Reticulocyte Hemoglobin-Equivalent Potentially Detects, Diagnoses and Discriminates between Stages of Iron Deficiency with High Sensitivity and Specificity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Blood Collection and Measurements
2.3. Definition of Anemia & Iron Deficiency Anemia
2.4. Statistical Analysis
3. Results
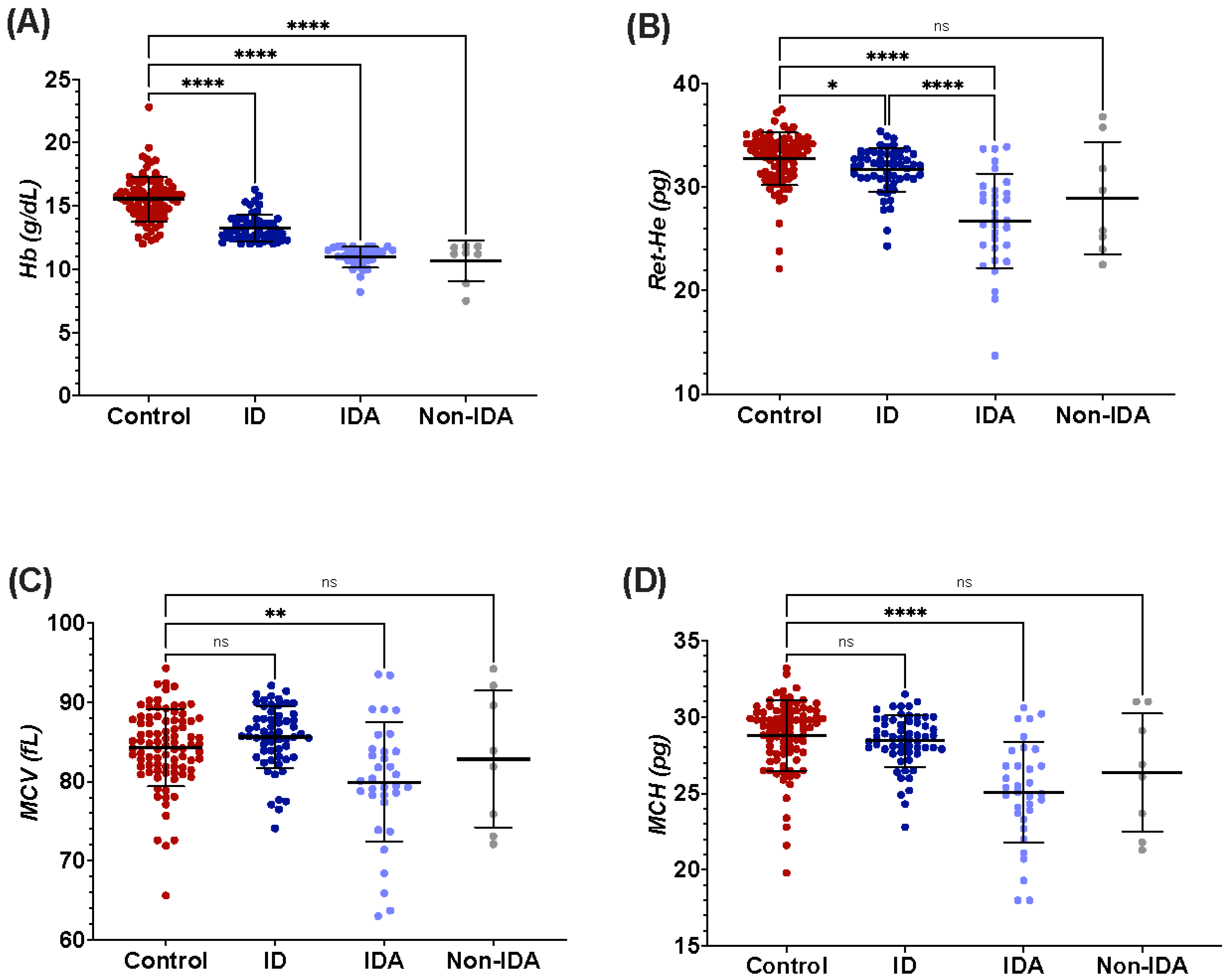
3.1. Participants’ Demographic and Clinical Information
3.2. Relationship between Ret-He and Iron Biomarkers
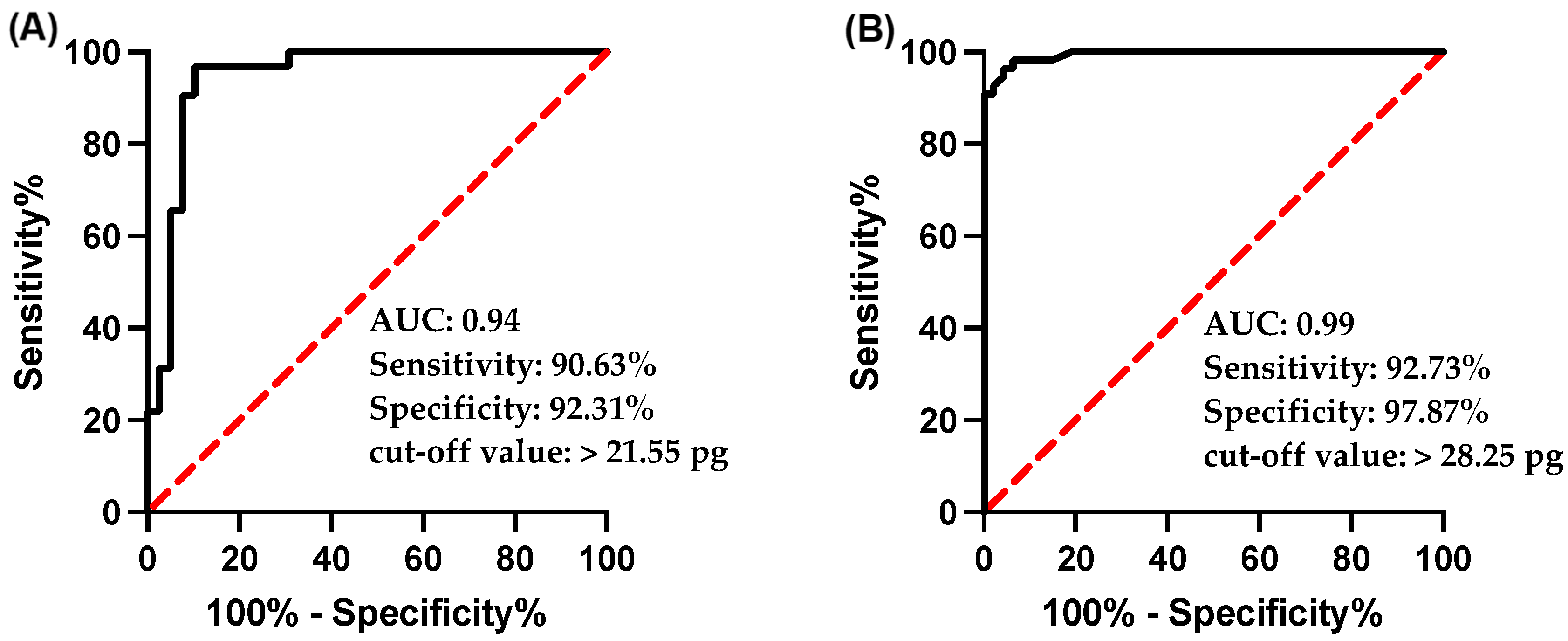
3.3. Ret-He as a Diagnostic Marker for ID and IDA
3.4. Ret-He as a Marker for Detecting Early Iron Deficiency
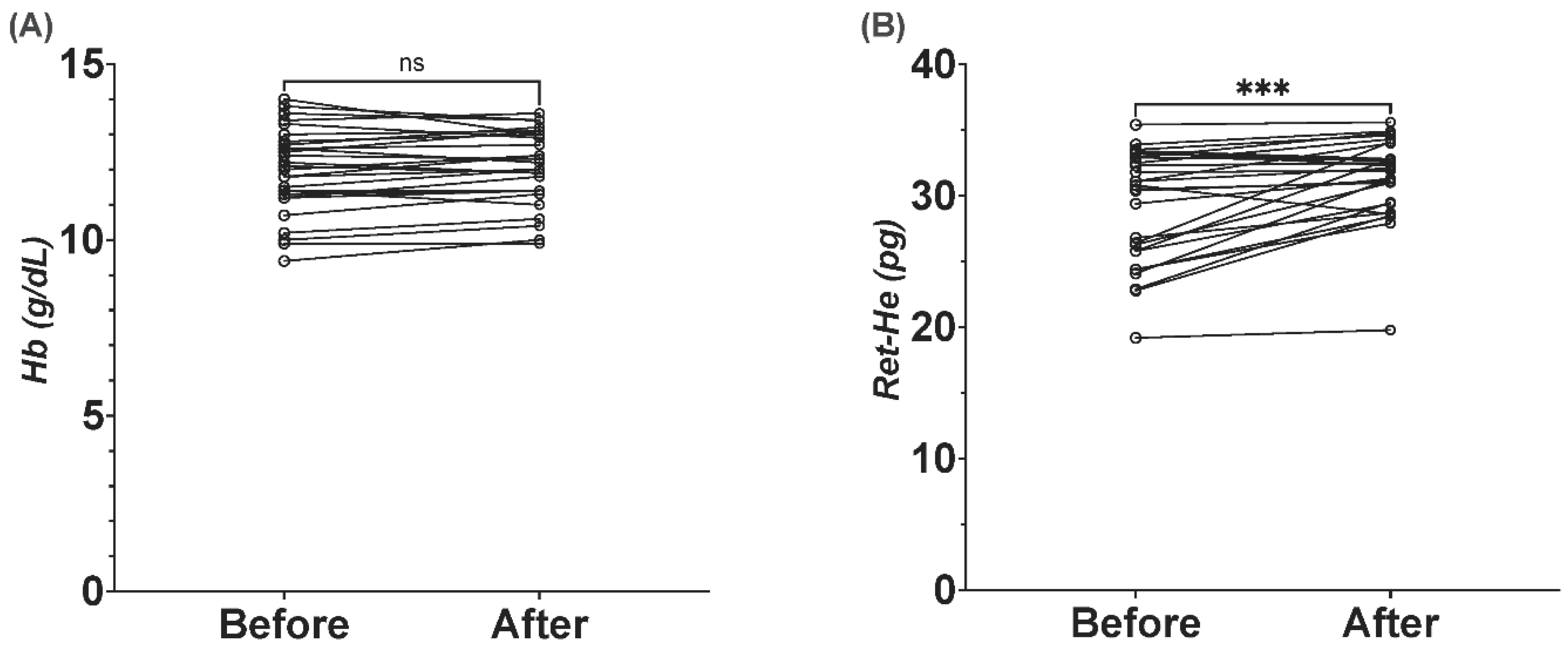
3.5. Ret-He as Marker for Response to Iron Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Benoist, B.; World Health Organization; Centers for Disease Control and Prevention (U.S.). Worldwide prevalence of anaemia 1993–2005. In WHO Global Database of Anaemia; World Health Organization: Geneva, Switzerland, 2008; Available online: http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf (accessed on 31 January 2020).
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Miller, J.L. Iron Deficiency Anemia: A Common and Curable Disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef] [PubMed]
- Verster, A.; Vander Pols, J.C. Anaemia in the Eastern Mediterranean Region. 1995. Available online: https://apps.who.int/iris/handle/10665/119272 (accessed on 11 January 2021).
