The Rate of Hospitalization of Pregnant Women with Multiple Sclerosis in Poland
Abstract
1. Introduction
2. Materials and Methods
3. Results
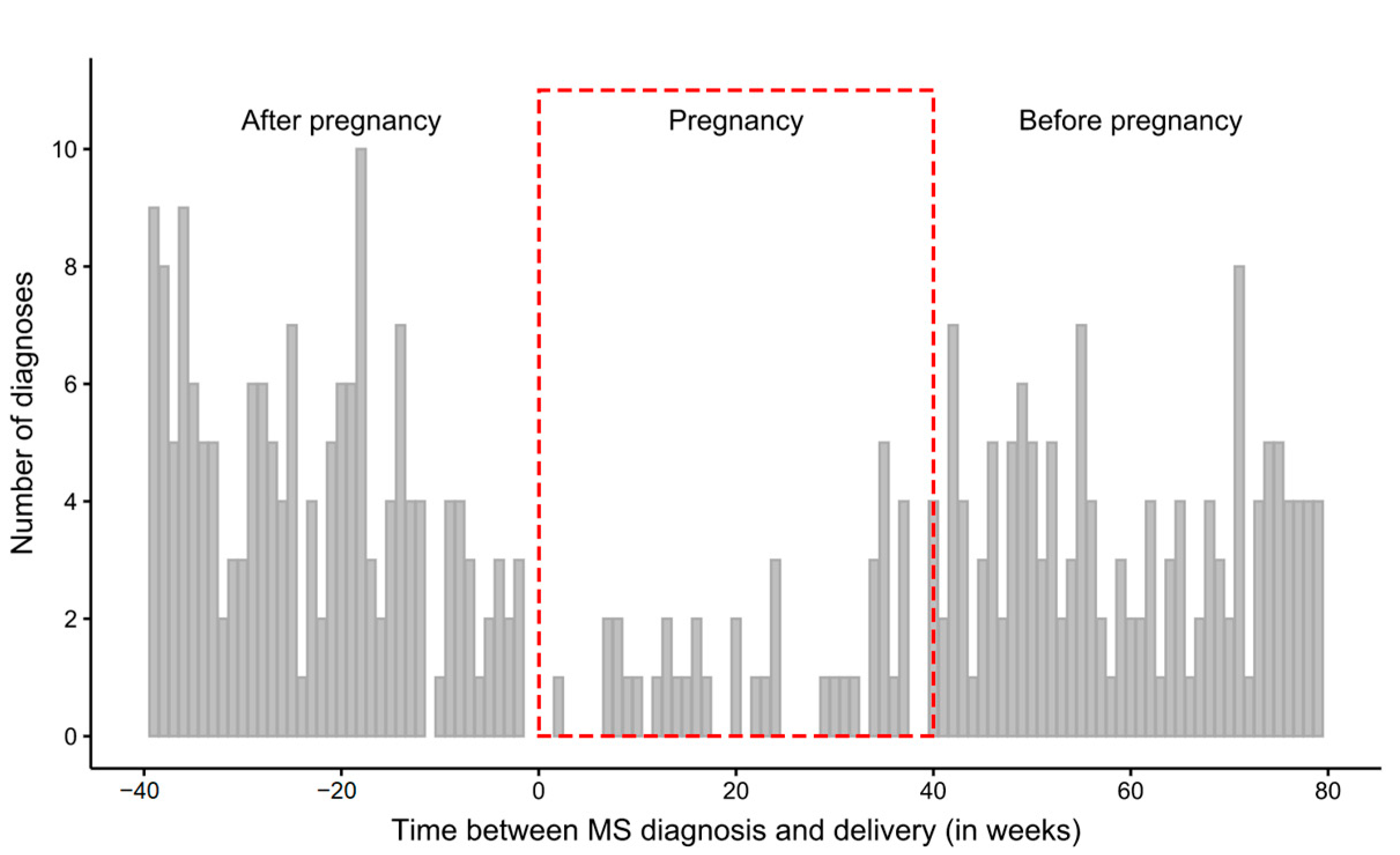
3.1. Time Intervals between the First Diagnosis and Delivery
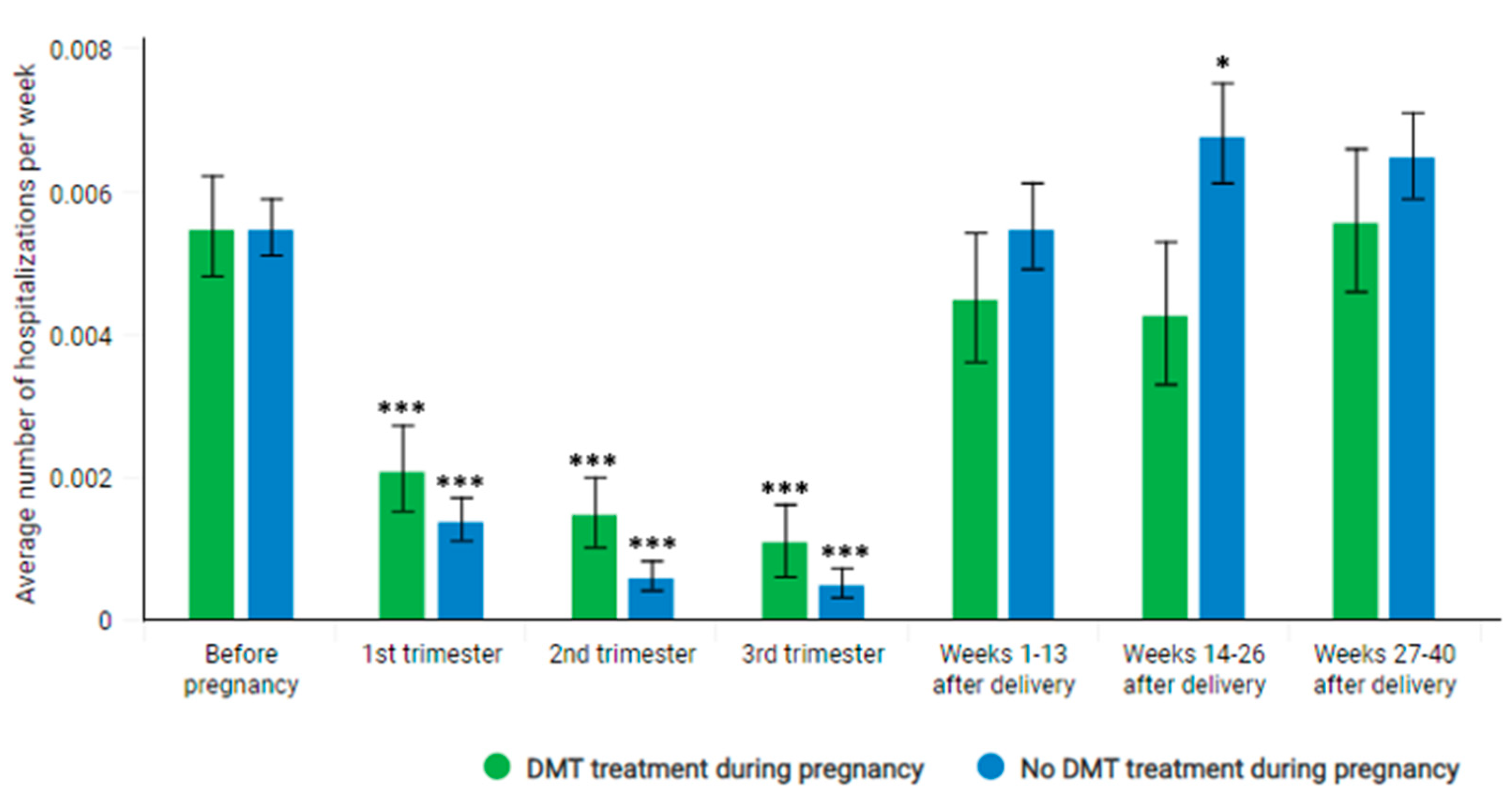
3.2. Number and Rate of Hospitalizations
3.3. Participation of Pregnant MS Patients in the DMT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonavita, S.; Lavorgna, L.; Worton, H.; Russell, S.; Jack, D. Family Planning Decision Making in People with Multiple Sclerosis. Front. Neurol. 2021, 12, 620772. [Google Scholar] [CrossRef]
- Alwan, S.; Yee, I.; Dybalski, M.; Guimond, C.; Dwosh, E.; Greenwood, T.; Butler, R.; Sadovnick, A. Reproductive decision making after the diagnosis of multiple sclerosis (MS). Mult. Scler. J. 2013, 19, 351–358. [Google Scholar] [CrossRef]
- Whitacre, C.C.; Reingold, S.C.; O’Looney, P.A. A gender gap in autoimmunity. Science 1999, 283, 1277–1278. [Google Scholar] [CrossRef]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef]
- Houtchens, M.K.; Edwards, N.C.; Schneider, G.; Stern, K.; Phillips, A.L. Pregnancy rates and outcomes in women with and without MS in the United States. Neurology 2018, 91, e1559–e1569. [Google Scholar] [CrossRef]
- Vukusic, S.; Michel, L.; Leguy, S.; Lebrun-Frenay, C. Pregnancy with multiple sclerosis. Rev. Neurol. 2021, 177, 180–194. [Google Scholar] [CrossRef]
- Spence, R.D.; Voskuhl, R.R. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocrinol. 2012, 33, 105–115. [Google Scholar] [CrossRef]
- Bebo, B.F., Jr.; Fyfe-Johnson, A.; Adlard, K.; Beam, A.G.; Vandenbark, A.A.; Offner, H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J. Immunol. 2001, 166, 2080–2089. [Google Scholar] [CrossRef]
- Kim, S.; Liva, S.M.; Dalal, M.A.; Verity, M.A.; Voskuhl, R.R. Estriol ameliorates autoimmune demyelinating disease: Implications for multiple sclerosis. Neurology 1999, 52, 1230–1238. [Google Scholar] [CrossRef]
- Palaszynski, K.M.; Liu, H.; Loo, K.K.; Voskuhl, R.R. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: Implications for multiple sclerosis. J. Neuroimmunol. 2004, 149, 84–89. [Google Scholar] [CrossRef]
