When Can an Emergency CTA Be Dispensed with for TIA Patients?
Abstract
1. Introduction
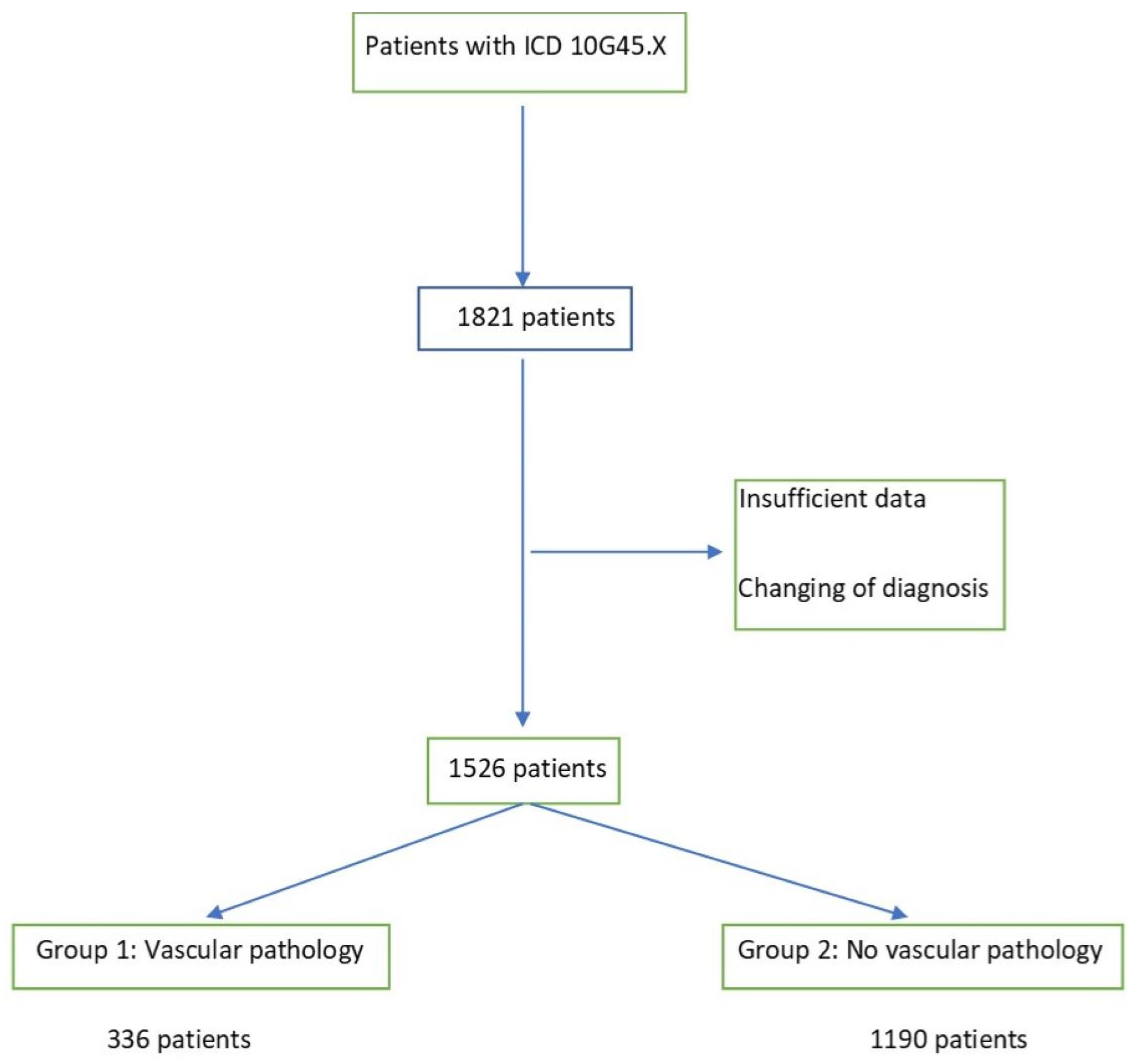
2. Material and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Pathology n = 336 (22%) | |
|---|---|
| Vessel occlusion | 14 |
| Stenosis > 50% (NASCET) | 297 |
| Vessel Dissection | 12 |
| Thrombus | 13 |
References
- Fonseca, A.C.; Merwick, Á.; Dennis, M.; Ferrari, J.; Ferro, J.M.; Kelly, P.; Lal, A.; Ois, A.; Olivot, J.M.; Purroy, F. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur. Stroke J. 2021, 6, CLXIII–CLXXXVI. [Google Scholar] [CrossRef] [PubMed]
- Coull, A.J.; Lovett, J.K.; Rothwell, P.M. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: Implications for public education and organisation of services. BMJ 2004, 328, 326. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Rothwell, P.M.; Nguyen-Huynh, M.N.; Giles, M.F.; Elkins, J.S.; Bernstein, A.L.; Sidney, S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007, 369, 283–292. [Google Scholar] [CrossRef]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Chalela, J.A.; Kidwell, C.S.; Nentwich, L.M.; Luby, M.; Butman, J.A.; Demchuk, A.M.; Hill, M.D.; Patronas, N.; Latour, L.; Warach, S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet 2007, 369, 293–298. [Google Scholar] [CrossRef]
- Von Kummer, R.; Allen, K.L.; Holle, R.; Bozzao, L.; Bastianello, S.; Manelfe, C.; Bluhmki, E.; Ringleb, P.; Meier, D.H.; Hacke, W. Acute stroke: Usefulness of early CT findings before thrombolytic therapy. Radiology 1997, 205, 327–333. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef]
- Hacke, W.; Donnan, G.; Fieschi, C.; Kaste, M.; von Kummer, R.; Broderick, J.P.; Brott, T.; Frankel, M.; Grotta, J.C.; Haley, E.C., Jr.; et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004, 363, 768–774. [Google Scholar] [CrossRef]
- Kelly, A.G.; Hellkamp, A.S.; Olson, D.; Smith, E.E.; Schwamm, L.H. Predictors of rapid brain imaging in acute stroke: Analysis of the Get with the Guidelines-Stroke program. Stroke 2012, 43, 1279–1284. [Google Scholar] [CrossRef]
- Hopyan, J.; Ciarallo, A.; Dowlatshahi, D.; Howard, P.; John, V.; Yeung, R.; Zhang, L.; Kim, J.; MacFarlane, G.; Lee, T.Y.; et al. Certainty of stroke diagnosis: Incremental benefit with CT perfusion over noncontrast CT and CT angiography. Radiology 2010, 255, 142–153. [Google Scholar] [CrossRef]
