Hochuekkito Combined with Pulmonary Rehabilitation in Apathetic Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
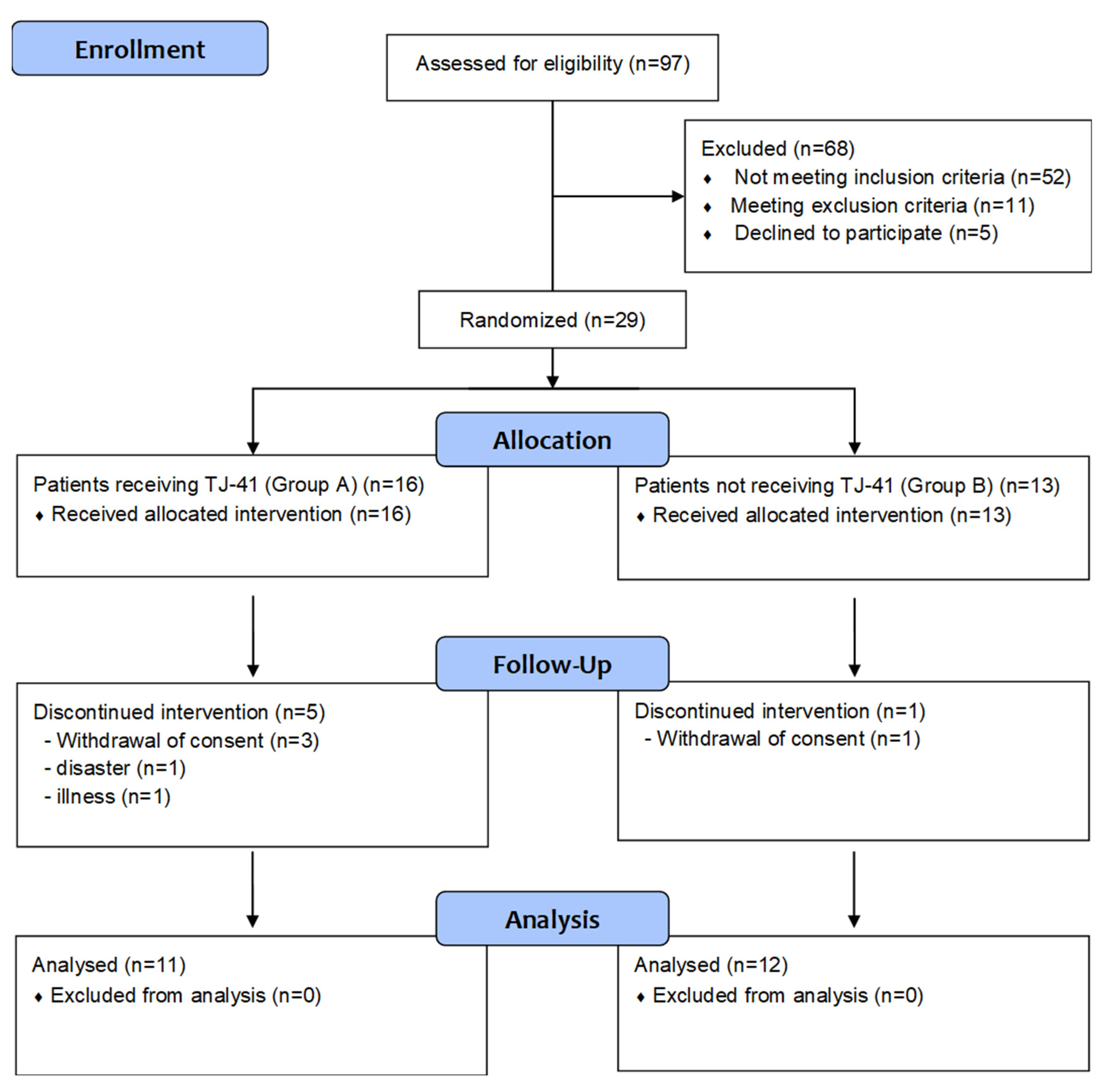
2.2. Patients
2.3. Study Protocol
2.4. Test Drug
2.5. Outcome Measurements
2.6. Safety Measurements
2.7. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
3.2. Outcomes
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease, Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2022. Available online: http://www.goldcopd.org (accessed on 14 August 2022).
