Percutaneous-Reinforced Osteoplasty: A Review of Emerging Treatment Strategies for Bone Interventions
Abstract
1. Introduction
| IR technique. | Description | Advantages and Disadvantages | Ref. |
|---|---|---|---|
| Mechanical stabilization | |||
| Cementoplasty | Use of bone cement for bone consolidation and pain palliation. | Widely available procedure at low cost. PMMA cement has low resistance to bending and twisting, toxicity, and cement leakage. | [9,10] |
| Reinforcement cementoplasty | Use of reinforcements such as K-wires, nails, and screws in addition to regular cementoplasty. | Provides additional mechanical stability. Infection, pain, and reinforcement failures. | [11,12,13,14] |
| Tumor destruction | |||
| Ablation—Radiofrequency (RFA) and microwave (MWA) | Tumor destruction with the application of high temperature (≥60 ° C). | RFA—Cost-effective, widely used. Suffers from local perfusion or vascular heat sink effects, less powerful than MWA, and non-homogeneous energy propagation. MWA—Minimal heat sink effect, independent of tissue non-conductivity, and larger ablation zones. Imprecise energy delivery with oval ablation effects, and overheating. | [15,16] |
| Cryoablation | Tumoricidal effects from exposure to extremely cold temperatures of super-cooled gases (<−40 °C). | Deep tissue penetration, ablated areas visible in imaging due to ice-ball formation (temperature difference), and less damage to tissue architecture. Costlier than RFA or MWA, cryoshock, and not indicated for all tumors. | |
| Embolization | Obstruction of tumor-feeding blood vessels that cuts off nutrients and oxygen supply (devascularization) choking tumor cells. | Less bleeding and rapid cut-off of blood supply. Infection, ischemia, and potential damage to healthy tissues from wrong delivery. | [15,17] |
| MR-guided Focused Ultrasound (MRgFUS) (also called, High-Intensity Focused Ultrasound [HIFU]). | Application of focussed ultrasonic pulses to lyse tumor cells with MR-based tissue identification and targeting. | Minimally invasive and does not require tissue contact via needlelike applicators to deliver ultrasonic waves. Shallow penetration depth. | |
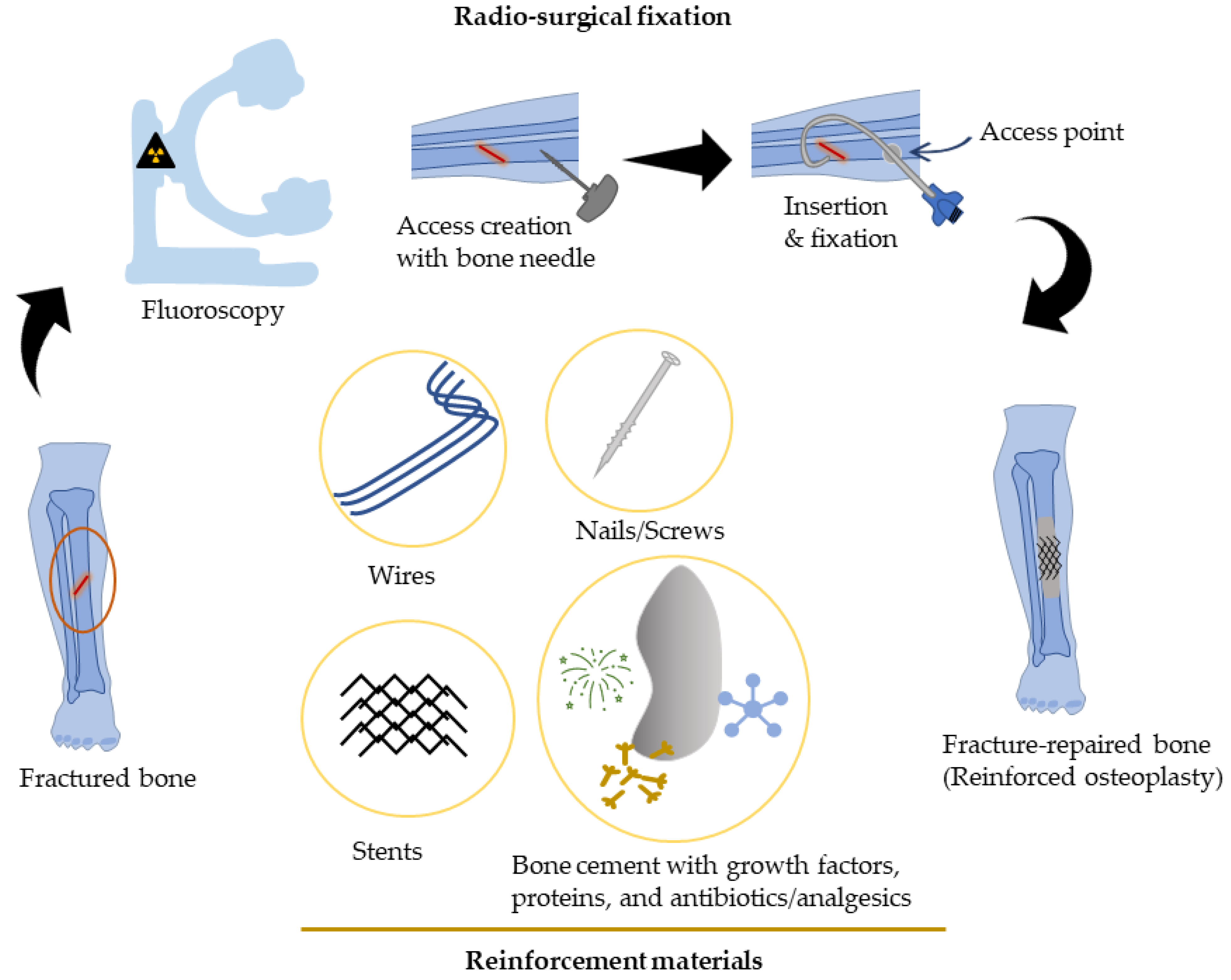
2. Percutaneous-Reinforced Bone Interventions
| Material | Evidence | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| PMMA bone cement | Preclinical Clinical | Readily available, ease of use, high axial compressive strength (80–94 MPa). | Low bending (67–72 MPa), tensile (36–47 MPa; Young’s modulus: ~2400 MPa), and shear strength (50–69 MPa). | [40] |
| Calcium phosphate cement | Clinical | Good osteoconductive properties, mimics natural mineral phase of bone, low toxicity. | More expensive than PMMA cement, and has low mechanical strength (compressive strength: 35 MPa). | [41,42] |
| Stents | Preclinical | Ease of deployment in the target site. | Low mechanical strength, and displacement. | [12,43,44] |
| Nails (Screws) | Preclinical Clinical | Bone consolidation, stability, and durability. | Breaking, loosening, or migration. | [45,46,47] |
| K-wires | Preclinical Clinical | Ease of deployment, deployable in bundles to boost strength. | Low mechanical strength. | [48,49] |
| Y STRUTS | Preclinical Clinical | Bone consolidation, reinforces mechanical stability. | Increase in procedural complexity and time, application limited to proximal femur. | [50,51] |
Percutaneous Bone Intervention Procedure
3. Approaches to Improve Strength for Percutaneous Bone Interventions
3.1. Metallic Materials
| Lesion Localization (Patients (n), (Year)) | Intervention Type | Outcome | Complications | Reference |
|---|---|---|---|---|
| Hip and neck (n = 11, (2022)) | Screw fixation and cementoplasty for pathologic bone fractures. | Significant decrease in pain score from 8.0 ± 2.7 to 1.6 ± 2.5, lower analgesic consumption from 70.9 ± 37 to 48.2 ± 46 mg/day, and improved EQ5D score from 42.5 ± 13.6 vs. 63.6 ± 10.3 (p < 0.05). | Minor subcutaneous hematoma (n = 1), and asymptomatic pulmonary cruciate embolism (n = 1). | [45] |