- Campbell, B.C.; Weir, L.; Desmond, P.M.; Tu, H.T.; Hand, P.J.; Yan, B.; Donnan, G.A.; Parsons, M.W.; Davis, S.M. CT perfusion improves diagnostic accuracy and confidence in acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2013, 84, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Köhrmann, M.; Schellinger, P.D. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: Pro MR imaging. Radiology 2009, 251, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Rowley, H.A.; Lev, M.H. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: Pro CT. Radiology 2009, 251, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Rowland Hill, C. Imaging assessment of acute ischaemic stroke: A review of radiological methods. Br. J. Radiol. 2018, 91, 20170573. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Latchaw, R.E.; Alberts, M.J.; Lev, M.H.; Connors, J.J.; Harbaugh, R.E.; Higashida, R.T.; Hobson, R.; Kidwell, C.S.; Koroshetz, W.J.; Mathews, V.; et al. Recommendations for imaging of acute ischemic stroke: A scientific statement from the American Heart Association. Stroke 2009, 40, 3646–3678. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Maier, I.L.; Herpertz, G.U.; Bähr, M.; Psychogios, M.N.; Liman, J. What is the added value of CT-angiography in patients with transient ischemic attack? BMC Neurol. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- North America Symptomatic Carotid Endarterectomy Trial Steering Committee. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991, 22, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Verma, A.; Kim, I.J.; Kujbid, N.; Si, K.; Casaubon, L.K.; Kapral, M.K.; Fang, J.; Symons, S.; Swartz, R.H.; et al. Multidisciplinary quality improvement initiative to optimize acute neurovascular imaging for transient ischemic attack or minor stroke. Can. J. Emerg. Med. 2021, 23, 820–827. [Google Scholar] [CrossRef]
- Jing, J.; Suo, Y.; Wang, A.; Zuo, Y.; Jiang, Y.; Liu, L.; Zhao, X.; Wang, Y.; Li, Z.; Li, H.; et al. Imaging Parameters Predict Recurrence After Transient Ischemic Attack or Minor Stroke Stratified by ABCD(2) Score. Stroke 2021, 52, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Timpone, V.M.; Jensen, A.; Poisson, S.N.; Trivedi, P.S. Compliance with Imaging Guidelines for Workup of Transient Ischemic Attack: Evidence from the Nationwide Emergency Department Sample. Stroke 2020, 51, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; Grams, A.; Freyschlag, C.F.; Gummerer, M.; Knoflach, M. Management and prognosis of acute extracranial internal carotid artery occlusion. Ann. Transl. Med. 2020, 8, 1268. [Google Scholar] [CrossRef]
- Mayer, L.; Ferrari, J.; Krebs, S.; Boehme, C.; Toell, T.; Matosevic, B.; Tinchon, A.; Brainin, M.; Gattringer, T.; Sommer, P.; et al. ABCD3-I score and the risk of early or 3-month stroke recurrence in tissue- and time-based definitions of TIA and minor stroke. J. Neurol. 2018, 265, 530–534. [Google Scholar] [CrossRef]