- Voskuhl, R.; Momtazee, C. Pregnancy: Effect on Multiple Sclerosis, Treatment Considerations, and Breastfeeding. Neurotherapeutics 2017, 14, 974–984. [Google Scholar] [CrossRef]
- D’Hooghe, M.B.; Haentjens, P.; Nagels, G.; D’Hooghe, T.; De Keyser, J.; Keyser, J. Menarche, oral contraceptives, pregnancy and progression of disability in relapsing onset and progressive onset multiple sclerosis. J. Neurol. 2012, 259, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Masera, S.; Cavalla, P.; Prosperini, L.; Mattioda, A.; Mancinelli, C.; Superti, G.; Chiavazza, C.; Vercellino, M.; Pinessi, L.; Pozzilli, C. Parity is associated with a longer time to reach irreversible disability milestones in women with multiple sclerosis. Mult. Scler. J. 2015, 21, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Voskuhl, R.R. Pregnancy and multiple sclerosis: From molecular mechanisms to clinical application. Semin. Immunopathol. 2016, 38, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.M. Pregnancy in patients with multiple sclerosis. J. Investig. Med. 2021, 70, 14–19. [Google Scholar] [CrossRef]
- Siroos, B.; Harirchian, M.H. Multiple sclerosis and pregnancy; What a neurologist may be asked for? Iran. J. Neurol. 2014, 13, 57–63. [Google Scholar]
- Bartnik, P.; Wielgoś, A.; Kacperczyk-Bartnik, J.; Szymusik, I.; Podlecka-Piętowska, A.; Zakrzewska-Pniewska, B.; Wielgos, M. Evaluation of reproductive health in female patients with multiple sclerosis in Polish population. J. Clin. Neurosci. 2018, 53, 117–121. [Google Scholar] [CrossRef]
- Korn-Lubetzki, I.; Kahana, E.; Cooper, G.; Abramsky, O. Activity of multiple sclerosis during pregnancy and puerperium. Ann. Neurol. 1984, 16, 229–231. [Google Scholar] [CrossRef]
- Vukusic, S.; Hutchinson, M.; Hours, M.; Moreau, T.; Cortinovis-Tourniaire, P.; Adeleine, P.; Confavreux, C. Pregnancy and multiple sclerosis (the PRIMS study): Clinical predictors of post-partum relapse. Brain 2004, 127, 1353–1360. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Smith, J.B.; Albers, K.B.; Xiang, A.H.; Wu, J.; Kerezsi, E.H.; McClearnen, K.; Gonzales, E.G.; Leimpeter, A.D.; Eeden, S.K.V.D. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology 2020, 94, e1939–e1949. [Google Scholar] [CrossRef]