| Pelvic Ring (n = 50, (2021)) | Percutaneous fixation with internal cemented screws (FICS) for prophylactic consolidation of large osteolytic tumors. |
| Self-resolving hematoma (n = 2), inflammatory sciatic pain (n = 1), and focal pain at the ischial tuberosity (n = 1). | [46] |
| Hip, shoulder, chest, and jaw (n = 94, total 110 fractures, (2021)) | Percutaneous image-guided screw fixation (PIGSF) of insufficiency, impending or pathological fractures. | Extremely low rates (<4%) of per-procedural (cement leak, induced fracture, or hematoma) and early complication (≤24 h) following PIGSF. | Delayed complications (>24 h, total: 18%) included infection (most frequent), focal pain, tumor seeding, screw loosening and fracture. | [47] |
| Periarticular load-bearing bones (n = 23, total lesion = 26, (2020)) | Ablation, osteoplasty, reinforcement, and internal fixation (AORIF) of osteolytic lesions of the pelvis, hip, knee, and ankle. |
| None reported. | [57] |
| Femoral neck (n = 61, (2020)) | FICS in metastatic patients with impending pathological fracture. |
|
| [58] |
| Sternum (n = 9, (2019)) | FICS for sternal fracture fixation or consolidation of osteolytic metastases. |
|
| [29] |
| Pelvic bone (n = 126, total 178 lesions, (2019)) | Percutaneous osteoplasty for treatment of pelvic bone metastases. |
|
| [25] |
| Spine (n = 69, total 102 spinal metastases, (2018)) | Microwave ablation and cementoplasty for treatment of painful spinal metastases. |
| S1 nerve thermal injury (n = 1) and skin burn (n = 1) (3%). | [59] |
| Pelvic bone and lower leg (n = 43, (2018)) | Extraspinal cementoplasty for bone metastasis. |
| Cement leakage (12%), hematoma (2%), and acute respiratory distress due to infection (2%). | [60] |
3.2. Regenerative Scaffolds
3.3. Bone Morphogenetic Proteins
4. Mechanical Characterization of Bone/Bone Implant Devices
4.1. Flexural Test
4.1.1. Three-Point Bending Test
4.1.2. Four-Point Bending Test
4.2. Potting Bone Ends to Comply with Four-Point Test and Multidirectional Testing
4.3. Torsional Test
4.4. Hardness/Indentation Test
4.5. In Silico Test
5. Summary and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordero, D.M.; Miclau, T.A.; Paul, A.V.; Morshed, S.; Martin, C.; Shearer, D.W. The global burden of musculoskeletal injury in low and lower-middle income countries. OTA Int. Open Access J. Orthop. Trauma 2020, 3, e062. [Google Scholar] [CrossRef]
- O’Hara, N.N.; Isaac, M.; Slobogean, G.P.; Klazinga, N.S. The socioeconomic impact of orthopaedic trauma: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227907. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Lipton, A.; Uzzo, R.; Amato, R.J.; Ellis, G.K.; Hakimian, B.; Roodman, G.D.; Smith, M.R. The Science and Practice of Bone Health in Oncology: Managing Bone Loss and Metastasis in Patients With Solid Tumors. J. Natl. Compr. Cancer Netw. 2009, 7 (Suppl. S7), S1–S29. [Google Scholar] [CrossRef]
- Huang, J.-F.; Shen, J.; Li, X.; Rengan, R.; Silvestris, N.; Wang, M.; Derosa, L.; Zheng, X.; Belli, A.; Zhang, X.-L.; et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: A large population-based study. Ann. Transl. Med. 2020, 8, 482. [Google Scholar] [CrossRef]
- Androjna, C.; Yee, C.S.; White, C.R.; Waldorff, E.I.; Ryaby, J.T.; Zborowski, M.; Alliston, T.; Midura, R.J. A comparison of alendronate to varying magnitude PEMF in mitigating bone loss and altering bone remodeling in skeletally mature osteoporotic rats. Bone 2021, 143, 115761. [Google Scholar] [CrossRef]
- Bailey, S.; Vashishth, D. Mechanical Characterization of Bone: State of the Art in Experimental Approaches—What Types of Experiments Do People Do and How Does One Interpret the Results? Curr. Osteoporos. Rep. 2018, 16, 423–433. [Google Scholar] [CrossRef]
- Nyman, J.S.; Granke, M.; Singleton, R.C.; Pharr, G.M. Tissue-Level Mechanical Properties of Bone Contributing to Fracture Risk. Curr. Osteoporos. Rep. 2016, 14, 138–150. [Google Scholar] [CrossRef]
- Hayek, G.; Kastler, B. Interventional radiology for treatment of bone metastases. Cancer Radiother. 2020, 24, 374–378. [Google Scholar] [CrossRef]
