Clinical Significance of Adenosine-Induced Atrial Fibrillation after Complete Pulmonary Vein Isolation
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Design
2.2. Electrophysiologic Studies and Catheter Ablation
2.3. Adenosine Testing
2.4. Post Ablation Follow-Up and Definition of Recurrence
2.5. Statistical Analysis
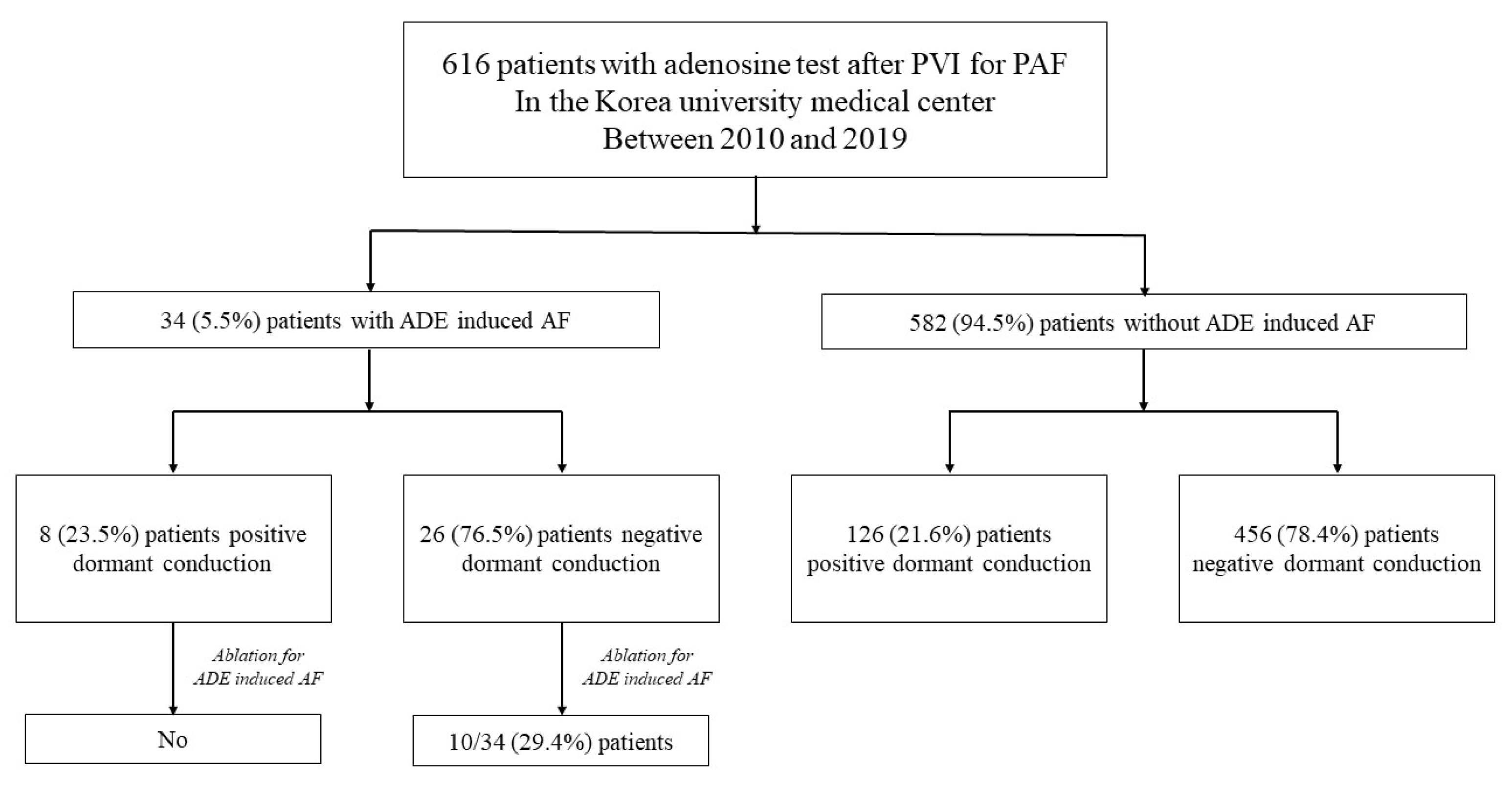
3. Results
3.1. Baseline Characteristics
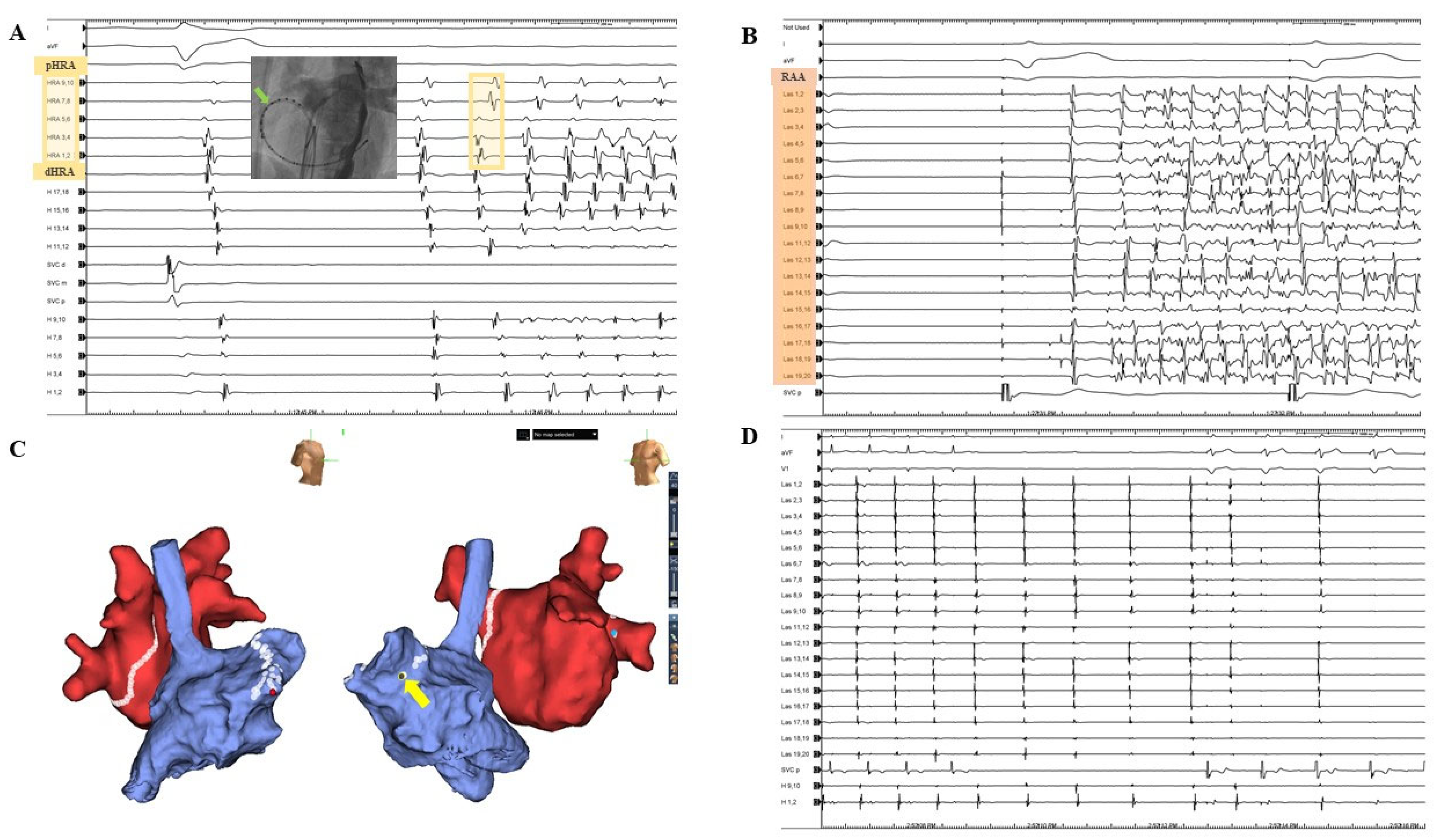
3.2. Dormant Conduction and Adenosine-Induced Atrial Fibrillation
3.3. Recurrence of Atrial Arrhythmia after RFCA
4. Discussion
4.1. Adenosine-Induced Atrial Fibrillation and Right Atrium
4.2. Sympathetic Excitatory Effect
4.3. Comparison to Isoproterenol for Confirming Non-PV Trigger
4.4. Clinical Implications
4.5. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, G.; Sinner, M.F.; Lutz, M.; Müller-Nurasyid, M.; Kääb, S.; Reinecke, H. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: A nationwide in-hospital analysis of administrative data for Germany in 2014. Eur. Heart J. 2018, 39, 4020–4029. [Google Scholar] [CrossRef] [PubMed]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010, 303, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kardiol. Pol. 2016, 74, 1359–1469. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Tabrizi, F.; Englund, A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: Data from Swedish health registries. Eur. Heart J. 2016, 37, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Choi, J.I.; Boo, K.Y.; Kim, D.Y.; Oh, S.K.; Park, H.S.; Lee, K.N.; Shim, J.; Kim, J.S.; Park, S.W.; et al. Clinical and Echocardiographic Risk Factors Predict Late Recurrence after Radiofrequency Catheter Ablation of Atrial Fibrillation. Sci. Rep. 2019, 9, 6890. [Google Scholar] [CrossRef] [PubMed]