- Musaiger, A.O. Iron deficiency anaemia among children and pregnant women in the Arab Gulf countries: The need for action. Nutr. Health 2002, 16, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, N.N. The prevalence of iron deficiency anemia in a Saudi University female students. J. Microsc. Ultrastruct. 2015, 3, 25–28. [Google Scholar] [CrossRef]
- AlSheikh, M.H. Prevalence and Risk Factors of Iron-Deficiency Anemia in Saudi Female Medical Students. 2018. Available online: https://www.saudijhealthsci.org/article.asp?issn=2278-0521;year=2018;volume=7;issue=3;spage=148;epage=152;aulast=AlSheikh (accessed on 11 January 2021).
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Hung, S.-C.; Tarng, D.-C. Bone Marrow Iron in CKD: Correlation With Functional Iron Deficiency. Am. J. Kidney Dis. 2010, 55, 617–621. [Google Scholar] [CrossRef]
- Daru, J.; Colman, K.; Stanworth, S.J.; de la Salle, B.; Wood, E.M.; Pasricha, S.-R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1634S–1639S. [Google Scholar] [CrossRef]
- Rocha, L.A.; Barreto, D.V.; Barreto, F.C.; Dias, C.B.; Moysés, R.; Silva, M.R.R.; Moura, L.A.; Draibe, S.A.; Jorgetti, V.; Carvalho, A.B.; et al. Serum Ferritin Level Remains a Reliable Marker of Bone Marrow Iron Stores Evaluated by Histomorphometry in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Hernandez, C.J.; Bruce, L.J. Reticulocyte