- Park, J.W.; Lim, H.-J.; Kang, H.G.; Kim, J.H.; Kim, H.-S. Percutaneous Cementoplasty for the Pelvis in Bone Metastasis: 12-Year Experience. Ann. Surg. Oncol. 2022, 29, 1413–1422. [Google Scholar] [CrossRef]
- Sgalambro, F.; Zugaro, L.; Bruno, F.; Palumbo, P.; Salducca, N.; Zoccali, C.; Barile, A.; Masciocchi, C.; Arrigoni, F. Interventional Radiology in the Management of Metastases and Bone Tumors. J. Clin. Med. 2022, 11, 3265. [Google Scholar] [CrossRef]
- Koirala, N.; McLennan, G. Percutaneous reinforced osteoplasty for long bone metastases: A feasibility study. Skelet. Radiol. 2020, 49, 375–382. [Google Scholar] [CrossRef]
- Kelekis, A.; Filippiadis, D.; Anselmetti, G.; Brountzos, E.; Mavrogenis, A.; Papagelopoulos, P.; Martin, J.-B. Percutaneous Augmented Peripheral Osteoplasty in Long Bones of Oncologic Patients for Pain Reduction and Prevention of Impeding Pathologic Fracture: The Rebar Concept. Cardiovasc. Interv. Radiol. 2016, 39, 90–96. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Garnon, J.; Tsoumakidou, G.; Koch, G.; Palussière, J.; Gangi, A.; Buy, X. Percutaneous image-guided screws meditated osteosynthesis of impeding and pathological/insufficiency fractures of the femoral neck in non-surgical cancer patients. Eur. J. Radiol. 2017, 90, 1–5. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bruno, F.; Zugaro, L.; Natella, R.; Cappabianca, S.; Russo, U.; Papapietro, V.R.; Splendiani, A.; Di Cesare, E.; Masciocchi, C.; et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed. 2018, 89, 166–174. [Google Scholar] [CrossRef]
- Brace, C. Thermal Tumor Ablation in Clinical Use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef]
- Bertrand, A.-S.; Iannessi, A.; Natale, R.; Beaumont, H.; Patriti, S.; Xiong-Ying, J.; Baudin, G.; Thyss, A. Focused ultrasound for the treatment of bone metastases: Effectiveness and feasibility. J. Ther. Ultrasound 2018, 6, 8. [Google Scholar] [CrossRef]
- Tian, Q.; Cheng, Y.; Wu, C. Percutaneous Osteoplasty for Extraspinal Metastases. J. Interv. Med. 2018, 1, 137–142. [Google Scholar] [CrossRef]
- Katsanos, K.; Sabharwal, T.; Adam, A. Percutaneous Cementoplasty. Semin. Interv. Radiol. 2010, 27, 137–147. [Google Scholar] [CrossRef]
- Tian, Q.-H.; Wu, C.-G.; Gu, Y.-F.; He, C.-J.; Li, M.-H.; Cheng, Y.-D. Combination Radiofrequency Ablation and Percutaneous Osteoplasty for Palliative Treatment of Painful Extraspinal Bone Metastasis: A Single-Center Experience. J. Vasc. Interv. Radiol. 2014, 25, 1094–1100. [Google Scholar] [CrossRef]
- Kelekis, A.; Filippiadis, D.K.; Kelekis, N.L.; Martin, J.-B. Percutaneous Augmented Osteoplasty of the Humeral Bone Using a Combination of MicroNeedles Mesh and Cement. J. Vasc. Interv. Radiol. 2015, 26, 595–597. [Google Scholar] [CrossRef]
- Seo, S.-S.; Park, J.-Y.; Kim, H.-J.; Yoon, J.-W.; Park, S.-H.; Kim, K.-H. Percutaneous osteoplasty for the treatment of a painful osteochondral lesion of the talus: A case report and literature review. Pain Phys. 2012, 15, E743–E748. [Google Scholar]
- Anselmetti, G.C. Osteoplasty: Percutaneous Bone Cement Injection beyond the Spine. Semin. Interv. Radiol. 2010, 27, 199–208. [Google Scholar] [CrossRef]
- Shi, G.; Liu, Q.; Chen, H.; Feng, F.; Jia, P.; Bao, L.; Tang, H. Percutaneous osteoplasty for the management of a humeral head metastasis. Medicine 2019, 98, e15727. [Google Scholar] [CrossRef]
- Liu, H.-F.; Wu, C.-G.; Tian, Q.-H.; Wang, T.; Yi, F. Application of Percutaneous Osteoplasty in Treating Pelvic Bone Metastases: Efficacy and Safety. Cardiovasc. Interv. Radiol. 2019, 42, 1738–1744. [Google Scholar] [CrossRef]
- Shi, G.; Tang, H. Percutaneous osteoplasty for the management of a pubic bone metastasis. Orthopade 2019, 48, 704–707. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.; Hu, J.-H.; Peng, Z.-H.; Chen, J.-Z.; Huang, J.-Q.; Jiang, Y.-N.; Luo, G.; Yi, G.-F.; Shen, J.; et al. Palliative pain relief and safety of percutaneous radiofrequency ablation combined with cement injection for bone metastasis. Jpn. J. Clin. Oncol. 2018, 48, 753–759. [Google Scholar] [CrossRef]