- Waschki, B.; Kirsten, A.; Holz, O.; Müller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Santos, E.; Frei, A.; Steurer-Stey, C.; de Batlle, J.; Rabinovich, R.A.; Raste, Y.; Hopkinson, N.S.; Polkey, M.I.; van Remoortel, H.; Troosters, T.; et al. Determinants and outcomes of physical activity in patients with COPD: A systematic review. Thorax 2014, 69, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Sekikawa, K.; Murakami, I.; Aimoto, K.; Kagawa, K.; Sumigawa, T.; Okusaki, K.; Dodo, T.; Awaya, Y.; Watanabe, M.; et al. Effects of Hochuekkito combined with pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Exp. Ther. Med. 2018, 16, 5236–5242. [Google Scholar] [CrossRef]
- Schneider, C.; Jick, S.S.; Bothner, U.; Meier, C.R. COPD and the risk of depression. Chest 2010, 137, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Müllerova, H.; Locantore, N.W.; Vestbo, J.; Watkins, M.L.; Wouters, E.F.; Rennard, S.I.; Sharafkhaneh, A. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators, Determinants of depression in the Eclipse chronic obstructive pulmonary disease cohort. Am. J. Respir. Crit. Care Med. 2011, 183, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Zhu, Y.; Chen, P.; Zhang, P.; Yu, J.; Zhang, N.; Chen, N.; Zhang, L.; Wu, H.; Zhao, J. Prevalence and correlations with depression, anxiety, and other features in outpatients with chronic obstructive pulmonary disease in China: A cross-sectional case control study. BMC Pulm. Med. 2012, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Tohda, M.; Mingmalairak, S. Evidence of antidepressive effects of a Wakan-yaku, Hochuekkito, in depression model mice with learned-helplessness behavior. Evid. Based Complement. Altern. Med. 2013, 2013, 319073. [Google Scholar] [CrossRef]
- Levy, M.L.; Cummings, J.L.; Fairbanks, L.A.; Masterman, D.; Miller, B.L.; Craig, A.H.; Paulsen, J.S.; Litvan, I. Apathy is not depression. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 314–319. [Google Scholar] [CrossRef]
- Van Reekum, R.; Stuss, D.T.; Ostrander, L. Apathy: Why care? J. Neuropsychiatry Clin. Neurosci. 2005, 17, 7–19. [Google Scholar] [CrossRef]
- Tay, J.; Morris, R.G.; Markus, H.S. Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment. Int. J. Stroke 2021, 16, 510–518. [Google Scholar] [CrossRef]
- Ishii, S.; Weintraub, N.; Mervis, J.R. Apathy: A common psychiatric syndrome in the elderly. J. Am. Med. Dir. Assoc. 2009, 10, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.A.; Richard, E.; Fransen, N.L.; Eurelings, L.S.; Beem, L.; Eikelenboom, P.; van Gool, W.A.; Moll van Charante, E.P. Association of vascular factors with apathy in community-dwelling elderly individuals. Arch. Gen. Psychiatry 2012, 69, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Padala, P.R.; Desouza, C.V.; Almeida, S.; Shivaswamy, V.; Ariyarathna, K.; Rouse, L.; Burke, W.J.; Petty, F. The impact of apathy on glycemic control in diabetes: A cross-sectional study. Diabetes Res. Clin. Pract. 2008, 79, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.G.; Nelson, M.E.; Mace, J.L.; Davis, W.A.; Davis, T.M.E.; Starkstein, S.E. Apathy in older patients with type 2 diabetes. Am. J. Geriatr. Psychiatry 2015, 23, 615–621. [Google Scholar] [CrossRef]