Maturation. Membranes 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C.; Laufer, M.R.; Friedman, A.J.; Bridges, K.; Platt, O. Reticulocyte hemoglobin content (CHr): Early indicator of iron deficiency and response to therapy. Blood 1994, 83, 3100–3101. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C.; Schiller, B.; Moran, J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin. Lab. Haematol. 2006, 28, 303–308. [Google Scholar] [CrossRef]
- Brugnara, C.; Zurakowski, D.; DiCanzio, J.; Boyd, T.; Platt, O. Reticulocyte hemoglobin content to diagnose iron deficiency in children. JAMA 1999, 281, 2225–2230. [Google Scholar] [CrossRef]
- Brugnara, C. Iron deficiency and erythropoiesis: New diagnostic approaches. Clin. Chem. 2003, 49, 573–1578. [Google Scholar] [CrossRef]
- Choudhary, M.; Sharma, D.; Shekhawat, D.S.; Dabi, D. Significance of Red Cell Distribution Width in the Diagnosis of Iron Deficiency Anemia: An Observational Study from India. J. Pediatr. Neonatal Care 2015, 3, 00102. [Google Scholar] [CrossRef][Green Version]
- Rahman, S. Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. In Assessing the Iron Status of Populations: Including Literature Reviews: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April 2004; World Health Organization: Geneva, Switzerland, 2007; Available online: https://apps.who.int/iris/handle/10665/75368 (accessed on 28 July 2022).