- Hartung, M.P.; Tutton, S.M.; Hohenwalter, E.J.; King, D.M.; Neilson, J.C. Safety and Efficacy of Minimally Invasive Acetabular Stabilization for Periacetabular Metastatic Disease with Thermal Ablation and Augmented Screw Fixation. J. Vasc. Interv. Radiol. 2016, 27, 682–688.e1. [Google Scholar] [CrossRef]
- Poussot, B.; Deschamps, F.; Varin, F.; Abed, A.; Moulin, B.; Prud’Homme, C.; Al Ahmar, M.; Teriitehau, C.; Hakime, A.; Laurent, S.; et al. Percutaneous Fixation by Internal Cemented Screws of the Sternum. Cardiovasc. Interv. Radiol. 2019, 43, 103–109. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Garnon, J.; Dalili, D.; Autrusseau, P.-A.; Auloge, P.; De Marini, P.; Buy, X.; Palussiere, J.; Gangi, A. Percutaneous osteoplasty in long bones: Current status and assessment of outcomes. Tech. Vasc. Interv. Radiol. 2022, 25, 100803. [Google Scholar] [CrossRef] [PubMed]
- Iannessi, A.; Amoretti, N.; Marcy, P.-Y.; Sedat, J. Percutaneous cementoplasty for the treatment of extraspinal painful bone lesion, a prospective study. Diagn. Interv. Imaging 2012, 93, 859–870. [Google Scholar] [CrossRef]
- Hierholzer, J.; Anselmetti, G.; Fuchs, H.; Depriester, C.; Koch, K.; Pappert, D. Percutaneous Osteoplasty as a Treatment for Painful Malignant Bone Lesions of the Pelvis and Femur. J. Vasc. Interv. Radiol. 2003, 14, 773–777. [Google Scholar] [CrossRef]
- Deib, G.; Deldar, B.; Hui, F.; Barr, J.S.; Khan, M.A. Percutaneous Microwave Ablation and Cementoplasty: Clinical Utility in the Treatment of Painful Extraspinal Osseous Metastatic Disease and Myeloma. Am. J. Roentgenol. 2019, 212, 1377–1384. [Google Scholar] [CrossRef]
- Plancarte-Sanchez, R.; Guajardo-Rosas, J.; Cerezo-Camacho, O.; Chejne-Gomez, F.; Gomez-Garcia, F.; Meneses-Garcia, A.; Armas-Plancarte, C.; Saldaña-Ramirez, G.; Medina-Santillan, R. Femoroplasty: A New Option for Femur Metastasis. Pain Pr. 2013, 13, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Plancarte, R.; Guajardo, J.; Meneses-Garcia, A.; Hernandez-Porras, C.; Chejne-Gomez, F.; Medina-Santillan, R.; Galindo-Hueso, G.; Nieves, U.; Cerezo, O. Clinical benefits of femoroplasty: A nonsurgical alternative for the management of femoral metastases. Pain Phys. 2014, 17, 227–234. [Google Scholar] [CrossRef]
- Lei, M.; Liu, Y.; Yang, S.; Jiang, W.; Cao, Y.; Liu, S. Percutaneous cementoplasty for painful osteolytic distal femur metastases: A case report. J. Pain Res. 2016, 9, 859–863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deschamps, F.; Farouil, G.; Hakime, A.; Teriitehau, C.; Barah, A.; de Baere, T. Percutaneous Stabilization of Impending Pathological Fracture of the Proximal Femur. Cardiovasc. Interv. Radiol. 2012, 35, 1428–1432. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Kang, H.G.; Kim, T.S.; Kim, S.-K.; Kim, J.H.; Kim, H.S. Palliative percutaneous stabilization of lower extremity for bone metastasis using flexible nails and bone cement. Surg. Oncol. 2014, 23, 192–198. [Google Scholar] [CrossRef]
- Kawai, N.; Sato, M.; Iwamoto, T.; Tanihata, H.; Minamiguti, H.; Nakata, K. Percutaneous Osteoplasty with Use of a Cement-filled Catheter for a Pathologic Fracture of the Humerus. J. Vasc. Interv. Radiol. 2007, 18, 805–809. [Google Scholar] [CrossRef]
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 851–857. [Google Scholar] [CrossRef]
- Palmer, I.; Nelson, J.; Schatton, W.; Dunne, N.J.; Buchanan, F.J.; Clarke, S.A. Biocompatibility of calcium phosphate bone cement with optimized mechanical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 308–315. [Google Scholar] [CrossRef]
- Fathi, M.; Kholtei, A.; EL Youbi, S.; EL Idrissi, B.C. Setting Properties of Calcium Phosphate Bone Cement. Mater. Today Proc. 2019, 13, 876–881. [Google Scholar] [CrossRef]
- Nakata, K.; Kawai, N.; Sato, M.; Cao, G.; Sahara, S.; Sonomura, T.; Takasaka, I.; Minamiguchi, H.; Nakai, M. Bone Marrow Nails Created by Percutaneous Osteoplasty for Long Bone Fracture: Comparisons Among Acrylic Cement Alone, Acrylic-Cement–Filled Bare Metallic Stent, and Acrylic-Cement–Filled Covered Metallic Stent. Cardiovasc. Interv. Radiol. 2011, 34, 609–614. [Google Scholar] [CrossRef]