- Langer-Gould, A.M. Pregnancy and Family Planning in Multiple Sclerosis. Continuum 2019, 25, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.E.; Spelman, T.; Gray, O.M.; Boz, C.; Trojano, M.; Lugaresi, A.; Izquierdo, G.; Duquette, P.; Girard, M.; Grand’Maison, F.; et al. Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult. Scler. J. 2014, 20, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Paavilainen, T.; Kurki, T.; Parkkola, R.; Färkkilä, M.; Salonen, O.; Dastidar, P.; Elovaara, I.; Airas, L. Magnetic resonance imaging of the brain used to detect early post-partum activation of multiple sclerosis. Eur. J. Neurol. 2007, 14, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, P.; Pol-Patil, J.; Manieri, M.C.; Mahlanza, T.; Beretich, B.; Berrios Morales, I.; Cabot, A.; Chaves, C.; Ionete, C.; Katz, J.; et al. Peri-partum MRI activity in multiple sclerosis: Interim analysis from PREG-MS cohort. In Proceedings of the ECTRIMS 2021 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Online, 13–15 October 2021. [Google Scholar]
- Lehmann, H.; Zveik, O.; Levin, N.; Brill, L.; Imbar, T.; Vaknin-Dembinsky, A. Brain MRI activity during the year before pregnancy can predict post-partum clinical relapses. In Proceedings of the ECTRIMS 2021 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Online, 13–15 October 2021. [Google Scholar]
- Kalinowska, A.; Kułakowska, A.; Adamczyk-Sowa, M.; Czajkowski, K.; Kurowska, K.; Pietrzak, B.; Radziszewski, P.; Rejdak, K.; Bartosik-Psujek, H. Recommendations for neurological, obstetrical and gynaecological care in women with multiple sclerosis: A statement by a working group convened by the Section of Multiple Sclerosis and Neuroimmunology of the Polish Neurological Society. Neurol. Neurochir. Pol. 2020, 54, 125–137. [Google Scholar] [CrossRef]
- Airas, L.; Saraste, M.; Rinta, S.; Elovaara, I.; Huang, Y.-H.; Wiendl, H. Immunoregulatory factors in multiple sclerosis patients during and after pregnancy: Relevance of natural killer cells. Clin. Exp. Immunol. 2008, 151, 235–243. [Google Scholar] [PubMed]
- Boskovic, R.; Wide, R.; Wolpin, J.; Bauer, D.J.; Koren, G. The reproductive effects of beta interferon therapy in pregnancy: A longitudinal cohort. Neurology 2005, 65, 807–811. [Google Scholar] [CrossRef]
