A New Look on Long-COVID Effects: The Functional Brain Fog Syndrome
Abstract
1. Introduction
1.1. Brain Fog and Fatigue
1.2. Brain Fog and Cognitive Symptoms
1.3. Brain Fog and Neuropsychiatric Symptoms
1.4. Aims and Hypotheses of the Study
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Assessment
2.3.1. Brain Fog and Subjective Cognitive Complaints
2.3.2. Positive Affect and Negative Affect Schedule (PANAS)
2.3.3. Depression, Anxiety and Stress Scale (DASS-21)
2.3.4. Impact of Event Scale-Revised (IES-R)
2.3.5. Beck Cognitive Insight Scale
2.3.6. Sleep Disorders
2.4. Statistical Analyses
3. Results
3.1. Neuropsychiatric Characteristics of HS, PBF, and FBF Groups
3.2. Correlates and Predictors of Brain Fog and Cognitive Complaints in Functional Brain Fog Group
4. Discussion
4.1. Brain Fog and Scc
4.2. The Role of Neuropsychiatric Symptoms
4.3. The Role of Demographic Variables
4.4. A Bio-Psycho-Social Model of Brain Fog
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Titze-de-Almeida, R.; da Cunha, T.R.; dos Santos Silva, L.D.; Ferreira, C.S.; Silva, C.P.; Ribeiro, A.P.; de Castro Moreira Santos Júnior, A.; de Paula Brandão, P.R.; Silva, A.P.B.; da Rocha, M.C.O.; et al. Persistent, New-Onset Symptoms and Mental Health Complaints in Long COVID in a Brazilian Cohort of Non-Hospitalized Patients. BMC Infect. Dis. 2022, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Costas-Carrera, A.; Sánchez-Rodríguez, M.M.; Cañizares, S.; Ojeda, A.; Martín-Villalba, I.; Primé-Tous, M.; Rodríguez-Rey, M.A.; Segú, X.; Valdesoiro-Pulido, F.; Borras, R.; et al. Neuropsychological Functioning in Post-ICU Patients after Severe COVID-19 Infection: The Role of Cognitive Reserve. Brain Behav. Immun.-Health 2022, 21, 100425. [Google Scholar] [CrossRef] [PubMed]
- Turana, Y.; Nathaniel, M.; Shen, R.; Ali, S.; Aparasu, R.R. Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications. Brain Sci. 2021, 12, 59. [Google Scholar] [CrossRef]
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent Symptoms after Covid-19: Qualitative Study of 114 “Long Covid” Patients and Draft Quality Principles for Services. BMC Health Serv. Res. 2020, 20, 1144. [Google Scholar] [CrossRef]
- Callan, C.; Ladds, E.; Husain, L.; Pattinson, K.; Greenhalgh, T. ‘I Can’t Cope with Multiple Inputs’: A Qualitative Study of the Lived Experience of ‘Brain Fog’ after COVID-19. BMJ Open 2022, 12, e056366. [Google Scholar] [CrossRef]
- Heiberg, K.E.; Heggestad, A.K.T.; Jøranson, N.; Lausund, H.; Breievne, G.; Myrstad, M.; Ranhoff, A.H.; Walle-Hansen, M.M.; Bruun-Olsen, V. ‘Brain Fog’, Guilt, and Gratitude: Experiences of Symptoms and Life Changes in Older Survivors 6 Months after Hospitalisation for COVID-19. Eur. Geriatr. Med. 2022, 13, 695–703. [Google Scholar] [CrossRef]
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive Profile Following COVID-19 Infection: Clinical Predictors Leading to Neuropsychological Impairment. Brain Behav. Immun.-Health 2020, 9, 100163. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent Neurologic Symptoms and Cognitive Dysfunction in Non-hospitalized Covid-19 “Long Haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID Syndrome-associated Brain Fog and Chemofog: Luteolin to the Rescue. BioFactors 2021, 47, 232–241. [Google Scholar] [CrossRef]
- Mantovani, E.; Mariotto, S.; Gabbiani, D.; Dorelli, G.; Bozzetti, S.; Federico, A.; Zanzoni, S.; Girelli, D.; Crisafulli, E.; Ferrari, S.; et al. Chronic Fatigue Syndrome: An Emerging Sequela in COVID-19 Survivors? J. Neurovirol. 2021, 27, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Granberg, T.; Andersen, P.; Jokhadar, E.; Kåhlin, J.; Granström, A.; Hallinder, H.; Schening, A.; Thunborg, C.; Walles, H.; et al. The Karolinska NeuroCOVID Study Protocol: Neurocognitive Impairment, Biomarkers and Advanced Imaging in Critical Care Survivors. Acta Anaesthesiol. Scand. 2022, 66, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and Long-Term Neurological and Neuropsychiatric Manifestations of Post-COVID-19 Syndrome: A Meta-Analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive Deficits in People Who Have Recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Krishnan, K.; Lin, Y.; Prewitt, K.-R.M.; Potter, D.A. Multidisciplinary Approach to Brain Fog and Related Persisting Symptoms Post COVID-19. J. Health Serv. Psychol. 2022, 48, 31–38. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Stefano, G.B. Historical Insight into Infections and Disorders Associated with Neurological and Psychiatric Sequelae Similar to Long COVID. Med. Sci. Monit. 2021, 27, e931447. [Google Scholar] [CrossRef]
- Franssen, F.M.E.; Janssen, D.J.A.; Spruit, M.A. Long COVID: The next Challenge for Health Care. Ned. Tijdschr. Geneeskd. 2022, 166, D6413. [Google Scholar]
- Hoffer, E.P. Long COVID: Does It Exist? What Is It? We Can We Do For Sufferers? Am. J. Med. 2021, 134, 1310–1311. [Google Scholar] [CrossRef]
- Yong, E. COVID-19 Can Last for Several Months. Available online: https://www.theatlantic.com/health/archive/2020/06/covid-19-coronavirus-longterm-symptoms-months/612679/ (accessed on 4 June 2020).