- Nakata, K.; Kawai, N.; Sato, M.; Cao, G.; Sahara, S.; Tanihata, H.; Takasaka, I.; Minamiguchi, H.; Nakai, T. Percutaneous Osteoplasty with a Bone Marrow Nail for Fractures of Long Bones: Experimental Study. J. Vasc. Interv. Radiol. 2010, 21, 1436–1441. [Google Scholar] [CrossRef]
- Bertholon, S.; Grange, R.; Thomas, T.; Tetard, M.-C.; Barral, F.-G.; Beneton, A.; Morisson, S.; Grange, S. Combination of Percutaneous Screw Fixation and Cementoplasty for Lytic Bone Metastases: Feasibility, Safety and Clinical Outcomes. Cardiovasc. Interv. Radiol. 2022, 45, 1129–1133. [Google Scholar] [CrossRef]
- Assouline, J.; Tselikas, L.; Roux, C.; Yevich, S.; Delpla, A.; Najafi, A.; Al Ahmar, M.; Bijot, J.-C.; de Baère, T.; Deschamps, F. Prophylactic Percutaneous Consolidation of Large Osteolytic Tumors of the Pelvic Ring Using Fixation by Internal Cemented Screws. Radiol. Imaging Cancer 2021, 3, e200137. [Google Scholar] [CrossRef] [PubMed]
- Autrusseau, P.-A.; Garnon, J.; Bertucci, G.; Dalili, D.; De Marini, P.; Auloge, P.; Koch, G.; Caudrelier, J.; Weiss, J.; Cazzato, R.L.; et al. Complications of percutaneous image-guided screw fixation: An analysis of 94 consecutive patients. Diagn. Interv. Imaging 2021, 102, 347–353. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Koch, G.; Garnon, J.; Ramamurthy, N.; Jégu, J.; Clavert, P.; Gangi, A. Biomechanical effects of osteoplasty with or without Kirschner wire augmentation on long bone diaphyses undergoing bending stress: Implications for percutaneous imaging-guided consolidation in cancer patients. Eur. Radiol. Exp. 2019, 3, 4. [Google Scholar] [CrossRef]
- Abdel-Aal, A.K.; Underwood, E.S.; Saddekni, S. Use of Cryoablation and Osteoplasty Reinforced with Kirschner Wires in the Treatment of Femoral Metastasis. Cardiovasc. Interv. Radiol. 2012, 35, 1211–1215. [Google Scholar] [CrossRef]
- Szpalski, M.; Gunzburg, R.; Aebi, M.; Delimoge, C.; Graf, N.; Eberle, S.; Vienney, C. A new approach to prevent contralateral hip fracture: Evaluation of the effectiveness of a fracture preventing implant. Clin. Biomech. 2015, 30, 713–719. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Tselikas, L.; Carteret, T.; Lapuyade, B.; De Baere, T.; Le Huec, J.C.; Deschamps, F. Percutaneous internal fixation with Y-STRUT® device to prevent both osteoporotic and pathological hip fractures: A prospective pilot study. J. Orthop. Surg. Res. 2017, 12, 27. [Google Scholar] [CrossRef][Green Version]
- Koirala, N.; Duffy, S.; McLennan, G. A biomechanical testing model for evaluating the feasibility of percutaneous osteoplasty in weight-bearing bones. J. Vasc. Interv. Radiol. 2016, 27, S135. [Google Scholar] [CrossRef]
- Mifsut, D.; Renovell, P.; Gomar, F.; Saravia, M. Percutaneous osteoplasty in treatment of bone lymphangiomatosis. Indian J. Orthop. 2013, 47, 515–518. [Google Scholar] [CrossRef]
- Tian, Q.; He, C.; Xiao, Q. Clinical Study of Percutaneous Osteoplasty with and without Interventional Internal Fixation for Impending Pathological Fracture of the Proximal Femur. Chin. J. Radiol. 2015, 49, 52–56. [Google Scholar] [CrossRef]
- Kelekis, A.; Martin, J.-B.; Anselmetti, G.; Filipiadis, D. Regarding “Percutaneous Augmented Peripheral Osteoplasty in Long Bones of Oncologic Patients for Pain Reduction and Prevention of Impeding Pathologic Fracture: The Rebar Concept”: Reply. Cardiovasc. Interv. Radiol. 2016, 39, 479–480. [Google Scholar] [CrossRef]
- Bensoussan, S.; Premat, K.; Shotar, E.; Cormier, É.; Al Raasi, A.; Spano, J.-P.; Morardet, L.; Bonaccorsi, R.; Morel, V.; Mathout, J.; et al. Percutaneous reinforced cementoplasty using spindles as a palliative option for malignant fractures of the humerus. Diagn. Interv. Imaging 2022, 103, 375–377. [Google Scholar] [CrossRef]