- Buttarello, M.; Temporin, V.; Ceravolo, R.; Farina, G.; Bulian, P. The New Reticulocyte Parameter (RET-Y) of the Sysmex XE 2100: Its Use in the Diagnosis and Monitoring of Posttreatment Sideropenic Anemia. Am. J. Clin. Pathol. 2004, 121, 489–495. [Google Scholar] [CrossRef]
- Soppi, E.T. Iron deficiency without anemia—A clinical challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef]
- Mast, A.E.; Blinder, M.A.; Gronowski, A.M.; Chumley, C.; Scott, M.G. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin. Chem. 1998, 44, 45–51. [Google Scholar] [CrossRef]
- Al-Quaiz, J.M. Iron deficiency anemia. A study of risk factors. Saudi Med. J. 2001, 22, 490–496. [Google Scholar]
- Aedh, A.; Elfaki, N.; Sounni, E. Iron Deficiency Anemia and Associated Risk Factors among Teenagers in Najran, Saudi Arabia. Int. J. Med. Res. Health Sci. 2019, 8, 108–114. [Google Scholar]
- Ahluwalia, N. Diagnostic utility of serum transferrin receptors measurement in assessing iron status. Nutr. Rev. 1998, 56 Pt 1, 133–141. [Google Scholar] [CrossRef]
- Ansari, T.; Ali, L.; Aziz, T.; Ara, J.; Liaquat, N.; Tahir, H. Nutritional iron deficiency in women of child bearing age--what to do? J. Ayub Med. Coll. Abbottabad JAMC 2009, 21, 17–20. [Google Scholar] [PubMed]
- Hamali, H.A.; Mobarki, A.A.; Saboor, M.; Alfeel, A.; Madkhali, A.M.; Akhter, M.S.; Dobie, G. Prevalence of Anemia Among Jazan University Students. Int. J. Gen. Med. 2020, 13, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamea, L.; Woodman, A.; Elnagi, E.A.; Al-Amri, S.S.; Al-Zahrani, A.A.; Al-shammari, N.H.; Al-zahrani, R.A.; Al-Yami, F.S.; Al-Ameri, S.A. Prevalence of Iron-deficiency anemia and its associated risk factors in female undergraduate students at prince sultan military college of health sciences. J. Appl. Hematol. 2019, 10, 126. [Google Scholar] [CrossRef]
- Alswailem, A.M.; Alahmad, S.M.; Alshehri, M.A. The Prevalence of Iron Deficiency Anemia and its Associated Risk Factors among a Sample of Females in Riyadh, Saudi Arabia. Egypt. J. Hosp. Med. 2018, 72, 4625–4629. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef]
- Toki, Y.; Ikuta, K.; Kawahara, Y.; Niizeki, N.; Kon, M.; Enomoto, M.; Tada, Y.; Hatayama, M.; Yamamoto, M.; Ito, S. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron deficiency. Int. J. Hematol. 2017, 106, 116–125. [Google Scholar] [CrossRef]
- Uçar, M.A.; Falay, M.; Dağdas, S.; Ceran, F.; Urlu, S.M.; Özet, G. The Importance of RET-He in the Diagnosis of Iron Deficiency and Iron Deficiency Anemia and the Evaluation of Response to Oral Iron Therapy. J. Med. Biochem. 2019, 38, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Bhardwaj, G.; Arora, D.; Aggarwal, G.; Pabbi, S.; Dara, R.C.; Sachdev, R.; Raizada, A.; Sethi, M. Applying newer parameter Ret-He (reticulocyte haemoglobin equivalent) to assess latent iron deficiency (LID) in blood donors–study at a tertiary care hospital in India. Vox Sang. 2018, 113, 639–646. [Google Scholar] [CrossRef] [PubMed]