- The Lancet. Facing up to Long COVID. Lancet 2020, 396, 1861. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, T.; Edwards, M.J.; Isaacs, J.D. A Unifying Theory for Cognitive Abnormalities in Functional Neurological Disorders, Fibromyalgia and Chronic Fatigue Syndrome: Systematic Review. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1308–1319. [Google Scholar] [CrossRef]
- Badenoch, J.B.; Rengasamy, E.R.; Watson, C.; Jansen, K.; Chakraborty, S.; Sundaram, R.D.; Hafeez, D.; Burchill, E.; Saini, A.; Thomas, L.; et al. Persistent Neuropsychiatric Symptoms after COVID-19: A Systematic Review and Meta-Analysis. Brain Commun. 2022, 4, fcab297. [Google Scholar] [CrossRef] [PubMed]
- Duerlund, L.S.; Shakar, S.; Nielsen, H.; Bodilsen, J. Positive Predictive Value of the ICD-10 Diagnosis Code for Long-COVID. Clin. Epidemiol. 2022, 14, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Maralakunte, M.; Garg, S.; Dhooria, S.; Sehgal, I.; Bhalla, A.S.; Vijayvergiya, R.; Grover, S.; Bhatia, V.; Jagia, P.; et al. The Conundrum of ‘Long-COVID-19ʹ: A Narrative Review. Int. J. Gen. Med. 2021, 14, 2491–2506. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, Ó.J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef]
- Stefano, G.B.; Büttiker, P.; Weissenberger, S.; Martin, A.; Ptacek, R.; Kream, R.M. Editorial: The Pathogenesis of Long-Term Neuropsychiatric COVID-19 and the Role of Microglia, Mitochondria, and Persistent Neuroinflammation: A Hypothesis. Med. Sci. Monit. 2021, 27, e933015. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Miller, A.K.; Reiter, K.; Bonner-Jackson, A. Neurocognitive Profiles in Patients with Persisting Cognitive Symptoms Associated with COVID-19. Arch. Clin. Neuropsychol. 2022, 37, 729–737. [Google Scholar] [CrossRef]
- Iosifescu, D.V. The Relation between Mood, Cognition and Psychosocial Functioning in Psychiatric Disorders. Eur. Neuropsychopharmacol. 2012, 22, S499–S504. [Google Scholar] [CrossRef]
- Pan, Z.; Park, C.; Brietzke, E.; Zuckerman, H.; Rong, C.; Mansur, R.B.; Fus, D.; Subramaniapillai, M.; Lee, Y.; McIntyre, R.S. Cognitive Impairment in Major Depressive Disorder. CNS Spectr. 2019, 24, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Barch, D.M.; Price, J.L.; Rundle, M.M.; Vaishnavi, S.N.; Snyder, A.Z.; Mintun, M.A.; Wang, S.; Coalson, R.S.; Raichle, M.E. The Default Mode Network and Self-Referential Processes in Depression. Proc. Natl. Acad. Sci. USA 2009, 106, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Tanno, Y. Self-Rumination, Self-Reflection, and Depression: Self-Rumination Counteracts the Adaptive Effect of Self-Reflection. Behav. Res. Ther. 2009, 47, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Amicucci, G.; Salfi, F.; D’Atri, A.; Viselli, L.; Ferrara, M. The Differential Impact of COVID-19 Lockdown on Sleep Quality, Insomnia, Depression, Stress, and Anxiety among Late Adolescents and Elderly in Italy. Brain Sci. 2021, 11, 1336. [Google Scholar] [CrossRef]
- Salfi, F.; Lauriola, M.; Amicucci, G.; Corigliano, D.; Viselli, L.; Tempesta, D.; Ferrara, M. Gender-Related Time Course of Sleep Disturbances and Psychological Symptoms during the COVID-19 Lockdown: A Longitudinal Study on the Italian Population. Neurobiol. Stress 2020, 13, 100259. [Google Scholar] [CrossRef]