- Lee, F.Y.; Latich, I.; Toombs, C.; Mungur, A.; Conway, D.; Alder, K.; Ibe, I.; Lindskog, D.; Friedlaender, G. Minimally Invasive Image-Guided Ablation, Osteoplasty, Reinforcement, and Internal Fixation (AORIF) for Osteolytic Lesions in the Pelvis and Periarticular Regions of Weight-Bearing Bones. J. Vasc. Interv. Radiol. 2020, 31, 649–658.e1. [Google Scholar] [CrossRef]
- Dassa, M.; Roux, C.; Tselikas, L.; Delpla, A.; Yevich, S.; Faron, M.; Teriitehau, C.; Hakime, A.; Al Ahmar, M.; de Baère, T.; et al. Image-guided Percutaneous Fixation with Internal Cemented Screws of Impending Femoral Neck Pathologic Fractures in Patients with Metastatic Cancer: Safety, Efficacy, and Durability. Radiology 2020, 297, 721–729. [Google Scholar] [CrossRef]
- Khan, M.; Deib, G.; Deldar, B.; Patel, A.; Barr, J. Efficacy and Safety of Percutaneous Microwave Ablation and Cementoplasty in the Treatment of Painful Spinal Metastases and Myeloma. Am. J. Neuroradiol. 2018, 39, 1376–1383. [Google Scholar] [CrossRef]
- Couraud, G.; Gaston, A.-P.; Thuillier, T.; Eymard, F.; Hourdille, A.; Chevalier, X.-J.; Boussion, H.; Guignard, S. Evaluation of short-term efficacy of extraspinal cementoplasty for bone metastasis: A monocenter study of 31 patients. J. Bone Oncol. 2018, 13, 136–142. [Google Scholar] [CrossRef]
- Huang, S.-L.; Wen, B.; Bian, W.-G.; Yan, H.-W. Reconstruction of comminuted long-bone fracture using CF/CPC scaffolds manufactured by rapid prototyping. Med. Sci. Monit. 2012, 18, BR435–BR440. [Google Scholar] [CrossRef]
- McDonald, E.; Chu, T.; Tufaga, M.; Marmor, M.; Singh, R.; Yetkinler, D.; Matityahu, A.; Buckley, J.M.; McClellan, R.T. Tibial Plateau Fracture Repairs Augmented With Calcium Phosphate Cement Have Higher In Situ Fatigue Strength Than Those With Autograft. J. Orthop. Trauma 2011, 25, 90–95. [Google Scholar] [CrossRef]
- Libicher, M.; Hillmeier, J.; Liegibel, U.; Sommer, U.; Pyerin, W.; Vetter, M.; Meinzer, H.-P.; Grafe, I.; Meeder, P.; Nöldge, G.; et al. Osseous integration of calcium phosphate in osteoporotic vertebral fractures after kyphoplasty: Initial results from a clinical and experimental pilot study. Osteoporos. Int. 2006, 17, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Seeherman, H.J.; Bouxsein, M.; Kim, H.; Li, R.; Li, X.J.; Aiolova, M.; Wozney, J.M. Recombinant Human Bone Morphogenetic Protein-2 Delivered in an Injectable Calcium Phosphate Paste Accelerates Osteotomy-Site Healing in a Nonhuman Primate Model. J. Bone Jt. Surg. 2004, 86, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, D.; Soroori, S.; Hasaraki, S.; Jafari, N. Radiographic Evaluations of the Tetra-Calcium Phosphate and Diacalcium Phosphate with Bone Plate in Osseo-Integration of Bone Repair in Rabbit. Am. J. Anim. Vet. Sci. 2009, 4, 80–84. [Google Scholar] [CrossRef]
- Ambard, A.J.; Mueninghoff, L. Calcium Phosphate Cement: Review of Mechanical and Biological Properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef]
- Seeherman, H.; Wozney, J.M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345. [Google Scholar] [CrossRef]
- Steiner, M.; Volkheimer, D.; Meyers, N.; Wehner, T.; Wilke, H.-J.; Claes, L.; Ignatius, A. Comparison between Different Methods for Biomechanical Assessment of Ex Vivo Fracture Callus Stiffness in Small Animal Bone Healing Studies. PLoS ONE 2015, 10, e0119603. [Google Scholar] [CrossRef]
- Fatihhi, S.; Rabiatul, A.; Harun, M.; Kadir, M.R.A.; Kamarul, T.; Syahrom, A. Effect of torsional loading on compressive fatigue behaviour of trabecular bone. J. Mech. Behav. Biomed. Mater. 2016, 54, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, R.; Müller, R. Three-dimensional analysis of nonhuman primate trabecular architecture using micro-computed tomography. Am. J. Phys. Anthr. 2001, 115, 327–336. [Google Scholar] [CrossRef]
- Niebur, G.L.; Feldstein, M.J.; Yuen, J.C.; Chen, T.J.; Keaveny, T.M. High-resolution finite element models with tissue strength asymmetry accurately predict failure of trabecular bone. J. Biomech. 2000, 33, 1575–1583. [Google Scholar] [CrossRef]