- Elayi, C.S.; Di Biase, L.; Barrett, C.; Ching, C.K.; al Aly, M.; Lucciola, M.; Bai, R.; Horton, R.; Fahmy, T.S.; Verma, A.; et al. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm. 2010, 7, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.J.; Kumar, S.; Smith, C.; Morton, J.B.; Kalman, J.M.; Kistler, P.M. The role of adenosine following pulmonary vein isolation in patients undergoing catheter ablation for atrial fibrillation: A systematic review. J. Cardiovasc. Electrophysiol. 2013, 24, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N.; Roh, S.Y.; Baek, Y.S.; Park, H.S.; Ahn, J.; Kim, D.H.; Lee, D.I.; Shim, J.; Choi, J.I.; Park, S.W.; et al. Long-Term Clinical Comparison of Procedural End Points After Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation: Elimination of Nonpulmonary Vein Triggers Versus Noninducibility. Circ. Arrhythm. Electrophysiol. 2018, 11, e005019. [Google Scholar] [CrossRef] [PubMed]
- Kuroi, A.; Miyazaki, S.; Usui, E.; Ichihara, N.; Kanaji, Y.; Takagi, T.; Iwasawa, J.; Nakamura, H.; Taniguchi, H.; Hachiya, H. Adenosine-Provoked Atrial Fibrillation Originating From Non–Pulmonary Vein Foci: The Clinical Significance and Outcome After Catheter Ablation. JACC Clin. Electrophysiol. 2015, 1, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, M.; Yamamoto, M.; Himi, T.; Kobayashi, Y. Long-term outcome of adenosine-induced atrial fibrillation after atrial fibrillation ablation: A propensity score matching analysis. Pacing Clin. Electrophysiol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Hansen, B.J.; Fedorenko, O.; Csepe, T.A.; Kalyanasundaram, A.; Li, N.; Hage, L.T.; Glukhov, A.V.; Billman, G.E.; Weiss, R.; et al. Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation 2014, 130, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation 2005, 112, 3697–3706. [Google Scholar] [CrossRef]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Sul, L.V.; Kalyanasundaram, A.; Zakharkin, S.O.; Zhao, J.; Guha, A.; Van Wagoner, D.R.; Kilic, A.; et al. Adenosine-Induced Atrial Fibrillation: Localized Reentrant Drivers in Lateral Right Atria due to Heterogeneous Expression of Adenosine A1 Receptors and GIRK4 Subunits in the Human Heart. Circulation 2016, 134, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Po, S.S.; Scherlag, B.J.; Yamanashi, W.S.; Edwards, J.; Zhou, J.; Wu, R.; Geng, N.; Lazzara, R.; Jackman, W.M. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm 2006, 3, 201–208. [Google Scholar] [CrossRef]
- Bettoni, M.; Zimmermann, M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002, 105, 2753–2759. [Google Scholar] [CrossRef]
- Biaggioni, I.; Killian, T.J.; Mosqueda-Garcia, R.; Robertson, R.M.; Robertson, D. Adenosine increases sympathetic nerve traffic in humans. Circulation 1991, 83, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Tutuianu, C.; Pap, R.; Riesz, T.; Bencsik, G.; Makai, A.; Saghy, L. Is adenosine useful for the identification of atrial fibrillation triggers? J. Cardiovasc. Electrophysiol. 2019, 30, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Elayi, C.S.; Di Biase, L.; Bai, R.; Burkhardt, J.D.; Mohanty, P.; Santangeli, P.; Sanchez, J.; Hongo, R.; Gallinghouse, G.J.; Horton, R.; et al. Administration of isoproterenol and adenosine to guide supplemental ablation after pulmonary vein antrum isolation. J. Cardiovasc. Electrophysiol. 2013, 24, 1199–1206. [Google Scholar] [CrossRef] [PubMed]


| Variables | AIAF | Non-AIAF | p-Value |
|---|---|---|---|
| (n = 34) | (n = 582) | ||
| Age, years | 53.9 ± 12.7 | 56.2 ± 10.9 | 0.305 |
| Male, n (%) | 28 (82.4) | 440 (75.6) | 0.370 |
| Bodyweight (kg) | 68.6 ± 10.9 | 71.6 ± 27.1 | 0.170 |
| Height (cm) | 169.6 ± 7.7 | 168.4 ± 8.8 | 0.420 |
| Body mass index (kg/m2) | 23.7 ± 2.6 | 25.2 ± 9.8 | 0.017 |
| Congestive Heart Failure, n (%) | 1 (2.9) | 23 (4.0) | 0.767 |
| Hypertension, n (%) | 9 (26.5) | 208 (35.7) | 0.271 |
| Diabetes mellitus, n (%) | 3 (8.8) | 43 (7.4) | 0.757 |
| Previous stroke, n (%) | 1 (2.9) | 47 (8.1) | 0.278 |
| Vascular disease, n (%) | 2 (5.9) | 24 (4.1) | 0.620 |
| CHA2DS2-VASc score | 0.9 ± 1.2 | 1.1 ± 1.1 | 0.195 |