- Gualano, M.R.; Lo Moro, G.; Voglino, G.; Bert, F.; Siliquini, R. Effects of Covid-19 Lockdown on Mental Health and Sleep Disturbances in Italy. Int. J. Environ. Res. Public Health 2020, 17, 4779. [Google Scholar] [CrossRef]
- Prati, G. Mental Health and Its Psychosocial Predictors during National Quarantine in Italy against the Coronavirus Disease 2019 (COVID-19). Anxiety Stress Coping 2021, 34, 145–156. [Google Scholar] [CrossRef]
- Salfi, F.; D’Atri, A.; Tempesta, D.; Ferrara, M. Sleeping under the Waves: A Longitudinal Study across the Contagion Peaks of the COVID-19 Pandemic in Italy. J. Sleep Res. 2021, 30, e13313. [Google Scholar] [CrossRef]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 Symptoms 6 Months after Acute Infection among Hospitalized and Non-Hospitalized Patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Saydah, S.H.; Brooks, J.T.; Jackson, B.R. Surveillance for Post-COVID Conditions Is Necessary: Addressing the Challenges with Multiple Approaches. J. Gen. Intern. Med. 2022, 37, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Pinto Pereira, S.M.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; Chalder, T.; et al. Physical and Mental Health 3 Months after SARS-CoV-2 Infection (Long COVID) among Adolescents in England (CLoCk): A National Matched Cohort Study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef]
- Tomasoni, D.; Bai, F.; Castoldi, R.; Barbanotti, D.; Falcinella, C.; Mulè, G.; Mondatore, D.; Tavelli, A.; Vegni, E.; Marchetti, G.; et al. Anxiety and Depression Symptoms after Virological Clearance of COVID-19: A Cross-sectional Study in Milan, Italy. J. Med. Virol. 2021, 93, 1175–1179. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Terracciano, A.; McCrae, R.R.; Costa, P.T., Jr. Factorial and Construct Validity of the Italian Positive and Negative Affect Schedule (PANAS). Eur. J. Psychol. Assess. 2003, 19, 131–141. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Lovibond, S.H. The Structure of Negative Emotional States: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Bottesi, G.; Ghisi, M.; Altoè, G.; Conforti, E.; Melli, G.; Sica, C. The Italian Version of the Depression Anxiety Stress Scales-21: Factor Structure and Psychometric Properties on Community and Clinical Samples. Compr. Psychiatry 2015, 60, 170–181. [Google Scholar] [CrossRef]
- Weiss, D.F.; Marmer, C.R. The Impact of Event Scale-Revised. In Assessing Psychological Trauma and PTSD: A Practitioners Handbook; Wilson, J.P., Keane, T.M., Eds.; Guilford Press: New York, NY, USA, 1997; pp. 399–411. [Google Scholar]
- APA. American Psychiatric Association. In Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; (Text Revision); American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Creamer, M.; Bell, R.; Failla, S. Psychometric Properties of the Impact of Event Scale—Revised. Behav. Res. Ther. 2003, 41, 1489–1496. [Google Scholar] [CrossRef]