- De Heras, V.L.; De Andrés, C.; Téllez, N.; Tintore, M. Pregnancy in multiple sclerosis patients treated with immunomodulators prior to or during part of the pregnancy: A descriptive study in the Spanish population. Mult. Scler. J. 2007, 13, 981–984. [Google Scholar] [CrossRef]
- Sandberg-Wollheim, M.; Frank, D.; Goodwin, T.M.; Giesser, B.; Lopez-Bresnahan, M.; Stam-Moraga, M.; Chang, P.; Francis, G.S. Pregnancy outcomes during treatment with interferon beta-1a in patients with multiple sclerosis. Neurology 2005, 65, 802–806. [Google Scholar] [CrossRef]
- Patti, F.; Cavallaro, T.; Fermo, S.L.; Nicoletti, A.; Cimino, V.; Vecchio, R.; Laisa, P.; Zarbo, R.; Zappia, M. Is in utero early-exposure to interferon beta a risk factor for pregnancy outcomes in multiple sclerosis? J. Neurol. 2008, 255, 1250–1253. [Google Scholar] [CrossRef]
- Amato, M.P.; Portaccio, E.; Ghezzi, A.; Hakiki, B.; Zipoli, V.; Martinelli, V.; Moiola, L.; Patti, F.; La Mantia, L.; Mancardi, G.L.; et al. Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology 2010, 75, 1794–1802. [Google Scholar] [CrossRef]
- Sandberg-Wollheim, M.; Alteri, E.; Moraga, M.S.; Kornmann, G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. J. 2011, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, K.; Geissbuehler, Y.; Sabidó, M.; Popescu, C.; Adamo, A.; Klinger, J.; Ornoy, A.; Huppke, P.; European Interferon-beta Pregnancy Study Group. Pregnancy outcomes in interferon-beta-exposed patients with multiple sclerosis: Results from the European Interferon-beta Pregnancy Registry. J. Neurol. 2020, 267, 1715–1723. [Google Scholar] [CrossRef]
- Salminen, H.J.; Leggett, H.; Boggild, M. Glatiramer acetate exposure in pregnancy: Preliminary safety and birth outcomes. J. Neurol. 2010, 257, 2020–2023. [Google Scholar] [CrossRef]
- Hoevenaren, I.A.; De Vries, L.C.; Rijnders, R.J.P.; Lotgering, F.K. Delivery of healthy babies after natalizumab use for multiple sclerosis: A report of two cases. Acta Neurol. Scand. 2011, 123, 430–433. [Google Scholar] [CrossRef]
- De Santis, M.; Straface, G.; Cavaliere, A.; Rosati, P.; Batocchi, A.; Caruso, A. The first case of mitoxantrone exposure in early pregnancy. Neurotoxicology 2007, 28, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.K. Multiple sclerosis and pregnancy prescriptions. Expert Opin. Drug Saf. 2014, 13, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Pretta, S.; Ragni, N. Multiple sclerosis: Management issues during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 3–9. [Google Scholar] [CrossRef]