- Takayama, S.; Kikuchi, A.; Makino, T.; Kainuma, M.; Namiki, T.; Ito, T. Basic pharmacological mechanisms and clinical evidence of the efficacy of hochuekkito against infectious diseases and its potential for use against COVID-19. Tradit. Kampo Med. 2021, 8, 3–21. [Google Scholar] [CrossRef]
- Tatsumi, K. Clinical usefulness of hochuekkito in patients with COPD. Kampo Med. 2021, 62, 329–336. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Belman, M.J. Exercise in patients with chronic obstructive pulmonary disease. Thorax 1993, 48, 936–946. [Google Scholar] [CrossRef]
- Hama, S.; Yamashita, H.; Shigenobu, M.; Watanabe, A.; Hiramoto, K.; Kurisu, K.; Yamawaki, S.; Kitaoka, T. Depression or apathy and functional recovery after stroke. Int. J. Geriatr. Psychiatry 2007, 22, 1046–1051. [Google Scholar] [CrossRef]
- Tokunaga, S.; Hotta, K.; Fujii, K.; Iwai, K.; Matsuda, T.; Fujita, Y.; Wakayama, S.; Okura, T. Impact of apathy on physical activity in community-dwelling elderly. Jpn. J. Health Promot. Phys. Ther. 2020, 10, 73–79. (In Japanese) [Google Scholar] [CrossRef]
- Starkstein, S.E.; Fedoroff, J.P.; Price, T.R.; Leiguarda, R.; Robinson, R.G. Apathy following cerebrovascular lesions. Stroke 1993, 24, 1625–1630. [Google Scholar] [CrossRef]
- Okada, K.; Kobayashi, S.; Yamagata, S.; Takahashi, K.; Yamaguchi, S. Poststroke apathy and regional cerebral blood flow. Stroke 1997, 28, 2437–2441. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Kohno, N.; Abe, S.; Toyoda, G.; Oguro, H.; Bokura, H.; Yamaguchi, S. Successful treatment of post-stroke apathy by the dopamine receptor agonist ropinirole. J. Clin. Neurosci. 2010, 17, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Miller, A.H. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Front. Neuroendocrinol. 2012, 33, 315–327. [Google Scholar] [CrossRef]
- Tatsumi, K.; Shinozuka, N.; Nakayama, K.; Sekiya, N.; Kuriyama, T.; Fukuchi, Y. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary disease. J. Am. Geriatr. Soc. 2009, 57, 169–170. [Google Scholar] [CrossRef]
- Isago, H.; Mitani, A.; Kohno, S.; Nagoshi, S.; Ishimori, T.; Saito, M.; Tamiya, H.; Miyashita, N.; Ishii, T.; Matsuzaki, H.; et al. The Japanese herbal (Kampo) medicine Hochuekkito attenuates lung inflammation in lung emphysema. Biol. Pharm. Bull. 2021, 44, 39–45. [Google Scholar] [CrossRef]
- Mantoani, L.C.; Rubio, N.; McKinstry, B.; MacNee, W.; Rabinovich, R.A. Interventions to modify physical activity in patients with COPD: A systematic review. Eur. Respir. J. 2016, 48, 69–81. [Google Scholar] [CrossRef]
- Robinson, S.A.; Shimada, S.L.; Quigley, K.S.; Moy, M.L. A web-based physical activity intervention benefits persons with low self-efficacy in COPD: Results from a randomized controlled trial. J. Behav. Med. 2019, 42, 1082–1090. [Google Scholar] [CrossRef]
- Watz, H.; Troosters, T.; Beeh, K.M.; Garcia-Aymerich, J.; Paggiaro, P.; Molins, E.; Notari, M.; Zapata, A.; Jarreta, D.; Garcia Gil, E. ACTIVATE: The effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2545–2558. [Google Scholar] [CrossRef]
- Troosters, T.; Maltais, F.; Leidy, N.; Lavoie, K.L.; Sedeno, M.; Janssens, W.; Garcia-Aymerich, J.; Erzen, D.; De Sousa, D.; Korducki, L.; et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1021–1032. [Google Scholar] [CrossRef]