- Craparo, G.; Faraci, P.; Rotondo, G.; Gori, A. The Impact of Event Scale – Revised: Psychometric Properties of the Italian Version in a Sample of Flood Victims. Neuropsychiatr. Dis. Treat. 2013, 2013, 1427–1432. [Google Scholar] [CrossRef]
- Beck, A. A New Instrument for Measuring Insight: The Beck Cognitive Insight Scale. Schizophr. Res. 2004, 68, 319–329. [Google Scholar] [CrossRef]
- Orfei, M.D.; Calatgirone, C.; Spalletta, G. I Disturbi Della Consapevolezza Nelle Malattie Neuropsichiatriche; Springer: Milan, Italy, 2007. [Google Scholar]
- Orfei, M.D.; Caltagirone, C.; Cacciari, C.; Assogna, F.; Spalletta, G. The Neuropsychological Correlates of Cognitive Insight in Healthy Participants. Appl. Cogn. Psychol. 2011, 25, 927–932. [Google Scholar] [CrossRef]
- Carse, T.; Langdon, R. Delusion Proneness in Nonclinical Individuals and Cognitive Insight: The Contributions of Rumination and Reflection. J. Nerv. Ment. Dis. 2013, 201, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Tastet, H.; Verdoux, H.; Bergua, V.; Destaillats, J.-M.; Prouteau, A. Cognitive Insight in Schizophrenia: The Missing Link Between Insight and Neurocognitive Complaint? J. Nerv. Ment. Dis. 2012, 200, 908–910. [Google Scholar] [CrossRef]
- Xu, L.; Cui, H.; Wei, Y.; Qian, Z.; Tang, X.; Hu, Y.; Wang, Y.; Hu, H.; Guo, Q.; Tang, Y.; et al. Relationships between Self-Reflectiveness and Clinical Symptoms in Individuals during Pre-Morbid and Early Clinical Stages of Psychosis. Gen. Psychiatry 2022, 35, e100696. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Torres-Macho, J.; Velasco-Arribas, M.; Arias-Navalón, J.A.; Guijarro, C.; Hernández-Barrera, V.; Canto-Diez, M.G. Similar Prevalence of Long-Term Post-COVID Symptoms in Patients with Asthma: A Case-Control Study. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]
- Orrù, G.; Bertelloni, D.; Diolaiuti, F.; Mucci, F.; Di Giuseppe, M.; Biella, M.; Gemignani, A.; Ciacchini, R.; Conversano, C. Long-COVID Syndrome? A Study on the Persistence of Neurological, Psychological and Physiological Symptoms. Healthcare 2021, 9, 575. [Google Scholar] [CrossRef]
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Burden of Post-COVID-19 Syndrome and Implications for Healthcare Service Planning: A Population-Based Cohort Study. PLoS ONE 2021, 16, e0254523. [Google Scholar] [CrossRef]
- The Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.-L.; Meyrignac, O.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525. [Google Scholar] [CrossRef]
- O’Keefe, J.B.; Minton, H.C.; Morrow, M.; Johnson, C.; Moore, M.A.; O’Keefe, G.A.D.; Benameur, K.; Higdon, J.; Fairley, J.K. Postacute Sequelae of SARS-CoV-2 Infection and Impact on Quality of Life 1–6 Months After Illness and Association With Initial Symptom Severity. Open Forum Infect. Dis. 2021, 8, ofab352. [Google Scholar] [CrossRef] [PubMed]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Kofler, M.; Lindner, A.; Mahlknecht, P.; Heim, B.; Limmert, V.; Sahanic, S.; Pizzini, A.; et al. Neurological Outcome and Quality of Life 3 Months after COVID-19: A Prospective Observational Cohort Study. Eur. J. Neurol. 2021, 28, 3348–3359. [Google Scholar] [CrossRef] [PubMed]