- Amato, M.P.; Bertolotto, A.; Brunelli, R.; Cavalla, P.; Goretti, B.; Marrosu, M.G.; Patti, F.; Pozzilli, C.; Provinciali, L.; Rizzo, N.; et al. Management of pregnancy-related issues in multiple sclerosis patients: The need for an interdisciplinary approach. Neurol. Sci. 2017, 38, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Varytė, G.; Arlauskienė, A.; Ramašauskaitė, D. Pregnancy and multiple sclerosis: An update. Curr. Opin. Obstet. Gynecol. 2021, 33, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Krysko, K.M.; Bove, R.; Dobson, R.; Jokubaitis, V.; Hellwig, K. Treatment of Women with Multiple Sclerosis Planning Pregnancy. Curr. Treat. Options Neurol. 2021, 23, 11. [Google Scholar] [CrossRef]



| Characteristic | Mean ± SD (Min–Max) |
|---|---|
| Age at the first MS diagnosis | 28.9 ± 5.4 (14–52) |
| Age at delivery | 30.7 ± 4.7 (17–46) |
| Duration of pregnancy (in weeks) | 38.6 ± 1.9 (23–47) * |
| % of multiple births | 2.5 |
| % of Ceaserian sections | 56.3 |
| Time | Sum of Diagnoses | Weekly Average |
|---|---|---|
| 40 weeks preceding pregnancy | 141 | 3.52 |
| 40–42 weeks of pregnancy | 39 | 0.98 |
| 40 weeks following pregnancy | 164 | 4.10 |
| Period | N | Sum of Hospitalizations | Number of Pregnancies for Which at Least 1 Hospitalization Occurred | Rate of Hospitalization per Week for Each Pregnancy Individually | ||
|---|---|---|---|---|---|---|
| Mean | SEM | p-Value | ||||
| All ≥ 1 day hospitalizations without reported DMT-products | ||||||
| Before pregnancy (40−1. week before pregnancy) | 1610 | 354 | 283 | 0.0055 | 0.0003 | Reference |
| Pregnancy | 1610 | 64 | 54 | 0.0010 | 0.0002 | <0.0001 |
| week 1–13 | 1610 | 33 | 31 | 0.0016 | 0.0003 | <0.0001 |
| week 14–26 | 1610−1605 | 18 | 18 | 0.0009 | 0.0002 | <0.0001 |
| week 27–42 | 1610−8 | 13 | 12 | 0.0007 | 0.0002 | <0.0001 |
| After pregnancy | 1610 | 381 | 300 | 0.0059 | 0.0004 | 0.3044 |
| week 1–13 | 1610 | 110 | 104 | 0.0053 | 0.0005 | 0.6570 |
| week 14–26 | 1610 | 129 | 117 | 0.0062 | 0.0006 | 0.2962 |
| week 27–40 | 1610 | 142 | 133 | 0.0063 | 0.0005 | 0.5023 |