| Variable | Gender and Age | Control Group (n = 87) | ID Group (n = 55) | IDA Group (n = 32) | Non-IDA Group (n = 8) |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Gender | Male | 75 (86.2) | 8 (14.5) | 0 (0) | 1 (12.5) |
| Female | 12 (13.8) | 47 (85.5) | 32 (100) | 7 (78.5) | |
| Age | 18–29 | 85 (97.7) | 53 (96.4) | 29 (90.6) | 5 (62.5) |
| 30–49 | 2 (2.3) | 2 (3.6) | 3 (9.4) | 1 (12.5) | |
| >50 | 2 (25) | ||||
| Parameters | Control (n = 87) | ID (n = 55) | IDA (n = 32) | Non-IDA (n = 8) | p-Value | |
|---|---|---|---|---|---|---|
| Hematological Parameters | Hb (g/dL) | 15.6 ± 1.8 | 13.3 ± 1.1 | 11.0 ± 0.8 | 10.7 ± 1.6 | <0.001 |
| MCV (fL) | 84.3 ± 4.9 | 85.5 ± 4.0 | 79.7 ± 7.6 | 82.9 ± 8.6 | <0.0001 | |
| MCH (pg) | 28.8 ± 2.3 | 28.4 ± 1.7 | 25.0 ± 3.3 | 26.4 ± 3.9 | <0.0001 | |
| Ret-He (pg) | 32.7 ± 2.7 | 31.7 ± 2.1 | 26.7 ± 4.5 | 29.0 ± 5.4 | <0.0001 | |
| Iron Parameters | Ferritin (ng/mL) | 71.1 ± 34.6 | 14.5 ± 7.8 | 8.7 ± 6.0 | 204.8 ± 288.0 | <0.0001 |
| Serum Fe (umol/L) | 16.9 ± 8.2 | 16.3 ± 15.3 | 8.0 ± 4.2 | 8.0 ± 6.8 | <0.0001 | |
| TIBC (umol/L) | 60.1 ± 6.6 | 65.4 ± 6.8 | 66.4 ± 13.3 | 50.6 ± 11.6 | <0.0001 | |
| TS (%) | 28.3 ± 13.7 | 25.8 ± 26.4 | 13.0 ± 8.9 | 0.9 ± 2.1 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almashjary, M.N.; Barefah, A.S.; Bahashwan, S.; Ashankyty, I.; ElFayoumi, R.; Alzahrani, M.; Assaqaf, D.M.; Aljabri, R.S.; Aljohani, A.Y.; Muslim, R.; et al. Reticulocyte Hemoglobin-Equivalent Potentially Detects, Diagnoses and Discriminates between Stages of Iron Deficiency with High Sensitivity and Specificity. J. Clin. Med. 2022, 11, 5675. https://doi.org/10.3390/jcm11195675
Almashjary MN, Barefah AS, Bahashwan S, Ashankyty I, ElFayoumi R, Alzahrani M, Assaqaf DM, Aljabri RS, Aljohani AY, Muslim R, et al. Reticulocyte Hemoglobin-Equivalent Potentially Detects, Diagnoses and Discriminates between Stages of Iron Deficiency with High Sensitivity and Specificity. Journal of Clinical Medicine. 2022; 11(19):5675. https://doi.org/10.3390/jcm11195675
Chicago/Turabian StyleAlmashjary, Majed N., Ahmed S. Barefah, Salem Bahashwan, Ibraheem Ashankyty, Refaat ElFayoumi, Majed Alzahrani, Duaa M. Assaqaf, Raghad S. Aljabri, Amera Y. Aljohani, Rema Muslim, and et al. 2022. "Reticulocyte Hemoglobin-Equivalent Potentially Detects, Diagnoses and Discriminates between Stages of Iron Deficiency with High Sensitivity and Specificity" Journal of Clinical Medicine 11, no. 19: 5675. https://doi.org/10.3390/jcm11195675
APA StyleAlmashjary, M. N., Barefah, A. S., Bahashwan, S., Ashankyty, I., ElFayoumi, R., Alzahrani, M., Assaqaf, D. M., Aljabri, R. S., Aljohani, A. Y., Muslim, R., Baawad, S. A., Bawazir, W. M., & Alharthy, S. A. (2022). Reticulocyte Hemoglobin-Equivalent Potentially Detects, Diagnoses and Discriminates between Stages of Iron Deficiency with High Sensitivity and Specificity. Journal of Clinical Medicine, 11(19), 5675. https://doi.org/10.3390/jcm11195675