- Søraas, A.; Bø, R.; Kalleberg, K.T.; Støer, N.C.; Ellingjord-Dale, M.; Landrø, N.I. Self-Reported Memory Problems 8 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2118717. [Google Scholar] [CrossRef] [PubMed]
- Amin-Chowdhury, Z.; Harris, R.J.; Aiano, F.; Zavala, M.; Bertran, M.; Borrow, R.; Linley, E.; Ahmad, S.; Parker, B.; Horsley, A.; et al. Characterising Post-COVID Syndrome More than 6 Months after Acute Infection in Adults; Prospective Longitudinal Cohort Study, England. medRxiv 2021. medRxiv: 03.18.21253633. [Google Scholar] [CrossRef]
- Rogers, J.P.; Watson, C.J.; Badenoch, J.; Cross, B.; Butler, M.; Song, J.; Hafeez, D.; Morrin, H.; Rengasamy, E.R.; Thomas, L.; et al. Neurology and Neuropsychiatry of COVID-19: A Systematic Review and Meta-Analysis of the Early Literature Reveals Frequent CNS Manifestations and Key Emerging Narratives. J. Neurol. Neurosurg. Psychiatry 2021, 92, 932–941. [Google Scholar] [CrossRef]
- Lee, M.H.; Perl, D.P.; Steiner, J.; Pasternack, N.; Li, W.; Maric, D.; Safavi, F.; Horkayne-Szakaly, I.; Jones, R.; Stram, M.N.; et al. Neurovascular Injury with Complement Activation and Inflammation in COVID-19. Brain 2022, 145, 2555–2568. [Google Scholar] [CrossRef]
- Priyanka; Choudhary, O.P.; Singh, I.; Patra, G. Aerosol Transmission of SARS-CoV-2: The Unresolved Paradox. Travel Med. Infect. Dis. 2020, 37, 101869. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG Brain PET Hypometabolism in Patients with Long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Shaik-Dasthagirisaheb, Y.; Conti, P. The Role of Mast Cells in Alzheimer’s Disease. Adv. Clin. Exp. Med. 2016, 25, 781–787. [Google Scholar] [CrossRef]
- Llach, C.-D.; Vieta, E. Mind Long COVID: Psychiatric Sequelae of SARS-CoV-2 Infection. Eur. Neuropsychopharmacol. 2021, 49, 119–121. [Google Scholar] [CrossRef]
- Orfei, M.D.; Assogna, F.; Pellicano, C.; Pontieri, F.E.; Caltagirone, C.; Pierantozzi, M.; Stefani, A.; Spalletta, G. Anosognosia for Cognitive and Behavioral Symptoms in Parkinson’s Disease with Mild Dementia and Mild Cognitive Impairment: Frequency and Neuropsychological/Neuropsychiatric Correlates. Parkinsonism Relat. Disord. 2018, 54, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Studer, J.; Donati, A.; Popp, J.; von Gunten, A. Subjective Cognitive Decline in Patients with Mild Cognitive Impairment and Healthy Older Adults: Association with Personality Traits: Subjective Decline in Healthy Older Adults and MCI. Geriatr. Gerontol. Int. 2014, 14, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Goeldner, C.; Ballard, T.M.; Knoflach, F.; Wichmann, J.; Gatti, S.; Umbricht, D. Cognitive Impairment in Major Depression and the MGlu2 Receptor as a Therapeutic Target. Neuropharmacology 2013, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Vannini, P.; Amariglio, R.; Hanseeuw, B.; Johnson, K.A.; McLaren, D.G.; Chhatwal, J.; Pascual-Leone, A.; Rentz, D.; Sperling, R.A. Memory Self-Awareness in the Preclinical and Prodromal Stages of Alzheimer’s Disease. Neuropsychologia 2017, 99, 343–349. [Google Scholar] [CrossRef]