| Hospitalizations reported with steroid injection procedures | ||||||
| Before pregnancy (40 − 1. week before pregnancy) | 1610 | 85 | 77 | 0.0013 | 0.0002 | Reference |
| Pregnancy | 1610 | 13 | 11 | 0.0002 | 0.0001 | <0.0001 |
| week 1–13 | 1610 | 7 | 7 | 0.0003 | 0.0001 | <0.0001 |
| week 14–26 | 1610−1605 | 4 | 4 | 0.0002 | 0.0001 | <0.0001 |
| week 27–42 | 1610−8 | 2 | 1 | 0.0001 | 0.0001 | <0.0001 |
| After pregnancy | 1610 | 76 | 64 | 0.0012 | 0.0002 | 0.3855 |
| week 1–13 | 1610 | 17 | 17 | 0.0008 | 0.0002 | 0.0083 |
| week 14–26 | 1610 | 23 | 23 | 0.0011 | 0.0002 | 0.1696 |
| week 27–40 | 1610 | 36 | 33 | 0.0016 | 0.0003 | 0.8422 |
| Hospitalizations in emergency department | ||||||
| Before pregnancy (week 1–40) | 1610 | 54 | 42 | 0.0008 | 0.0001 | Ref. |
| Pregnancy | 1610 | 21 | 17 | 0.0003 | 0.0001 | 0.0373 |
| week 1–13 | 1610 | 11 | 10 | 0.0005 | 0.0002 | 0.0351 |
| week 14–26 | 1610−1605 | 8 | 6 | 0.0004 | 0.0002 | 0.0008 |
| week 27–42 | 1610−8 | 2 | 2 | 0.0001 | 0.0001 | <0.0001 |
| After pregnancy | 1610 | 65 | 54 | 0.0010 | 0.0001 | 0.264 |
| week 1–13 | 1610 | 19 | 16 | 0.0009 | 0.0002 | 0.7534 |
| week 14–26 | 1610 | 24 | 21 | 0.0011 | 0.0003 | 0.4583 |
| week 27–40 | 1610 | 22 | 22 | 0.0010 | 0.0002 | 0.6805 |
| Period | N | Sum of Hospitalizations | Number of Pregnancies for Which at Least 1 Hospitalization Occurred | Rate of Hospitalization per Week for Each Pregnancy Individually | ||
|---|---|---|---|---|---|---|
| Mean | SEM | p-Value | ||||
| Patients participating in DMT during pregnancy | ||||||
| Before pregnancy (40−1. week before pregnancy) | 410 | 91 | 74 | 0.0055 | 0.0007 | Reference |
| Pregnancy | 410 | 25 | 21 | 0.0016 | 0.0004 | <0.0001 |
| week 1–13 | 410 | 11 | 11 | 0.0021 | 0.0006 | <0.0001 |
| week 14–26 | 410 | 8 | 8 | 0.0015 | 0.0005 | <0.0001 |
| week 27–42 | 410−2 | 6 | 5 | 0.0011 | 0.0005 | <0.0001 |
| After pregnancy | 410 | 79 | 68 | 0.0048 | 0.0006 | 0.3897 |
| week 1–13 | 410 | 24 | 24 | 0.0045 | 0.0009 | 0.2804 |
| week 14–26 | 410 | 23 | 21 | 0.0043 | 0.0010 | 0.1056 |
| week 27–40 | 410 | 32 | 32 | 0.0056 | 0.0010 | 0.7486 |
| Patients not participating in DMT during pregnancy | ||||||
| Before pregnancy (40−1. week before pregnancy) | 1200 | 263 | 209 | 0.0055 | 0.0004 | Reference |
