The Predictive Role of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Monocytes-to-Lymphocyte Ratio (MLR) and Gammaglobulins for the Development of Cutaneous Vasculitis Lesions in Primary Sjögren’s Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Groups
3.2. Dermatological Manifestations in Patients with pSS
3.3. Analysis of Hematological Parameters in pSS Patients with Cutaneous Vasculitis Lesions
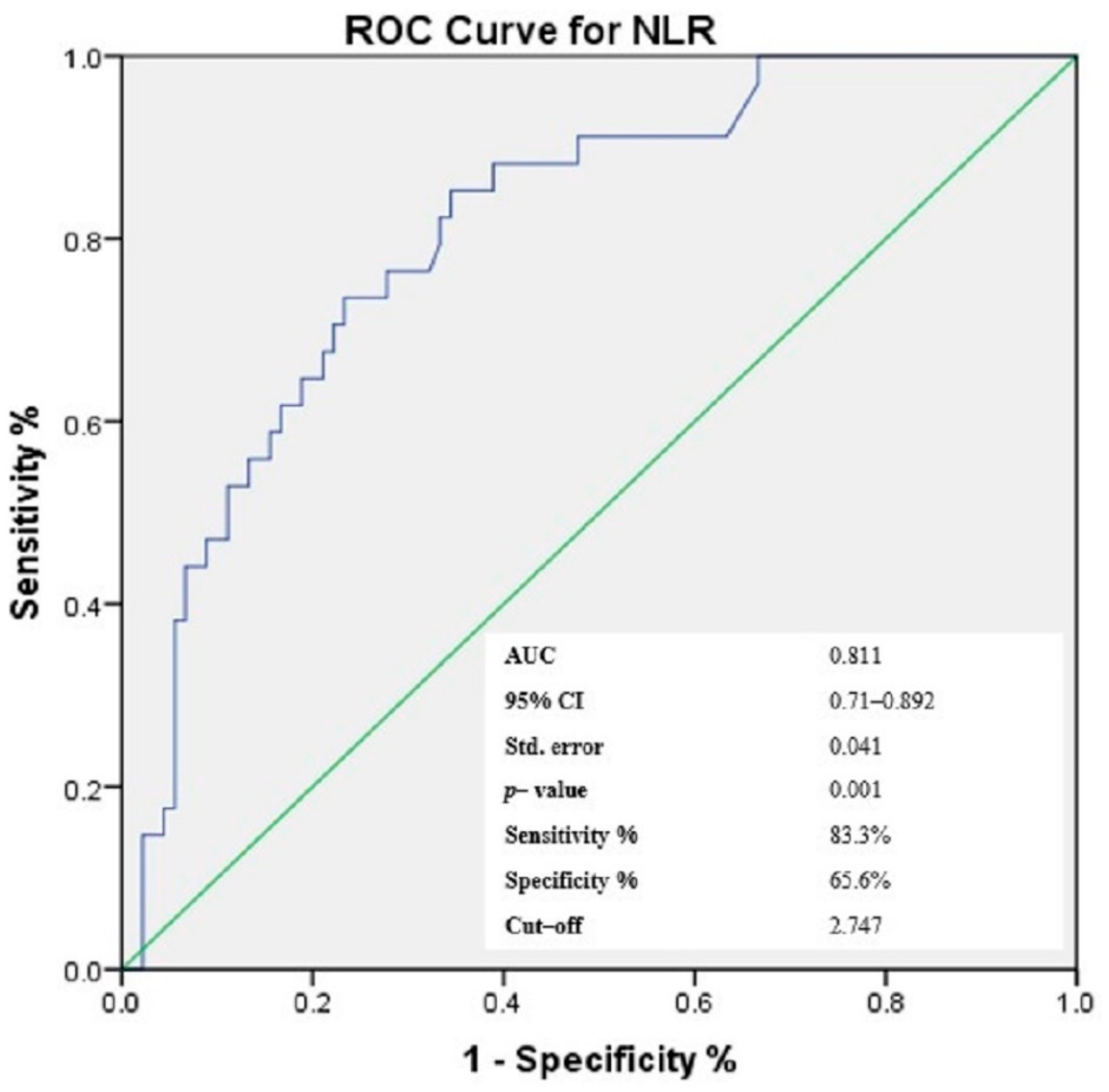
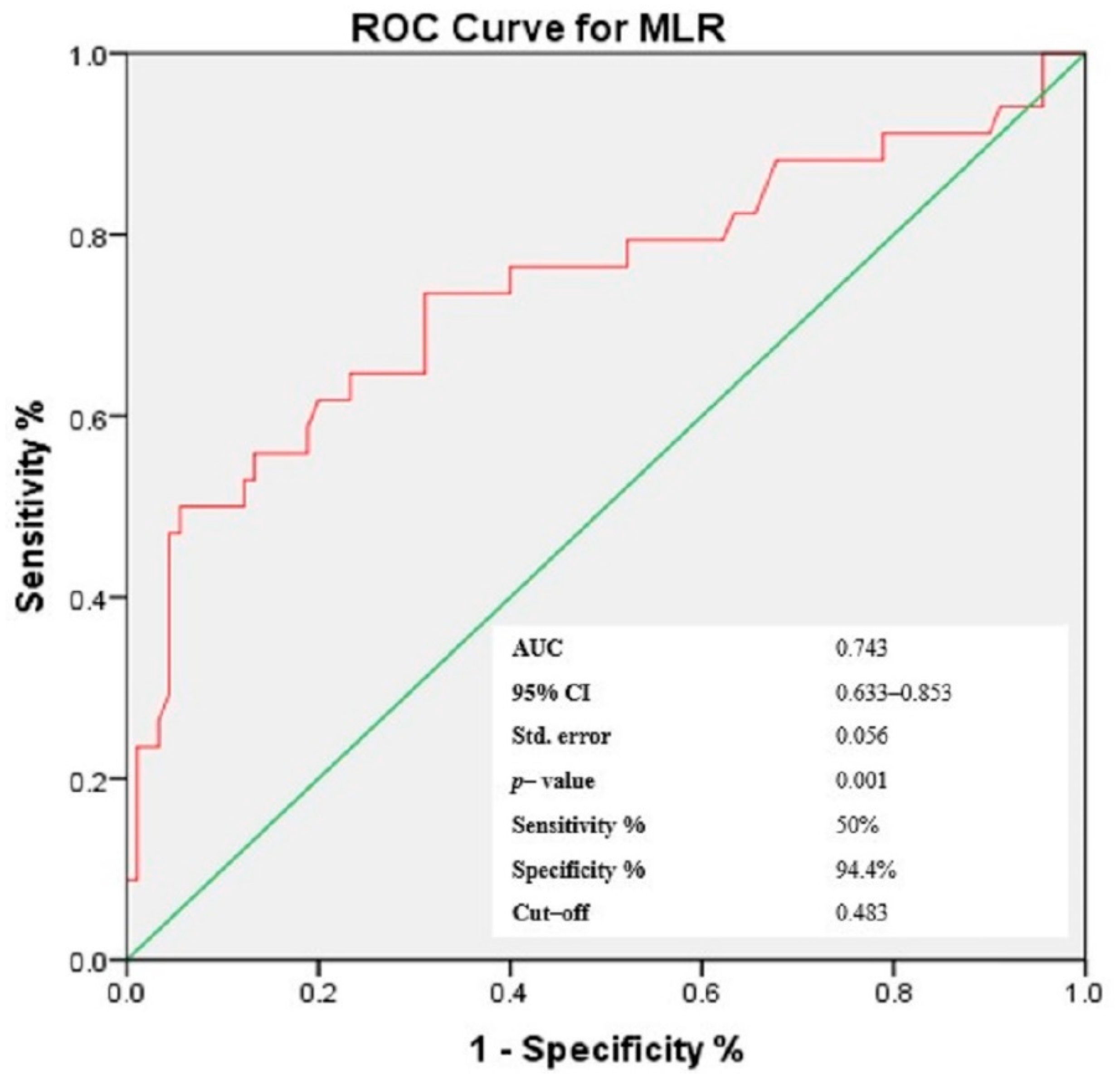
3.4. Receiver-Operating Characteristic (ROC) Curves of NLR, PLR, MLR and Gammaglobulins for the Prediction of Cutaneous Vasculitis in pSS Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, L.; Koh, V.; Thong, B.Y. Review of autoantigens in Sjögren’s syndrome: An update. J. Inflamm. Res. 2017, 10, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Shahane, A. The epidemiology of Sjögren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.C.; Alunno, A.; Cafaro, G.; Valentini, V.; Marcucci, E.; Bartoloni, E.; Gerli, R. The clinical spectrum of primary Sjögren’s syndrome: Beyond exocrine glands. Reumatismo 2017, 69, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kittridge, A.; Routhouska, S.B.; Korman, N.J. Dermatologic manifestations of Sjögren syndrome. J. Cutan. Med. Surg. 2011, 15, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Orgeolet, L.; Foulquier, N.; Misery, L.; Redou, P.; Pers, J.O.; Devauchelle-Pensec, V.; Saraux, A. Can artificial intelligence replace manual search for systematic literature? Review on cutaneous manifestations in primary Sjögren’s syndrome. Rheumatology 2020, 59, 811–819. [Google Scholar] [CrossRef]