- Casagrande, M.; Favieri, F.; Tambelli, R.; Forte, G. The Enemy Who Sealed the World: Effects Quarantine Due to the COVID-19 on Sleep Quality, Anxiety, and Psychological Distress in the Italian Population. Sleep Med. 2020, 75, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Tambelli, R.; Casagrande, M. The Enemy Which Sealed the World: Effects of COVID-19 Diffusion on the Psychological State of the Italian Population. J. Clin. Med. 2020, 9, 1802. [Google Scholar] [CrossRef]
- Orfei, M.D.; Bossi, F.; D’Arcangelo, S.; Maggi, F.; Lattanzi, N.; Malizia, A.P.; Ricciardi, E. Mental Health in the Post-Lockdown Pandemic Phase: Relief or Exacerbation of Psychological Distress? A Cross-Sectional Study in the General Population in Italy. Acta Psychol. (Amst.) 2022, 225, 103555. [Google Scholar] [CrossRef]
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Álvarez, A.; Gómez-Ruiz, N.; Yus, M.; Polidura, C.; Pérez-Izquierdo, C.; Marcos, A.; Gil, M.J.; Matías-Guiu, J.; et al. Examining Association of Personality Characteristics and Neuropsychiatric Symptoms in Post-COVID Syndrome. Brain Sci. 2022, 12, 265. [Google Scholar] [CrossRef]
- Choudhary, O.P.; Priyanka; Ali, R.K.; Maulud, S.Q.; Dhawan, M.; Mohammed, T.A. Will the next Spillover Pandemic Be Deadlier than the COVID-19?: A Wake-up Call. Int. J. Surg. 2022, 97, 106208. [Google Scholar] [CrossRef]
- Orfei, M.D.; Porcari, D.E.; D’Arcangelo, S.; Maggi, F.; Russignaga, D.; Lattanzi, N.; Malizia, A.P.; Ricciardi, E. COVID-19 and Stressful Adjustment to Work: A Long-Term Prospective Study About Homeworking for Bank Employees in Italy. Front. Psychol. 2022, 13, 843095. [Google Scholar] [CrossRef]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic Review and Meta-Analysis of the Prevalence of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Simani, L.; Ramezani, M.; Darazam, I.A.; Sagharichi, M.; Aalipour, M.A.; Ghorbani, F.; Pakdaman, H. Prevalence and Correlates of Chronic Fatigue Syndrome and Post-Traumatic Stress Disorder after the Outbreak of the COVID-19. J. Neurovirol. 2021, 27, 154–159. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent Fatigue Following SARS-CoV-2 Infection Is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef] [PubMed]
- The Long Covid Kids study group; Ortona, E.; Buonsenso, D.; Carfi, A.; Malorni, W. Long COVID: An Estrogen-Associated Autoimmune Disease? Cell Death Discov. 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. The Extended Autonomic System, Dyshomeostasis, and COVID-19. Clin. Auton. Res. 2020, 30, 299–315. [Google Scholar] [CrossRef]
- Wignall, E.L.; Dickson, J.M.; Vaughan, P.; Farrow, T.F.D.; Wilkinson, I.D.; Hunter, M.D.; Woodruff, P.W.R. Smaller Hippocampal Volume in Patients with Recent-Onset Posttraumatic Stress Disorder. Biol. Psychiatry 2004, 56, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Woon, F.L.; Sood, S.; Hedges, D.W. Hippocampal Volume Deficits Associated with Exposure to Psychological Trauma and Posttraumatic Stress Disorder in Adults: A Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1181–1188. [Google Scholar] [CrossRef]
- Jin, C.; Qi, R.; Yin, Y.; Hu, X.; Duan, L.; Xu, Q.; Zhang, Z.; Zhong, Y.; Feng, B.; Xiang, H.; et al. Abnormalities in Whole-Brain Functional Connectivity Observed in Treatment-Naive Post-Traumatic Stress Disorder Patients Following an Earthquake. Psychol. Med. 2014, 44, 1927–1936. [Google Scholar] [CrossRef]