| Pregnancy | 1200 | 39 | 33 | 0.0009 | 0.0002 | <0.0001 |
| week 1–13 | 1200 | 22 | 20 | 0.0014 | 0.0003 | <0.0001 |
| week 14–26 | 1200−1195 | 10 | 10 | 0.0006 | 0.0002 | <0.0001 |
| week 27–42 | 1194−6 | 7 | 7 | 0.0005 | 0.0002 | <0.0001 |
| After pregnancy | 1200 | 302 | 231 | 0.0063 | 0.0004 | 0.095 |
| week 1–13 | 1200 | 86 | 80 | 0.0055 | 0.0006 | 0.9295 |
| week 14–26 | 1200 | 106 | 96 | 0.0068 | 0.0007 | 0.0448 |
| week 27–40 | 1200 | 110 | 104 | 0.0065 | 0.0006 | 0.3486 |
| Drug Used | Number of Pregnancies for Which at Least One Visit Reported with DMT (with Given Drug) Was Observed | ||
|---|---|---|---|
| Week 1–13 (1st Trimester) | Week 14–26 (2nd Trimester) | Week 27–42 (3rd Trimester) | |
| IFN-ß 1b | 152 | 10 | 6 |
| IFN-ß 1a | 130 | 7 | 2 |
| Glatiramer acetate | 93 | 25 | 24 |
| Dimethyl fumarate | 46 | 2 | 3 |
| Fingolimod | 14 | 2 | 0 |
| Natalizumab | 6 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkiewicz, D.; Adamczyk, B.; Maluchnik, M.; Perwieniec, J.; Podwójcic, K.; Szeląg, M.; Zakrzewski, M.; Rejdak, K.; Słowik, A.; Wnuk, M.; et al. The Rate of Hospitalization of Pregnant Women with Multiple Sclerosis in Poland. J. Clin. Med. 2022, 11, 5615. https://doi.org/10.3390/jcm11195615
Walkiewicz D, Adamczyk B, Maluchnik M, Perwieniec J, Podwójcic K, Szeląg M, Zakrzewski M, Rejdak K, Słowik A, Wnuk M, et al. The Rate of Hospitalization of Pregnant Women with Multiple Sclerosis in Poland. Journal of Clinical Medicine. 2022; 11(19):5615. https://doi.org/10.3390/jcm11195615
Chicago/Turabian StyleWalkiewicz, Dorota, Bożena Adamczyk, Michał Maluchnik, Jakub Perwieniec, Krzysztof Podwójcic, Mateusz Szeląg, Michał Zakrzewski, Konrad Rejdak, Agnieszka Słowik, Marcin Wnuk, and et al. 2022. "The Rate of Hospitalization of Pregnant Women with Multiple Sclerosis in Poland" Journal of Clinical Medicine 11, no. 19: 5615. https://doi.org/10.3390/jcm11195615
APA StyleWalkiewicz, D., Adamczyk, B., Maluchnik, M., Perwieniec, J., Podwójcic, K., Szeląg, M., Zakrzewski, M., Rejdak, K., Słowik, A., Wnuk, M., & Adamczyk-Sowa, M. (2022). The Rate of Hospitalization of Pregnant Women with Multiple Sclerosis in Poland. Journal of Clinical Medicine, 11(19), 5615. https://doi.org/10.3390/jcm11195615