| Left ventricular ejection fraction (%) | 57.4 ± 1.7 | 55.5 ± 4.8 | <0.001 |
| Left atrial diameter (mm) | 37.0 ± 4.5 | 39.0 ± 5.3 | 0.014 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% Cl) | p-Value | |
| Age ≥ 65 (years) | 1.02 (0.65–1.60) | 0.940 | ||
| Male sex | 1.03 (0.66–1.63) | 0.885 | ||
| Body mass index | 0.99 (0.95–1.04) | 0.778 | ||
| Congestive heart failure | 2.63 (0.95–7.24) | 0.062 | ||
| Hypertension | 0.85 (0.57–1.28) | 0.435 | ||
| Diabetes mellitus | 0.93 (0.43–2.01) | 0.853 | ||
| Stroke/TIA | 1.20 (0.64–2.25) | 0.562 | ||
| Vascular disease | 1.33 (0.64–2.76) | 0.438 | ||
| ADE induced AF | 0.90 (0.37–2.22) | 0.827 | ||
| LA diameter ≥ 40 (mm) | 1.64 (1.12–2.41) | 0.011 | 2.10 (1.36–3.27) | 0.001 |
| LVEF < 50 (%) | 1.15 (0.64–2.06) | 0.647 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.Y.; Shim, J.; Kim, Y.G.; Min, K.; Roh, S.-Y.; Kim, J.S.; Choi, J.-I.; Kim, Y.-H. Clinical Significance of Adenosine-Induced Atrial Fibrillation after Complete Pulmonary Vein Isolation. J. Clin. Med. 2022, 11, 5679. https://doi.org/10.3390/jcm11195679
Choi YY, Shim J, Kim YG, Min K, Roh S-Y, Kim JS, Choi J-I, Kim Y-H. Clinical Significance of Adenosine-Induced Atrial Fibrillation after Complete Pulmonary Vein Isolation. Journal of Clinical Medicine. 2022; 11(19):5679. https://doi.org/10.3390/jcm11195679
Chicago/Turabian StyleChoi, Yun Young, Jaemin Shim, Yun Gi Kim, Kyongjin Min, Seung-Young Roh, Jin Seok Kim, Jong-Il Choi, and Young-Hoon Kim. 2022. "Clinical Significance of Adenosine-Induced Atrial Fibrillation after Complete Pulmonary Vein Isolation" Journal of Clinical Medicine 11, no. 19: 5679. https://doi.org/10.3390/jcm11195679
APA StyleChoi, Y. Y., Shim, J., Kim, Y. G., Min, K., Roh, S.-Y., Kim, J. S., Choi, J.-I., & Kim, Y.-H. (2022). Clinical Significance of Adenosine-Induced Atrial Fibrillation after Complete Pulmonary Vein Isolation. Journal of Clinical Medicine, 11(19), 5679. https://doi.org/10.3390/jcm11195679