- Blair, K.S.; Vythilingam, M.; Crowe, S.L.; McCaffrey, D.E.; Ng, P.; Wu, C.C.; Scaramozza, M.; Mondillo, K.; Pine, D.S.; Charney, D.S.; et al. Cognitive Control of Attention Is Differentially Affected in Trauma-Exposed Individuals with and without Post-Traumatic Stress Disorder. Psychol. Med. 2013, 43, 85–95. [Google Scholar] [CrossRef]
- van Rooij, S.J.H.; Rademaker, A.R.; Kennis, M.; Vink, M.; Kahn, R.S.; Geuze, E. Neural Correlates of Trauma-Unrelated Emotional Processing in War Veterans with PTSD. Psychol. Med. 2015, 45, 575–587. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What Is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tales, A.; Wilcock, G.K.; Phillips, J.E.; Bayer, A. Is There More to Subjective Cognitive Impairment than Meets the Eye? A Perspective. J. Alzheimer’s Dis. 2014, 41, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Kunthi, K.; Alwan, N.; Steves, C.; MacDermott, N.; Morsella, A. The Wake of the Pandemic Preparing for Long COVID; WHO Regional Office for Europe: Copenaghen, Denmark, 2021. [Google Scholar]
- Ocon, A.J. Caught in the Thickness of Brain Fog: Exploring the Cognitive Symptoms of Chronic Fatigue Syndrome. Front. Physiol. 2013, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the Long Term Effects of COVID-19: Summary of NICE, SIGN, and RCGP Rapid Guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Burke, M.J.; del Rio, C. Long COVID Has Exposed Medicine’s Blind-Spot. Lancet Infect. Dis. 2021, 21, 1062–1064. [Google Scholar] [CrossRef]
- Yelin, D.; Moschopoulos, C.D.; Margalit, I.; Gkrania-Klotsas, E.; Landi, F.; Stahl, J.-P.; Yahav, D. ESCMID Rapid Guidelines for Assessment and Management of Long COVID. Clin. Microbiol. Infect. 2022, 28, 955–972. [Google Scholar] [CrossRef]
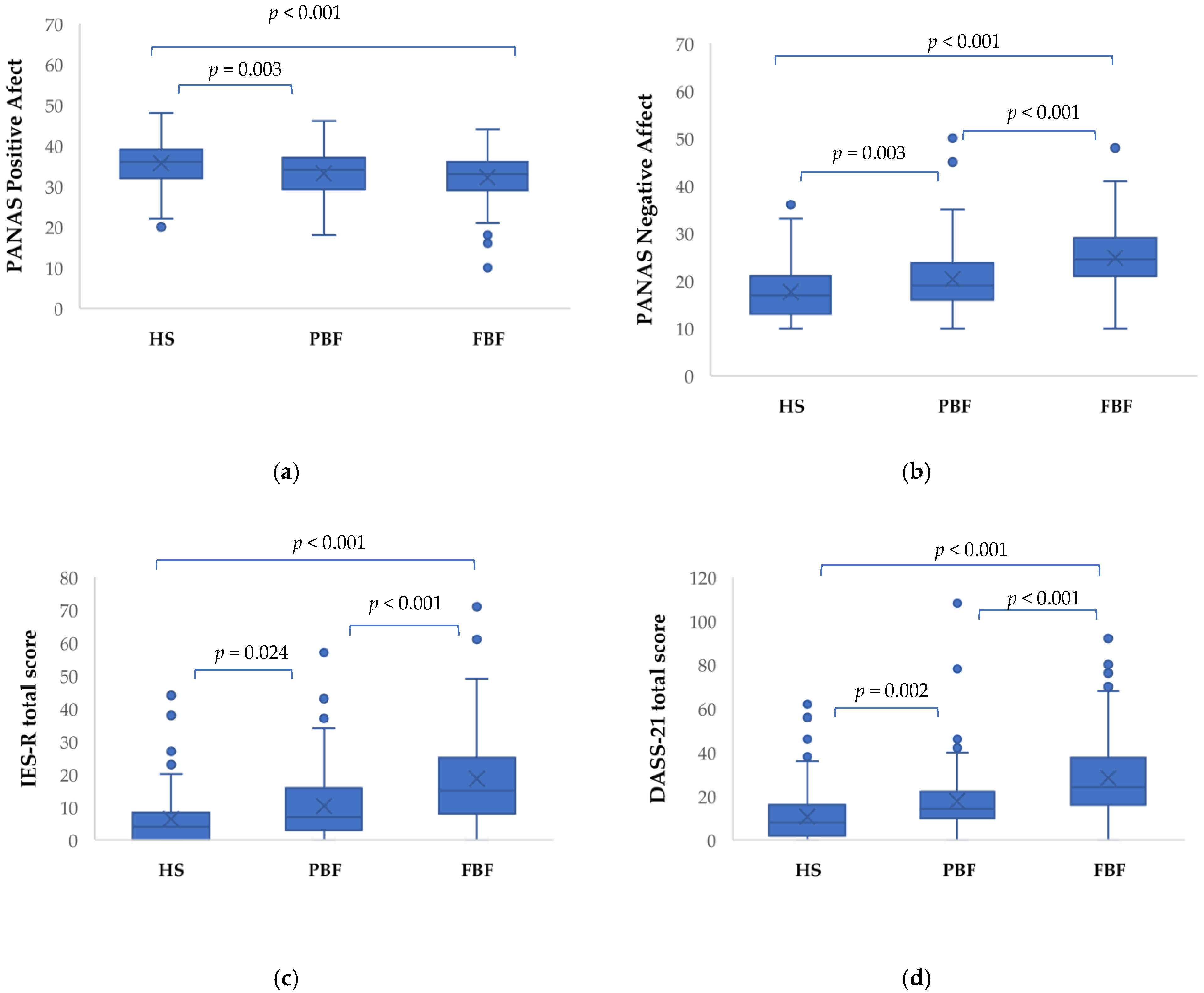
| Variable | HS n= 147 | PBF n = 117 | FBF n = 177 | p | HS vs. PBF | HS vs. FBF | PBF vs. FBF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Crit. Diff. | p | Crit. Diff. | p | Crit. Diff. | p | |||||
| Females n (%) | 39 (26.5) | 43 (36.8) | 89 (50.3) | <0.001 | - | - | - | - | - | - |
| Age (years ± SD) | 47.84 ± 9.6 | 47.93 ± 10.0 | 45.72 ± 8.8 | 0.063 | - | - | - | - | - | - |
| Sleep disorders Yes n (%) | 43 (29.3) | 55 (47) | 134 (75.7) | <0.001 | - | - | - | - | - | - |
| BCIS Self-Reflectiveness (mean ± SD) | 9.67 ± 3.1 | 10.56 ± 3.0 | 10.9 ± 3.2 | 0.003 | −0.876 | 0.075 | −1.197 | 0.003 | −0.321 | 1.000 |
| BCIS Self-Certainty (mean ± SD) | 7.88 ± 2.9 | 8.45 ± 2.6 | 8.40 ± 2.7 | 0.132 | - | - | - | - | - | - |
| BCIS R-C Index (mean ± SD) | 1.8 ± 4.2 | 2.11 ± 4.2 | 2.51 ± 4.50 | 0.430 | - | - | - | - | - | - |
| PANAS Positive Affect (mean ± SD) | 35.6 ± 5.7 | 33.16 ± 5.8 | 32.17 ± 5.65 | <0.001 | 2.361 | 0.003 | 3.245 | <0.001 | 0.884 | 0.595 |
| PANAS Negative Affect (mean ± SD) | 17.63 ± 5.6 | 20.36 ± 6.6 | 24.87 ± 6.7 | <0.001 | −2.574 | 0.003 | −6.882 | <0.001 | −4.308 | <0.001 |
| IES-R Avoidance (mean ± SD) | 0.30 ± 0.4 | 0.50 ± 0.5 | 0.83 ± 0.65 | <0.001 | −0.165 | 0.053 | −0.523 | <0.001 | −0.358 | <0.001 |