- Sewell, L.; Singh, S.J.; Williams, J.E.; Collier, R.; Morgan, M.D.L. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest 2005, 128, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Probst, V.S.; Kovelis, D.; Hernandes, N.A.; Camillo, C.A.; Cavalheri, V.; Pitta, F. Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respir. Care 2011, 56, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Borges-Santos, E.; Wada, J.T.; da Silva, C.M.; Silva, R.A.; Stelmach, R.; Carvalho, C.R.; Lunardi, A.C. Anxiety and depression are related to dyspnea and clinical control but not with thoracoabdominal mechanics in patients with COPD. Respir. Physiol. Neurobiol. 2015, 210, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Espín, I.; Demeyer, H.; Gimeno-Santos, E.; Polkey, M.I.; Hopkinson, N.S.; Rabinovich, R.A.; Dobbels, F.; Karlsson, N.; Troosters, T.; Garcia-Aymerich, J. Depression symptoms reduce physical activity in COPD patients: A prospective multicenter study. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1287–1295. [Google Scholar] [CrossRef]
- Hama, S.; Yamashita, H.; Shigenobu, M.; Watanabe, A.; Kurisu, K.; Yamawaki, S.; Kitaoka, T. Post-stroke affective or apathetic depression and lesion location: Left frontal lobe and bilateral basal ganglia. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 149–152. [Google Scholar] [CrossRef]
- Shimodaira, H.; Nozaki, M.; Kwon, Y.; Kamimura, N.; Kaiho, F. Analysis of adverse reaction in Kampo-Medicines using JADER database of PMDA. Jpn. J. Drug Inform. 2014, 16, 16–22. (In Japanese) [Google Scholar]
- Negishi, R.; Ichikawa, T.; Tama, Y.; Fujimura, A.; Tanaka, S.; Tenmoku, A.; Akazawa, K.; Kanno, M.; Sasaki, H.; Okubo, S.; et al. Liver injury and hepatic encephalopathy induced by the herbal medicine Hochuekkito. Jpn. J. Gastroenterol. 2014, 111, 1149–1156. (In Japanese) [Google Scholar]


| Variables | Group A (n = 11) | Group B (n = 12) | p-Value |
|---|---|---|---|
| Age (years) | 72.5 ± 5.7 | 76.4 ± 7.1 | 0.169 |
| Sex | |||
| Male | 10 (90.9) | 11 (91.7) | 0.949 |
| Female | 1 (9.1) | 1 (8.3) | |
| Height (cm) | 161.1 ± 4.6 | 164.4 ± 6.4 | 0.413 |
| Body weight (kg) | 53.3 ± 10.3 | 61.1 ± 7.9 | 0.151 |
| Body mass index | 20.5 ± 3.2 | 22.9 ± 2.7 | 0.069 |
| GOLD stage | |||
| Stage II | 5 (45.5) | 5 (41.7) | 1.000 |
| Stage III | 6 (54.5) | 7 (58.3) | |
| Apathy Scale score | 20.9 ± 3.3 | 20.5 ± 3.1 | 0.833 |
| PHQ-9 | 4.1 ± 2.2 | 4.2 ± 2.4 | 0.976 |
| Number of daily steps | 5056.5 ± 3929.2 | 3368.2 ± 1651.6 | 0.606 |
| Medical treatment | |||
| Use of LABA | 6 (54.5) | 9 (75.0) | 0.304 |
| Use of LAMA | 10 (90.9) | 12 (100.0) | 0.286 |
| Use of ICS | 1 (9.1) | 3 (25.0) | 0.315 |
| Home oxygen therapy | 0 (0) | 3 (25.0) | 0.075 |
| Variable | Group A (n = 11) | Group B (n = 12) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | p-Value | Baseline | 12 Weeks | p-Value | |
| Body weight (kg) | 53.3 ± 10.3 | 52.9 ± 11.1 | 0.484 | 61.1 ± 7.9 | 60.5 ± 7.2 | 0.230 |
| Body mass index | 20.5 ± 3.2 | 20.3 ± 3.6 | 0.594 | 22.9 ± 2.7 | 22.7 ± 2.4 | 0.508 |
| Ideal body weight (% predicted) | 92.9 ± 14.6 | 92.1 ± 16.2 | 0.594 | 103.1 ± 12.0 | 102.3 ± 10.6 | 0.563 |