- Huan, Y.; Chaoyang, Z.; Kai, D.; Chunhua, S.; Xin, Z.; Yue, Z. Predictive Value of Head-Neck CTA Combined with ABCD2 Scale Score for Patients with Cerebral Infarction of Vertebrobasilar Transient Ischemic Attack (TIA). Med. Sci. Monit. 2018, 24, 9001–9006. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Zhang, D.; Zhang, Y.; Deng, X.; Zhao, J. Comparison of Stroke Prediction Accuracy of ABCD2 and ABCD3-I in Patients with Transient Ischemic Attack: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2387–2395. [Google Scholar] [CrossRef]
- Knoflach, M.; Lang, W.; Seyfang, L.; Fertl, E.; Oberndorfer, S.; Daniel, G.; Seifert-Held, T.; Brainin, M.; Krebs, S.; Matosevic, B.; et al. Predictive value of ABCD2 and ABCD3-I scores in TIA and minor stroke in the stroke unit setting. Neurology 2016, 87, 861–869. [Google Scholar] [CrossRef]
- Kiyohara, T.; Kamouchi, M.; Kumai, Y.; Ninomiya, T.; Hata, J.; Yoshimura, S.; Ago, T.; Okada, Y.; Kitazono, T. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke 2014, 45, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Bjellerup, J.; Wester, P. Prediction of recurrent stroke with ABCD2 and ABCD3 scores in patients with symptomatic 50–99% carotid stenosis. BMC Neurol. 2014, 14, 223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Merwick, A.; Albers, G.W.; Amarenco, P.; Arsava, E.M.; Ay, H.; Calvet, D.; Coutts, S.B.; Cucchiara, B.L.; Demchuk, A.M.; Furie, K.L.; et al. Addition of brain and carotid imaging to the ABCD² score to identify patients at early risk of stroke after transient ischaemic attack: A multicentre observational study. Lancet Neurol. 2010, 9, 1060–1069. [Google Scholar] [CrossRef]
- Fothergill, A.; Christianson, T.J.; Brown, R.D., Jr.; Rabinstein, A.A. Validation and refinement of the ABCD2 score: A population-based analysis. Stroke 2009, 40, 2669–2673. [Google Scholar] [CrossRef] [PubMed]
- Hause, S.; Oldag, A.; Breja, A.; Neumann, J.; Wilcke, J.; Schreiber, S.; Heinze, H.J.; Skalej, M.; Halloul, Z.; Goertler, M. Acute symptomatic extracranial internal carotid occlusion-natural course and clinical impact. Vasa 2020, 49, 31–38. [Google Scholar] [CrossRef]
- Chaudhary, D.; Abedi, V.; Li, J.; Schirmer, C.M.; Griessenauer, C.J.; Zand, R. Clinical Risk Score for Predicting Recurrence Following a Cerebral Ischemic Event. Front. Neurol. 2019, 10, 1106. [Google Scholar] [CrossRef]
- Bibok, M.B.; Votova, K.; Balshaw, R.F.; Penn, M.; Lesperance, M.L.; Harris, D.R.; Sedgwick, C.; Nealis, M.; Farrell, B.; Mathieson, J.R.; et al. Retrospective evaluation of a clinical decision support tool for effective computed tomography angiography utilization in urgent brain imaging of suspected TIA/minor stroke in the emergency department. Can. J. Emerg. Med. 2019, 21, 343–351. [Google Scholar] [CrossRef]
- Uehara, T.; Ohara, T.; Minematsu, K.; Nagatsuka, K.; Toyoda, K. Predictors of Stroke Events in Patients with Transient Ischemic Attack Attributable to Intracranial Stenotic Lesions. Intern. Med. 2018, 57, 295–300. [Google Scholar] [CrossRef]