| IES-R Intrusion (mean ± SD) | 0.29 ± 0.4 | 0.48 ± 0.6 | 0.82 ± 0.7 | <0.001 | −0.180 | 0.054 | −0.517 | <0.001 | −0.337 | <0.001 |
| IES-R Hyperarousal (mean ± SD) | 0.25 ± 0.4 | 0.44 ± 0.5 | 0.88 ± 0.8 | <0.001 | −0.191 | 0.04 | −0.621 | <0.001 | −0.430 | <0.001 |
| IES-R Total score (mean ± SD) | 6.33 ± 8.7 | 10.32 ± 10.5 | 18.57 ± 12.8 | <0.001 | −3.904 | 0.024 | −12.047 | <0.001 | −8.142 | <0.001 |
| DASS-21 Stress (mean ± SD) | 2.50 ± 2.6 | 4.32 ± 3.1 | 6.12 ± 3.2 | <0.001 | −1.772 | <0.001 | −3.524 | <0.001 | −1.752 | <0.001 |
| DASS-21 Anxiety (mean ± SD) | 0.84 ± 1.7 | 1.43 ± 2.3 | 2.82 ± 3.3 | <0.001 | −0.553 | 0.265 | −1.890 | <0.001 | −1.337 | <0.001 |
| DASS-21 Depression (mean ± SD) | 1.93 ± 2.5 | 3.09 ± 3.2 | 5.19 ± 4.2 | <0.001 | −1.067 | 0.038 | −3.048 | <0.001 | −1.981 | <0.001 |
| DASS-21 Total score (mean ± SD) | 10.54 ± 11.8 | 17.68 ± 15.1 | 28.27 ± 19.1 | <0.001 | −6.784 | 0.002 | −16.923 | <0.001 | −10.139 | <0.001 |
| Variable | FBF N = 177 | |
|---|---|---|
| r | p | |
| Age | 0.043 | 0.573 |
| Gender | 0.195 | 0.009 |
| Sleep disorders | 0.215 | 0.004 |
| DASS-21 total score | 0.443 | <0.001 |
| IES-R total score | 0.324 | <0.001 |
| PANAS NA | 0.312 | <0.001 |
| BCIS Self-Reflectiveness | 0.095 | 0.208 |
| Variable | ||
|---|---|---|
| β (t) | 95% CI | |
| Step 1 | ||
| DASS-21 total score | 0.527 (8.179) | [0.048, 0.078] |
| Gender | 0.185 (2.914) | |
| IES-R total score | 0.162 (2.218) | |
| Sleep disorders | 0.127 (1.935) | |
| PANAS NA | −0.032 (−0.315) | |
| R2 | 0.278 | |
| P | <0.001 | |
| Step 2 | ||
| DASS-21 total score | 0.503 (7.910) | [0.045, 0.075] |
| Gender | 0.185 (2.914) | [0.314, 1.460] |
| IES-R total score | 0.178 (2.501) | |
| Sleep disorders | 0.101 (1.544) | |
| PANAS NA | −0.029 (−0.298) | |
| R2 | 0.311 | |
| p | 0.004 | |
| Step 3 | ||
| DASS-21 total score | 0.415 (5.779) | [0.033, 0.066] |
| Gender | 0.197 (3.136) | [0.375, 1.506] |
| IES-R total score | 0.178 (2.501) | [0.006, 0.051] |
| Sleep disorders | 0.103 (1.605) | |
| PANAS NA | −0.077 (−0.786) | |
| R2 | 0.336 | |
| p | 0.013 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orfei, M.D.; Porcari, D.E.; D’Arcangelo, S.; Maggi, F.; Russignaga, D.; Ricciardi, E. A New Look on Long-COVID Effects: The Functional Brain Fog Syndrome. J. Clin. Med. 2022, 11, 5529. https://doi.org/10.3390/jcm11195529
Orfei MD, Porcari DE, D’Arcangelo S, Maggi F, Russignaga D, Ricciardi E. A New Look on Long-COVID Effects: The Functional Brain Fog Syndrome. Journal of Clinical Medicine. 2022; 11(19):5529. https://doi.org/10.3390/jcm11195529
Chicago/Turabian StyleOrfei, Maria Donata, Desirée Estela Porcari, Sonia D’Arcangelo, Francesca Maggi, Dario Russignaga, and Emiliano Ricciardi. 2022. "A New Look on Long-COVID Effects: The Functional Brain Fog Syndrome" Journal of Clinical Medicine 11, no. 19: 5529. https://doi.org/10.3390/jcm11195529
APA StyleOrfei, M. D., Porcari, D. E., D’Arcangelo, S., Maggi, F., Russignaga, D., & Ricciardi, E. (2022). A New Look on Long-COVID Effects: The Functional Brain Fog Syndrome. Journal of Clinical Medicine, 11(19), 5529. https://doi.org/10.3390/jcm11195529