| Fat free mass (kg) | 40.6 ± 5.8 | 40.5 ± 6.1 | 0.878 | 44.2 ± 6.4 | 43.9 ± 6.1 | 0.638 |
| FVC (L) | 2.8 ± 0.7 | 2.9 ± 0.6 | 0.415 | 2.9 ± 0.7 | 2.8 ± 0.8 | 0.328 |
| FEV1 (L) | 1.4 ± 0.4 | 1.4 ± 0.3 | 0.221 | 1.3 ± 0.4 | 1.3 ± 0.5 | 0.473 |
| FEV1 (% predicted) | 57.7 ± 16.2 | 58.1 ± 13.0 | 0.657 | 51.6 ± 18.6 | 51.8 ± 19.3 | 0.929 |
| FEV1/FVC (%) | 50.8 ± 8.6 | 49.5 ± 8.5 | 0.374 | 45.7 ± 10.9 | 47.1 ± 10.1 | 0.209 |
| Apathy Scale score | 20.9 ± 3.3 | 17.0 ± 7.3 | 0.049 * | 20.5 ± 3.1 | 20.0 ± 5.8 | 0.959 |
| PHQ-9 | 4.1 ± 2.2 | 2.5 ± 1.6 | 0.031 * | 4.2 ± 2.4 | 2.9 ± 2.2 | 0.050 |
| mMRC dyspnea scale score | 1.4 ± 0.9 | 1.6 ± 0.9 | 0.480 | 1.6 ± 0.6 | 1.8 ± 0.8 | 0.317 |
| VAS score for dyspnea | 5.6 ± 2.9 | 2.4 ± 2.2 | 0.021 * | 4.4 ± 1.6 | 3.5 ± 2.0 | 0.071 |
| VAS score for fatigue | 3.9 ± 2.3 | 2.1 ± 2.1 | 0.058 | 4.5 ± 2.4 | 2.5 ± 2.0 | 0.023 * |
| Total CAT score | 13.8 ± 7.0 | 10.6 ± 6.2 | 0.220 | 15.6 ± 4.7 | 12.8 ± 3.5 | 0.152 |
| Score of cough | 1.2 ± 1.1 | 1.1 ± 0.8 | 0.792 | 1.8 ± 0.7 | 1.6 ± 1.7 | 0.729 |
| Score of production of phlegm | 1.7 ± 1.4 | 1.3 ± 1.2 | 0.194 | 1.4 ± 1.1 | 1.4 ± 1.0 | 1.000 |
| Score of chest tightness | 2.0 ± 0.9 | 1.7 ± 1.0 | 0.380 | 2.3 ± 1.2 | 2.0 ± 1.0 | 0.395 |
| Score of breathlessness | 3.5 ± 1.6 | 2.8 ± 1.7 | 0.167 | 3.7 ± 1.0 | 3.8 ± 0.9 | 0.480 |
| Score of activity limitation | 0.9 ± 1.1 | 0.7 ± 0.8 | 0.577 | 1.4 ± 0.9 | 0.5 ± 0.9 | 0.005 * |
| Score of confidence | 1.0 ± 1.4 | 0.9 ± 1.2 | 0.914 | 1.7 ± 1.3 | 1.1 ± 1.3 | 0.160 |
| Score of sleep | 1.2 ± 1.3 | 0.9 ± 1.4 | 0.732 | 1.3 ± 1.2 | 0.8 ± 0.9 | 0.132 |
| Score of energy | 2.3 ± 0.9 | 1.2 ± 1.1 | 0.026 * | 2.1 ± 1.0 | 1.6 ± 1.1 | 0.296 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, H.; Sekikawa, K.; Okusaki, K.; Dodo, T.; Kagawa, K.; Sumigawa, T.; Awaya, Y.; Sakimoto, N.; Shioya, S.; Hakozaki, K.; et al. Hochuekkito Combined with Pulmonary Rehabilitation in Apathetic Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Pilot Trial. J. Clin. Med. 2022, 11, 5673. https://doi.org/10.3390/jcm11195673
Hamada H, Sekikawa K, Okusaki K, Dodo T, Kagawa K, Sumigawa T, Awaya Y, Sakimoto N, Shioya S, Hakozaki K, et al. Hochuekkito Combined with Pulmonary Rehabilitation in Apathetic Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Pilot Trial. Journal of Clinical Medicine. 2022; 11(19):5673. https://doi.org/10.3390/jcm11195673
Chicago/Turabian StyleHamada, Hironobu, Kiyokazu Sekikawa, Ken Okusaki, Takefumi Dodo, Kazuyoshi Kagawa, Tatsuya Sumigawa, Yoshikazu Awaya, Naoki Sakimoto, Sachiko Shioya, Keisuke Hakozaki, and et al. 2022. "Hochuekkito Combined with Pulmonary Rehabilitation in Apathetic Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Pilot Trial" Journal of Clinical Medicine 11, no. 19: 5673. https://doi.org/10.3390/jcm11195673
APA StyleHamada, H., Sekikawa, K., Okusaki, K., Dodo, T., Kagawa, K., Sumigawa, T., Awaya, Y., Sakimoto, N., Shioya, S., Hakozaki, K., Kadowaki, T., Kakimoto, M., Ito, R., Kawamichi, K., Kondo, K., Namba, H., Iwamoto, H., & Hattori, N. (2022). Hochuekkito Combined with Pulmonary Rehabilitation in Apathetic Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Pilot Trial. Journal of Clinical Medicine, 11(19), 5673. https://doi.org/10.3390/jcm11195673