- Althomali, M.A.; Wille, M.-L.; Shortell, M.P.; Langton, C.M. Estimation of mechanical stiffness by finite element analysis of ultrasound computed tomography (UCT-FEA); a comparison with X-ray µCT based FEA in cancellous bone replica models. Appl. Acoust. 2018, 133, 8–15. [Google Scholar] [CrossRef]
- E Martin, D.; E Severns, A.; Kabo, J. Determination of mechanical stiffness of bone by pQCT measurements: Correlation with non-destructive mechanical four-point bending test data. J. Biomech. 2004, 37, 1289–1293. [Google Scholar] [CrossRef]
- D20 Committee. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar] [CrossRef]
- Flexural Strength (MOR) and Flexural Modulus (MOE) of Composite Materials and Profiles. In Wood-Plastic Composites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 225–318. ISBN 978-0-470-16593-5.
- Compressive and Tensile Strength and Modulus of Composite Profiles. In Wood-Plastic Composites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 319–332. ISBN 978-0-470-16593-5.
- D20 Committee. Standard Test Method for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials by Four-Point Bending; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- D20 Committee. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastic Lumber and Related Products; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Bramer, J.A.; Barentsen, R.H.; Elst, M.V.; Lange, E.S.; Patka, P.; Haarman, H.J. Representative assessment of long bone shaft biomechanical properties: An optimized testing method. J. Biomech. 1998, 31, 741–745. [Google Scholar] [CrossRef]
- Foux, A.; Black, R.C.; Uhthoff, H.K. Quantitative Measures for Fracture Healing: An In-Vitro Biomechanical Study. J. Biomech. Eng. 1990, 112, 401–406. [Google Scholar] [CrossRef]
- Dragomir-Daescu, D.; Rezaei, A.; Uthamaraj, S.; Rossman, T.; Bronk, J.T.; Bolander, M.; Lambert, V.; McEligot, S.; Entwistle, R.; Giambini, H.; et al. Proximal Cadaveric Femur Preparation for Fracture Strength Testing and Quantitative CT-based Finite Element Analysis. J. Vis. Exp. 2017, 121, e54925. [Google Scholar] [CrossRef]
- Ivarsson, B.J.; Genovese, D.; Crandall, J.R.; Bolton, J.R.; Untaroiu, C.D.; Bose, D. The Tolerance of the Femoral Shaft in Combined Axial Compression and Bending Loading. Stapp. Car. Crash J. 2009, 53, 251–290. [Google Scholar] [CrossRef]
- Inacio, J.V.; Cristino, D.; Hast, M.W.; Dailey, H. An Adaptable Ct-Derived 3D-Printed Alignment Fixture Minimizes Errors in Whole-Bone Biomechanical Testing. J. Biomech. Eng. 2021, 143, 111006. [Google Scholar] [CrossRef]
- Jepsen, K.J.; Silva, M.; Vashishth, D.; Guo, X.E.; van der Meulen, M.C.H. Establishing Biomechanical Mechanisms in Mouse Models: Practical Guidelines for Systematically Evaluating Phenotypic Changes in the Diaphyses of Long Bones. J. Bone Miner. Res. 2015, 30, 951–966. [Google Scholar] [CrossRef]
- Quinnan, S.; Seiter, M.; Al-Barghouthi, A.; Milne, E.; Latta, L.; Travascio, F. Does coating an intramedullary nail with polymethylmethacrylate improve mechanical stability at the fracture site? Clin. Biomech. 2021, 83, 105293. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, Y.; Chen, W.; Li, S.; Yin, B.; Wang, J.; Zhang, X.; Liu, G.; Hu, Z.; Zhang, Y. Bone Hardness of Different Anatomical Regions of Human Radius and its Impact on the Pullout Strength of Screws. Orthop. Surg. 2019, 11, 270–276. [Google Scholar] [CrossRef]
- Broitman, E. Indentation Hardness Measurements at Macro-, Micro-, and Nanoscale: A Critical Overview. Tribol. Lett. 2016, 65, 23. [Google Scholar] [CrossRef]
- Ibrahim, A.; Magliulo, N.; Groben, J.; Padilla, A.; Akbik, F.; Hamid, Z.A. Hardness, an Important Indicator of Bone Quality, and the Role of Collagen in Bone Hardness. J. Funct. Biomater. 2020, 11, 85. [Google Scholar] [CrossRef]
- Defranoux, N.A.; Stokes, C.L.; Young, D.L.; Kahn, A.J. In Silico Modeling and Simulation of Bone Biology: A Proposal. J. Bone Miner. Res. 2005, 20, 1079–1084. [Google Scholar] [CrossRef]
- Madden, J.C.; Enoch, S.J.; Paini, A.; Cronin, M.T. A Review of In Silico Tools as Alternatives to Animal Testing: Principles, Resources and Applications. Altern. Lab. Anim. 2020, 48, 146–172. [Google Scholar] [CrossRef]
