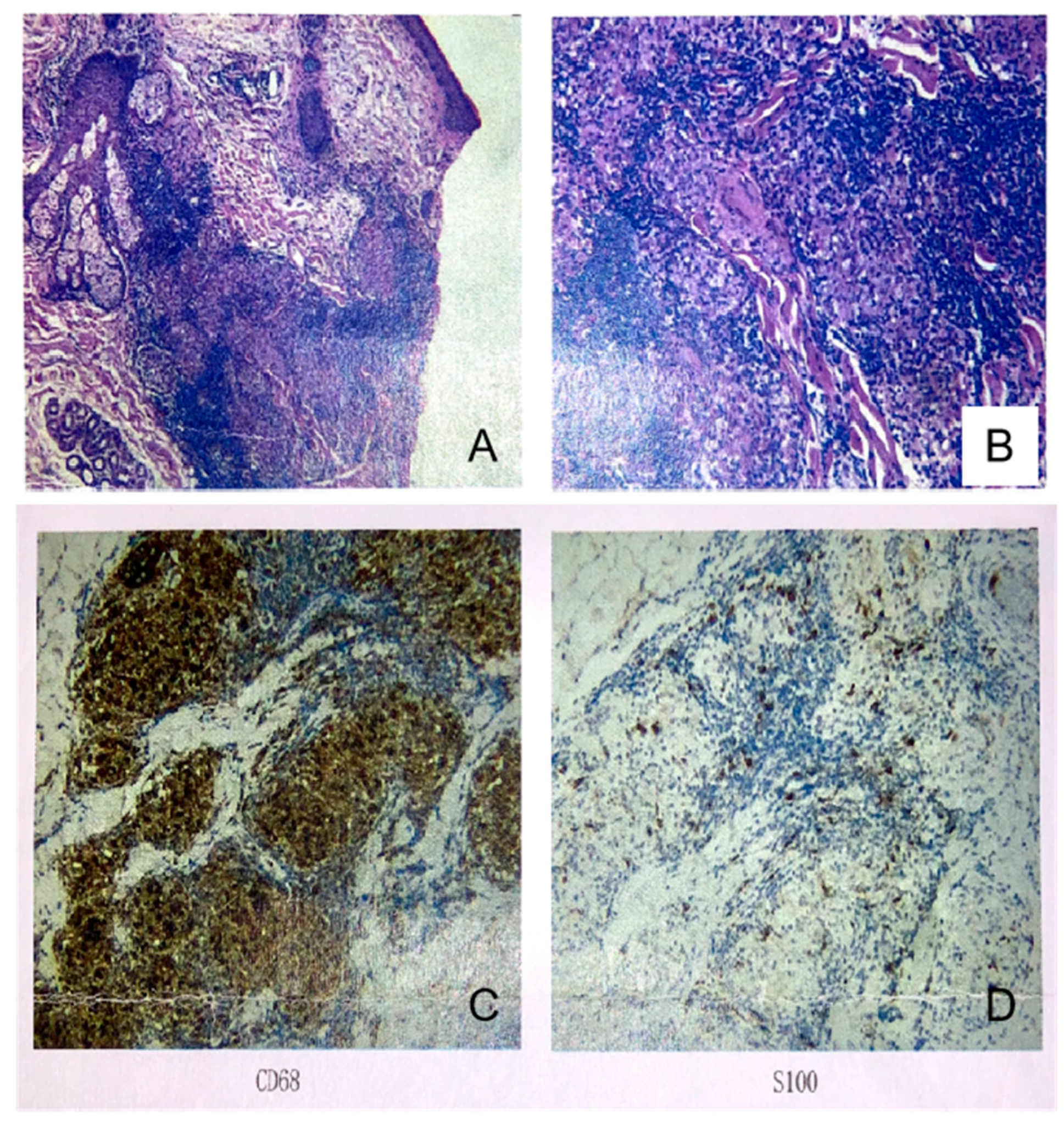
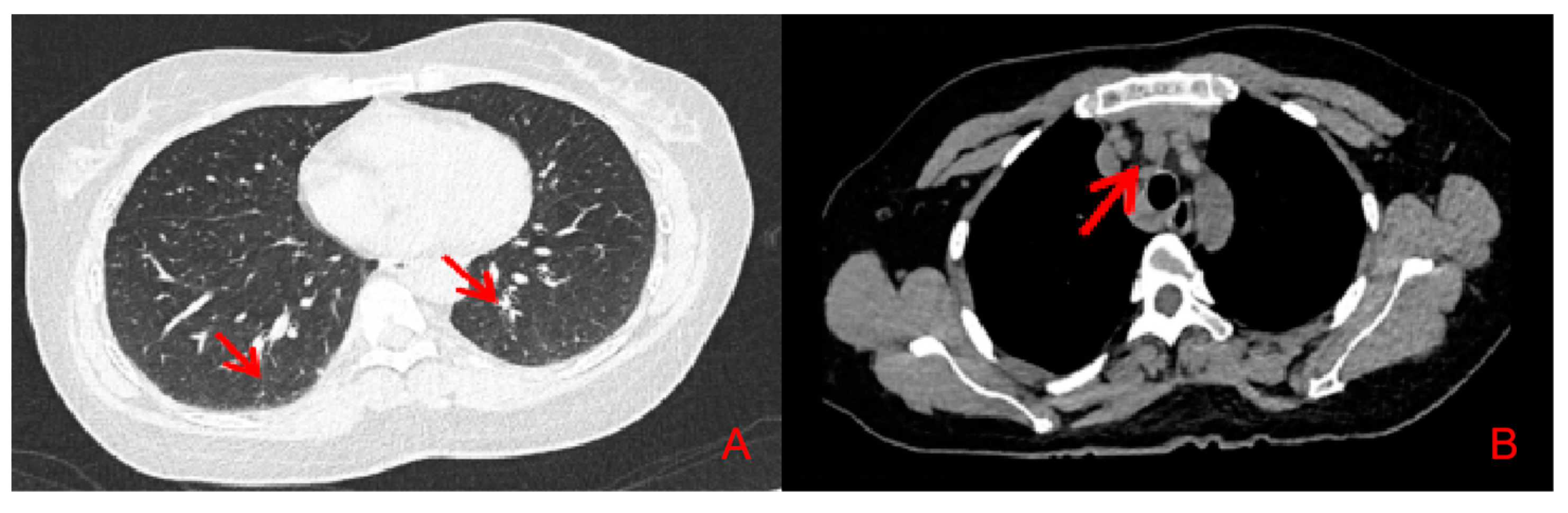
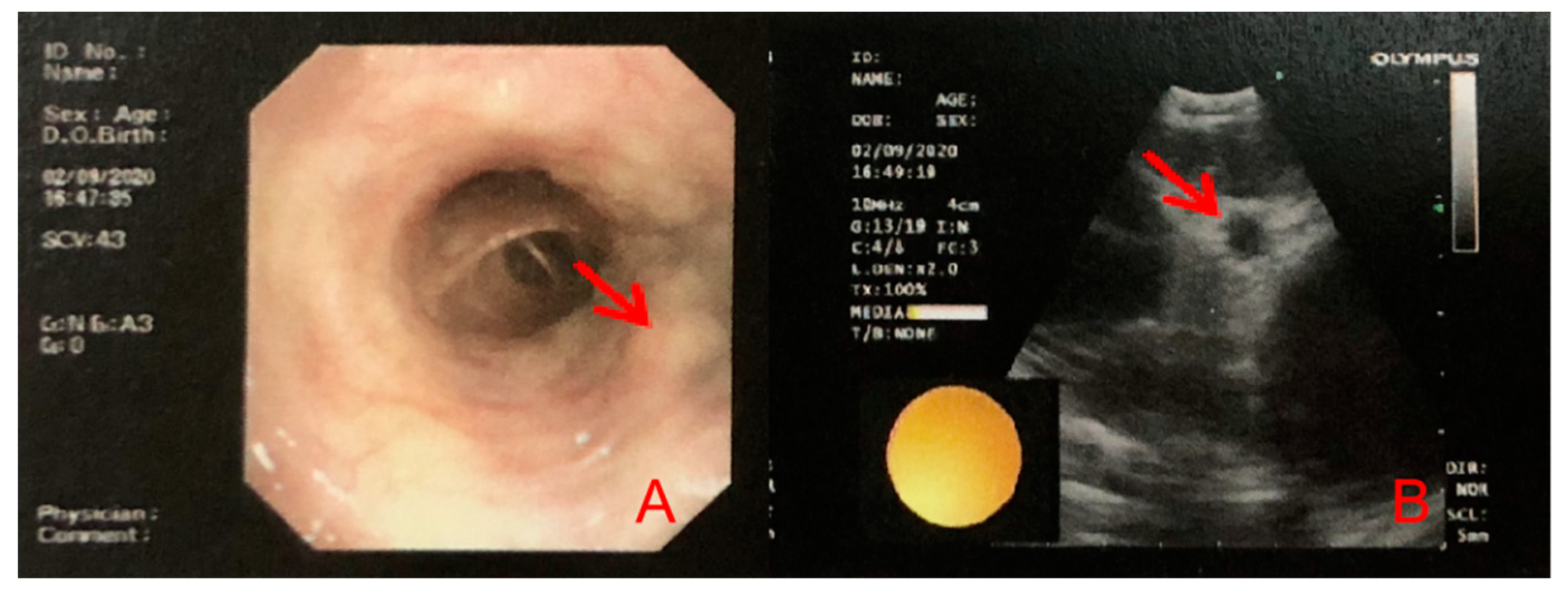
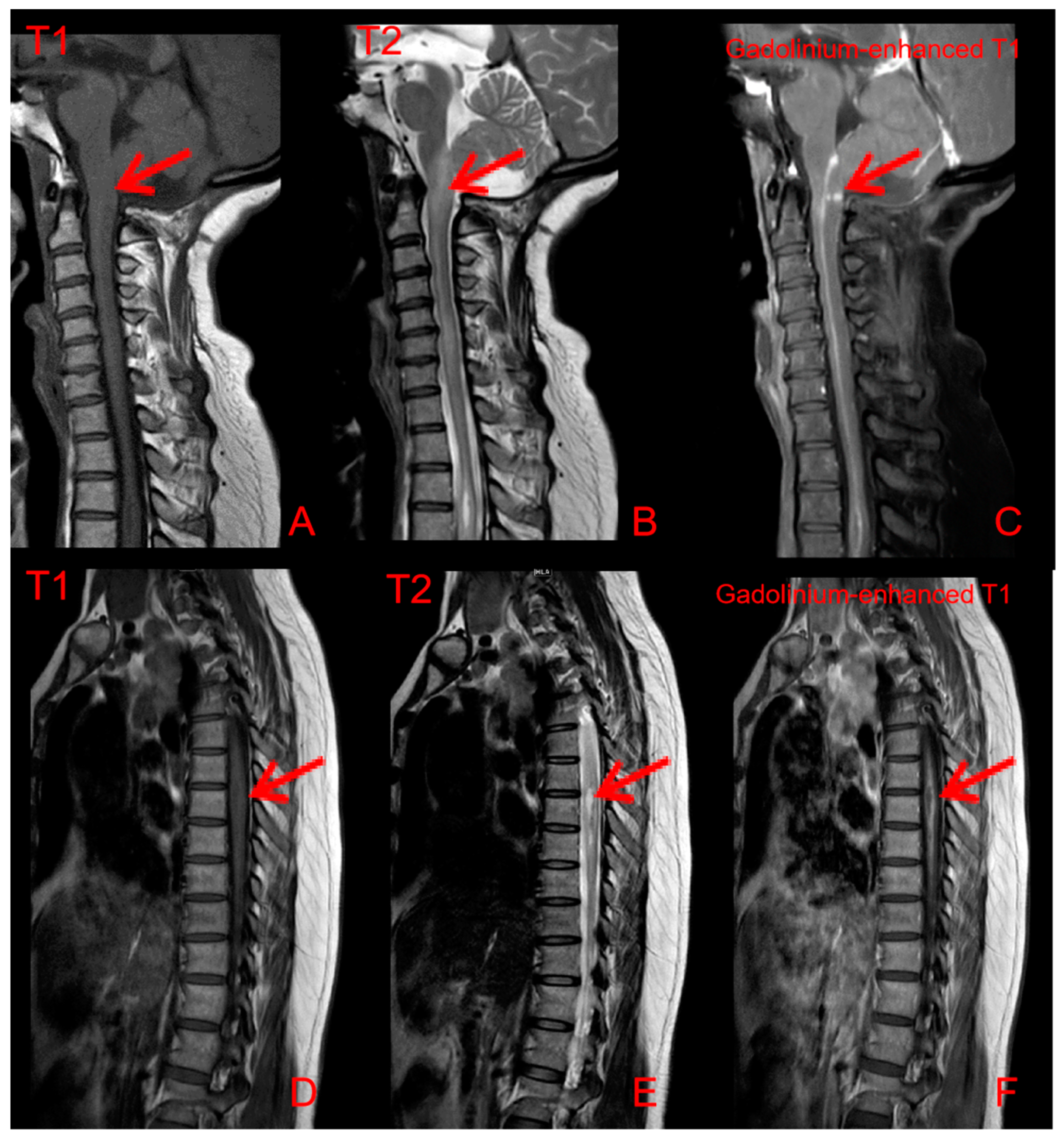
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.J.; Pawate, S.; Koth, L.L.; Cho, T.A.; Gelfand, J.M. Neurosarcoidosis: Pathophysiology, Diagnosis, and Treatment. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1084. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P.; Garcia-Carrasco, M.; Font, J. Sarcoidosis or Sjogren syndrome? Clues to defining mimicry or coexistence in 59 cases. Medicine 2004, 83, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Patoulias, D.; Keryttopoulos, P. Diagnostic dilemma between sarcoidosis and primary Sjögren syndrome: Mimicry, concomitance or coincidence? An up-to-date clinician’s perspective. Folia Med. Cracov. 2018, 58, 5–23. [Google Scholar] [PubMed]