- Kvickström, P.; Lindblom, B.; Bergström, G.; Zetterberg, M. Amaurosis fugax: Risk factors and prevalence of significant carotid stenosis. Clin. Ophthalmol. 2016, 10, 2165–2170. [Google Scholar] [CrossRef]
- Smit, R.L.; Baarsma, G.S.; Koudstaal, P.J. The source of embolism in amaurosis fugax and retinal artery occlusion. Int. Ophthalmol. 1994, 18, 83–86. [Google Scholar] [CrossRef]
- Purroy, F.; Jiménez Caballero, P.E.; Gorospe, A.; Torres, M.J.; Alvarez-Sabin, J.; Santamarina, E.; Martínez-Sánchez, P.; Cánovas, D.; Freijo, M.J.; Egido, J.A.; et al. Recurrent transient ischaemic attack and early risk of stroke: Data from the PROMAPA study. J. Neurol. Neurosurg. Psychiatry 2013, 84, 596–603. [Google Scholar] [CrossRef] [PubMed]
| Major Pathology n = 336 (22%) | Minor Pathology n = 1190 (78%) | p-Value | |
|---|---|---|---|
| Age, years ± SD | 72 ± 8 | 63 ± 9 | 0.011 |
| Sex (male, %) | 68% | 51% | 0.028 |
| NIHSS | 0 | 0 | 0.729 |
| Duration of symptoms >60 min | 45% | 59% | <0.001 |
| Arterial hypertension | 85% | 65% | <0.001 |
| Hyperlipidemia | 75% | 61% | <0.001 |
| Diabetes mellitus | 27% | 24% | 0.734 |
| Coronary heart disease | 27% | 12% | 0.001 |
| Obesity | 22% | 19% | 0.243 |
| History of myocardial infarction | 19% | 12% | 0.016 |
| Atrial fibrillation | 23% | 24% | 0.422 |
| History of Atherosclerotic peripheral vascular disease | 21% | 11% | <0.001 |
| History of smoking | 32% | 18% | 0.013 |
| Antiplatelet medication | 61% | 38% | <0.001 |
| DOAK | 7% | 8% | 0.192 |
| Marcumar | 10% | 9% | 0.433 |
| History of stroke | 29% | 18% | 0.013 |
| History of TIA | 34% | 21% | 0.021 |
| ABCD2 | 4 (3–5) | 5 (4–6) | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altenbernd, J.-C.; Gramada, R.; Kessler, E.; Skatulla, J.; Geppert, E.; Eyding, J.; Nordmeyer, H. When Can an Emergency CTA Be Dispensed with for TIA Patients? J. Clin. Med. 2022, 11, 5686. https://doi.org/10.3390/jcm11195686
Altenbernd J-C, Gramada R, Kessler E, Skatulla J, Geppert E, Eyding J, Nordmeyer H. When Can an Emergency CTA Be Dispensed with for TIA Patients? Journal of Clinical Medicine. 2022; 11(19):5686. https://doi.org/10.3390/jcm11195686
Chicago/Turabian StyleAltenbernd, Jens-Christian, Razvan Gramada, Eugen Kessler, Jakob Skatulla, Eduard Geppert, Jens Eyding, and Hannes Nordmeyer. 2022. "When Can an Emergency CTA Be Dispensed with for TIA Patients?" Journal of Clinical Medicine 11, no. 19: 5686. https://doi.org/10.3390/jcm11195686
APA StyleAltenbernd, J.-C., Gramada, R., Kessler, E., Skatulla, J., Geppert, E., Eyding, J., & Nordmeyer, H. (2022). When Can an Emergency CTA Be Dispensed with for TIA Patients? Journal of Clinical Medicine, 11(19), 5686. https://doi.org/10.3390/jcm11195686