- Osuna, L.G.G.; Soares, C.J.; Vilela, A.B.F.; Irie, M.S.; Versluis, A.; Soares, P.B.F. Influence of bone defect position and span in 3-point bending tests: Experimental and finite element analysis. Braz. Oral Res. 2021, 35, e001. [Google Scholar] [CrossRef]
- Premat, K.; Clarençon, F.; Bonaccorsi, R.; Degos, V.; Cormier, É.; Chiras, J. Reinforced cementoplasty using dedicated spindles in the management of unstable malignant lesions of the cervicotrochanteric region. Eur. Radiol. 2017, 87, 1810–3982. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Anselmetti, G.C.; Zoarski, G.; Manca, A.; Masala, S.; Eminefendic, H.; Russo, F.; Regge, D. Percutaneous Vertebroplasty and Bone Cement Leakage: Clinical Experience with a New High-Viscosity Bone Cement and Delivery System for Vertebral Augmentation in Benign and Malignant Compression Fractures. Cardiovasc. Interv. Radiol. 2008, 31, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Georgy, B.A. Feasibility, Safety and Cement Leakage in Vertebroplasty of Osteoporotic and Malignant Compression Fractures Using Ultra-Viscous Cement and Hydraulic Delivery System. Pain Phys. 2012, 15, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Garnon, J.; Meylheuc, L.; Harrer, L.; Koch, G.; Gangi, A.; Bayle, B. Injection Device for Percutaneous Osteoplasty. In New Trends in Medical and Service Robotics; Rauter, G., Cattin, P.C., Zam, A., Riener, R., Carbone, G., Pisla, D., Eds.; Mechanisms and Machine Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 93, pp. 81–88. ISBN 978-3-030-58103-9. [Google Scholar]
- Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, Y.; Ren, Y.; Zou, D. Prevention and treatment of bone cement-related complications in patients receiving percutaneous kyphoplasty. Int. J. Clin. Exp. Med. 2015, 8, 2371–2377. [Google Scholar]
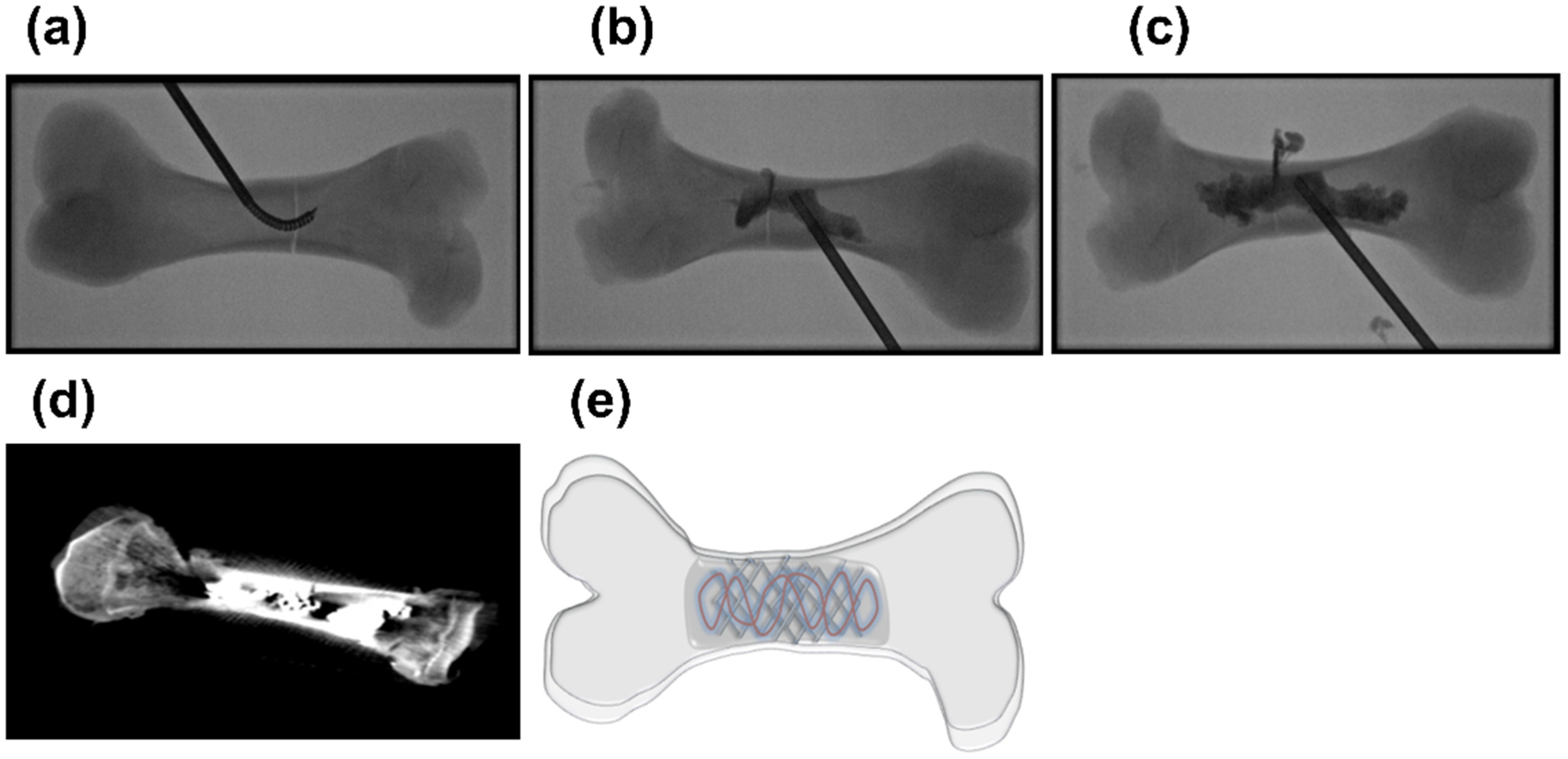
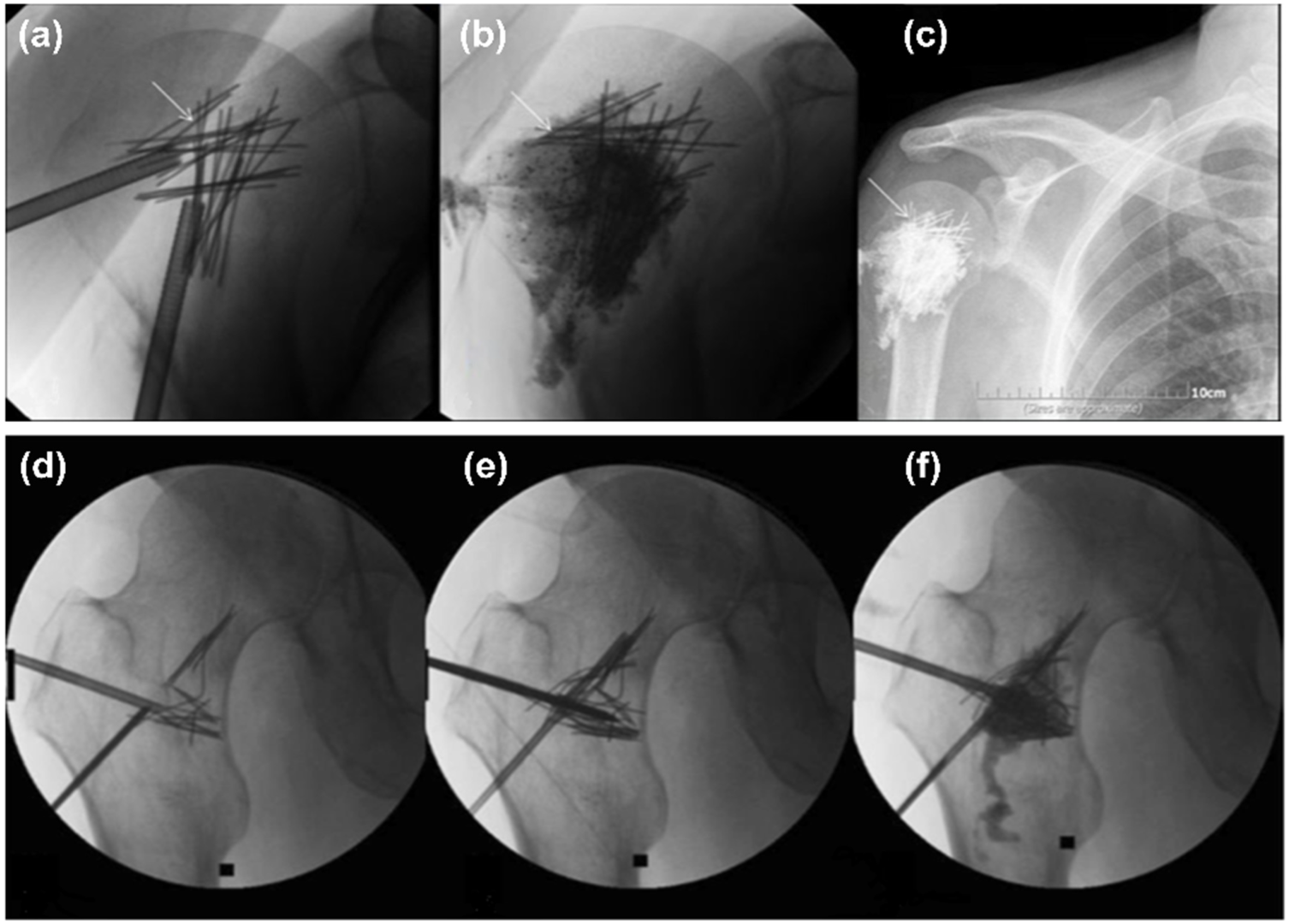

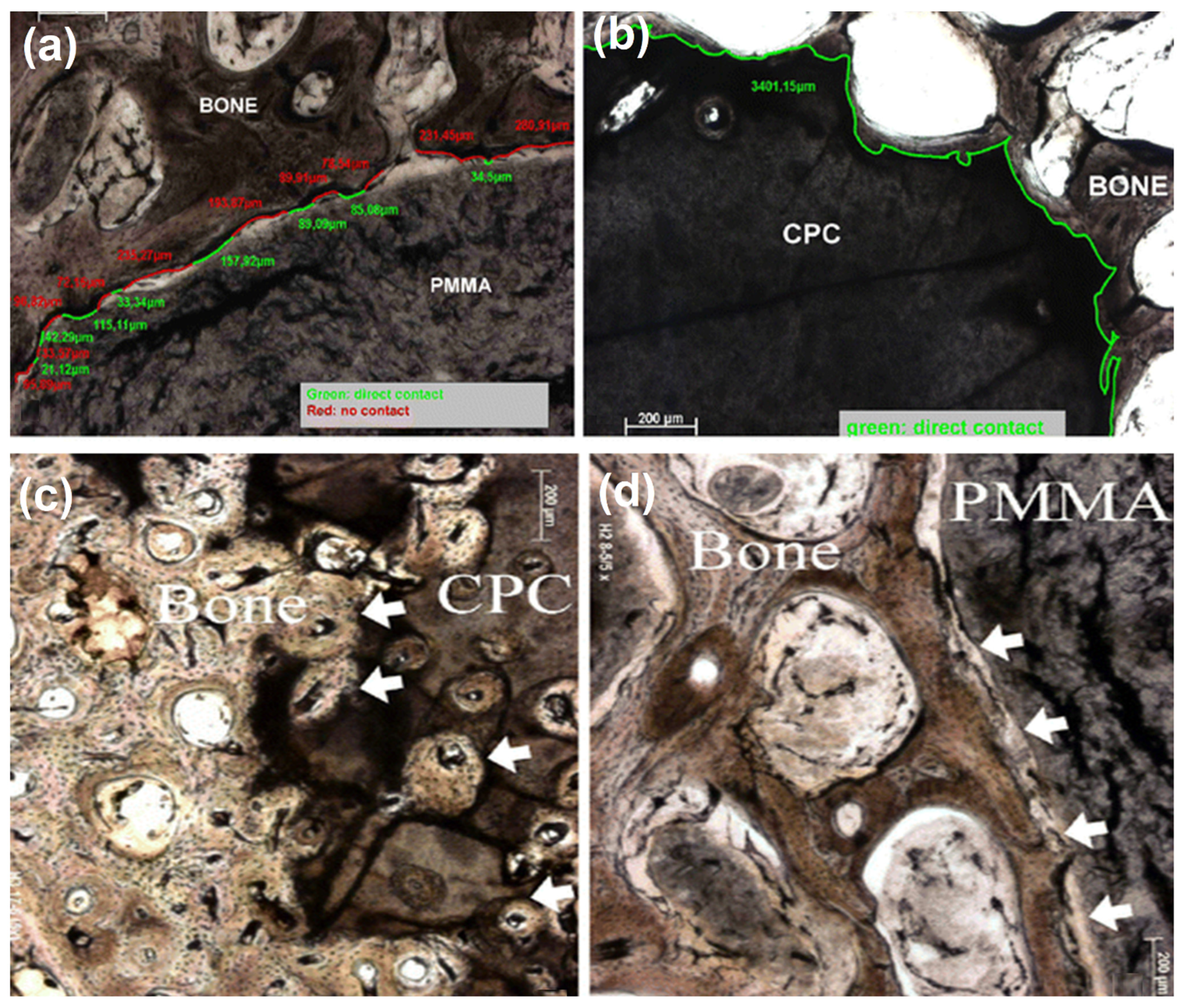

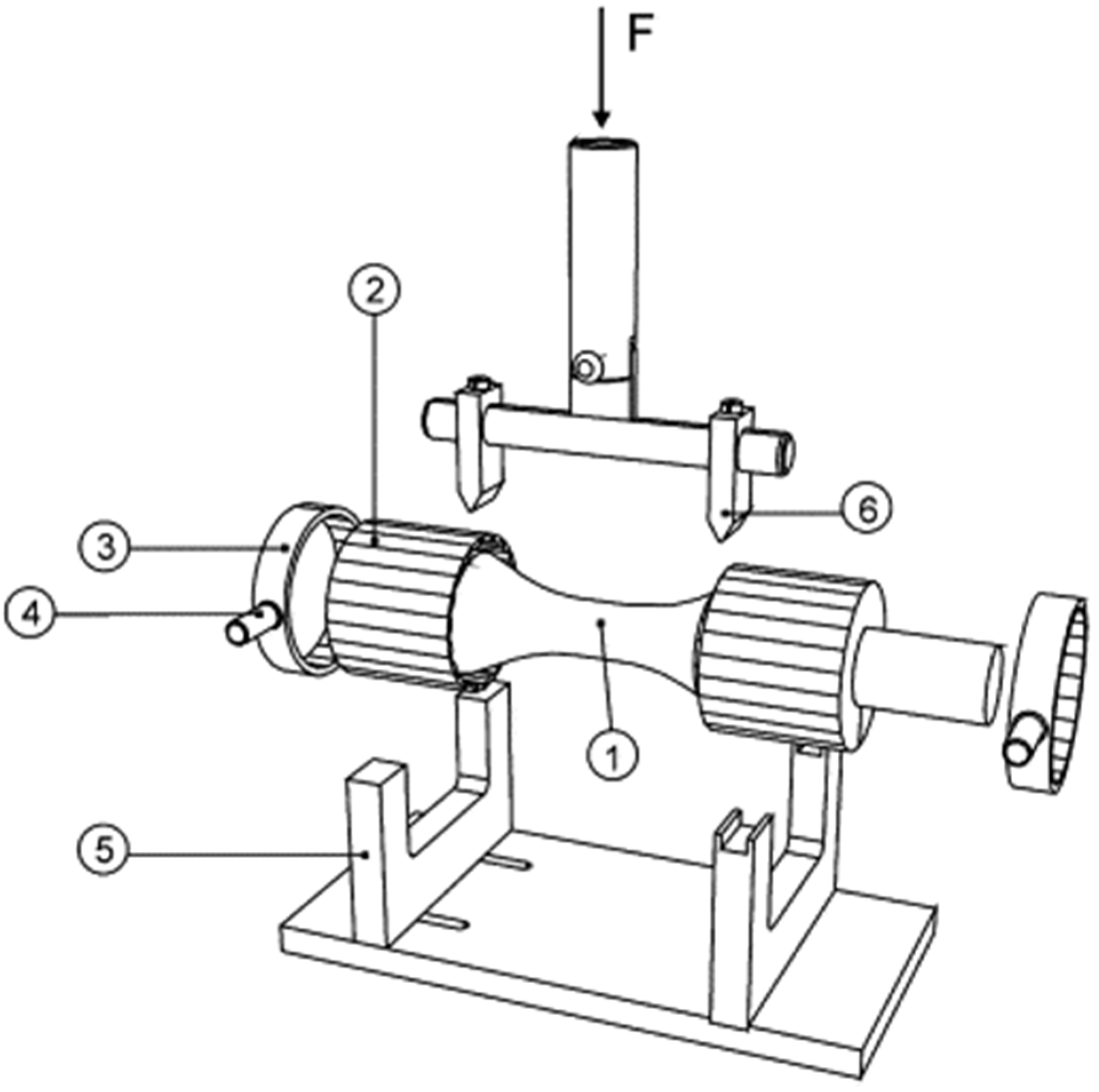
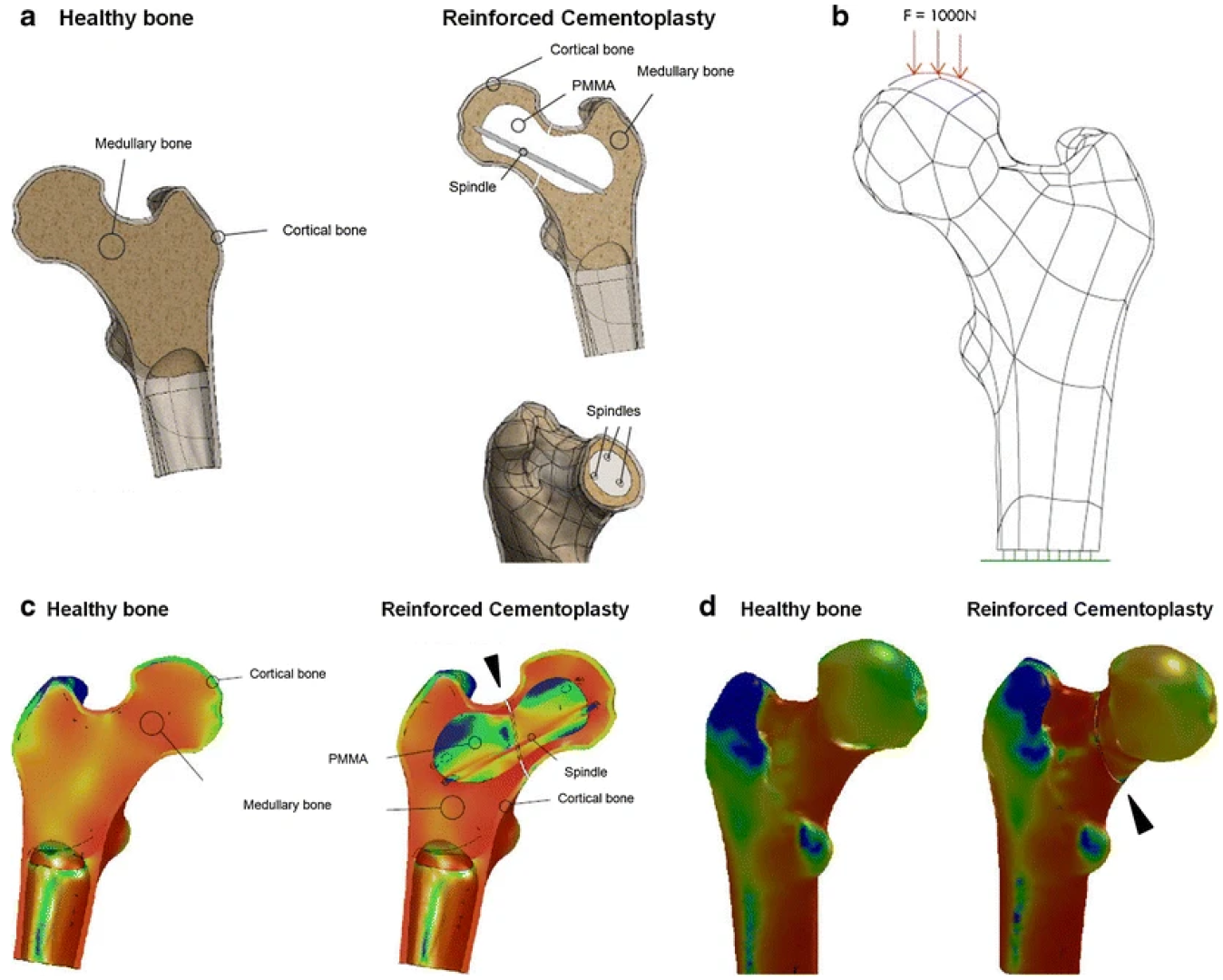
| Type | Examples | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Natural | Collagen, Hyaluronans, fibrin, chitosan, silk | Biocompatible, Bioresorbable | Procurement, disease transmission, immunogenicity | [68] |
| Synthetic | Polylactide, polyglycolide, PLGA | Design flexibility, no disease transmission | Inflammatory response, poor clearance due to high molecular weight | |
| Inorganic | Calcium orthophosphates, Bioglass | Biocompatible, Bioresorbable, osteoconductive | Low mechanical property, lack of macroporosity for cell infiltration | |
| Composite | Composite materials that include natural, synthetic, and/or inorganic components | Biocompatibility, improved handling | Phase separation |
| Parameter | Center Point Load (3-Point Test) | 4-Point Test | Reference | |
|---|---|---|---|---|
| Third-Point Load | Quarter-Point Load | |||
| Flexural strength (Bending stress) | [75,76,77,78,79] | |||
| Flexural modulus | ||||
| Deflection | ||||
| Bending moment | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koirala, N.; Joshi, J.; Duffy, S.F.; McLennan, G. Percutaneous-Reinforced Osteoplasty: A Review of Emerging Treatment Strategies for Bone Interventions. J. Clin. Med. 2022, 11, 5572. https://doi.org/10.3390/jcm11195572
Koirala N, Joshi J, Duffy SF, McLennan G. Percutaneous-Reinforced Osteoplasty: A Review of Emerging Treatment Strategies for Bone Interventions. Journal of Clinical Medicine. 2022; 11(19):5572. https://doi.org/10.3390/jcm11195572
Chicago/Turabian StyleKoirala, Nischal, Jyotsna Joshi, Stephen F. Duffy, and Gordon McLennan. 2022. "Percutaneous-Reinforced Osteoplasty: A Review of Emerging Treatment Strategies for Bone Interventions" Journal of Clinical Medicine 11, no. 19: 5572. https://doi.org/10.3390/jcm11195572
APA StyleKoirala, N., Joshi, J., Duffy, S. F., & McLennan, G. (2022). Percutaneous-Reinforced Osteoplasty: A Review of Emerging Treatment Strategies for Bone Interventions. Journal of Clinical Medicine, 11(19), 5572. https://doi.org/10.3390/jcm11195572