- Tuisku, I.S.; Konttinen, Y.T.; Soinila, S.; Karma, A.; Tervo, T.M. Neurosarcoidosis mimicking Sjogren’s syndrome. Acta Ophthalmol. Scand. 2004, 82, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Kandemirli, S.G.; Bathla, G. Neuroimaging findings in rheumatologic disorders. J. Neurol. Sci. 2021, 427, 117531. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Paul, F.; Weinshenker, B.G.; Levy, M.; Kim, H.J.; Wildemann, B. Neuromyelitis optica. Nat. Rev. Dis. Prim. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Zella, S.; Kneiphof, J.; Haghikia, A.; Gold, R.; Woitalla, D.; Thöne, J. Successful therapy with rituximab in three patients with probable neurosarcoidosis. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418805732. [Google Scholar] [CrossRef] [PubMed]
- Bomprezzi, R.; Pati, S.; Chansakul, C.; Vollmer, T. A case of neurosarcoidosis successfully treated with rituximab. Neurology 2010, 75, 568–570. [Google Scholar] [CrossRef] [PubMed]

| Subject | Results | Reference Ranges | Meanings of Results |
|---|---|---|---|
| CSF OCB | Positive | Different from serum, type II | |
| Serum OCB | Negative | ||
| BBB permeability | 21.01 × 10−3 | <5.0 × 10−3 | ↑ |
| IgG index | 0.99 | <0.85 | ↑ |
| IgG-Syn (mg/24 h) | 50.27 | <7.0 | ↑ |
| CSF MBP (µg/L) | 1.92 | <3.5 | |
| Serum MBP (µg/L) | 9.47 | <2.5 | ↑ |
| VSF MBP.Ab | 0.296 | <0.650 | |
| Serum MBP.Ab | 1.517 | <0.750 | ↑ |
| CSF MOG.Ab | 0.337 | <0.560 | |
| Serum MOG.Ab | 0.413 | <0.640 | |
| CSF NSE (µg/L) | 12.44 | <11.0 | ↑ |
| Serum NSE (µg/L) | 8.67 | <9.0 | |
| CSF S100β (µg/L) | 1.68 | <0.45 | ↑ |
| Serum S100β (µg/L) | 10.00 | <0.35 | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Li, R.; He, J.; Shao, M.; Li, Z. A Rare Case of Neurosarcoidosis Overlapped with Sjogren’s Syndrome. J. Clin. Med. 2022, 11, 5709. https://doi.org/10.3390/jcm11195709
Cai W, Li R, He J, Shao M, Li Z. A Rare Case of Neurosarcoidosis Overlapped with Sjogren’s Syndrome. Journal of Clinical Medicine. 2022; 11(19):5709. https://doi.org/10.3390/jcm11195709
Chicago/Turabian StyleCai, Wenxin, Ru Li, Jing He, Miao Shao, and Zhanguo Li. 2022. "A Rare Case of Neurosarcoidosis Overlapped with Sjogren’s Syndrome" Journal of Clinical Medicine 11, no. 19: 5709. https://doi.org/10.3390/jcm11195709
APA StyleCai, W., Li, R., He, J., Shao, M., & Li, Z. (2022). A Rare Case of Neurosarcoidosis Overlapped with Sjogren’s Syndrome. Journal of Clinical Medicine, 11(19), 5709. https://doi.org/10.3390/jcm11195709