- Nishishinya, M.B.; Pereda, C.A.; Muñoz-Fernández, S.; Pego-Reigosa, J.M.; Rúa-Figueroa, I.; Andreu, J.L.; Fernández-Castro, M.; Rosas, J.; Loza Santamaría, E. Identification of lymphoma predictors in patients with primary Sjögren’s syndrome: A systematic literature review and meta-analysis. Rheumatol. Int. 2015, 35, 17–26. [Google Scholar] [CrossRef]
- Micheletti, R.G.; Werth, V.P. Small vessel vasculitis of the skin. Rheum. Dis. Clin. N. Am. 2015, 41, 21–32. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef]
- Soliman, W.M.; Sherif, N.M.; Ghanima, I.M.; El-Badawy, M.A. Neutrophil to lymphocyte and platelet to lymphocyte ratios in systemic lupus erythematosus: Relation with disease activity and lupus nephritis. Reumatol. Clin. 2020, 16, 255–261. [Google Scholar] [CrossRef]
- Tezcan, D.; Körez, M.K.; Gülcemal, S.; Hakbilen, S.; Akdağ, T.; Yılmaz, S. Evaluation of diagnostic performance of haematological parameters in Behçet’s disease. Int. J. Clin. Pract. 2021, 75, e14638. [Google Scholar] [CrossRef]
- Lian, L.; Xia, Y.Y.; Zhou, C.; Shen, X.M.; Li, X.L.; Han, S.G.; Zheng, Y.; Mao, Z.Q.; Gong, F.R.; Wu, M.Y.; et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark 2015, 15, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.D.; Sun, Y.; Guo, J.; Huang, Y.L.; Qin, B.D.; Gao, Q.; Qin, Q.; Deng, A.M.; Zhong, R.Q. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjögren’s syndrome. Clin. Biochem. 2014, 47, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, F.; Gökmen, O. Haematologic indices and disease activity index in primary Sjogren’s syndrome. Int. J. Clin. Pract. 2021, 75, e13992. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Aparcı, M.; Ozturk, C.; Demirkol, S.; Celik, T. Neutrophil-lymphocyte ratio as an useful mortality marker. Am. J. Emerg. Med. 2014, 32, 1546–1547. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Yang, X.; Chen, L.; Yang, Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int. Immunopharmacol. 2016, 36, 94–99. [Google Scholar] [CrossRef]
- Celikbilek, M.; Dogan, S.; Ozbakır, O.; Zararsız, G.; Kücük, H.; Gürsoy, S.; Yurci, A.; Güven, K.; Yücesoy, M. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J. Clin. Lab Anal. 2013, 27, 72–76. [Google Scholar] [CrossRef]
- Caruntu, A.; Moraru, L.; Lupu, M.; Taubner, L.; Caruntu, C.; Tanase, C. The Hidden Treasures of Preoperative Blood Assessment in Oral Cancer: A Potential Source of Biomarkers. Cancers 2021, 13, 4475. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Lin, F.; Ren, Y.; Liu, D.; Zhong, R.; Liang, Y. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil- lymphocyte ratios among various systemic autoimmune rheumatic diseases. Apmis 2017, 125, 863–871. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Villon, C.; Orgeolet, L.; Roguedas, A.M.; Misery, L.; Gottenberg, J.E.; Cornec, D.; Jousse-Joulin, S.; Seror, R.; Berthelot, J.M.; Dieude, P.; et al. Epidemiology of cutaneous involvement in Sjögren syndrome: Data from three French pSS populations (TEARS, ASSESS, diapSS). Joint Bone Spine 2021, 88, 105162. [Google Scholar] [CrossRef] [PubMed]
- Scofield, R.H. Vasculitis in Sjögren’s Syndrome. Curr. Rheumatol. Rep. 2011, 13, 482–488. [Google Scholar] [CrossRef]
- Retamozo, S.; Brito-Zerón, P.; Ramos-Casals, M. Prognostic markers of lymphoma development in primary Sjögren syndrome. Lupus 2019, 28, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Vassiliou, V.A.; Moutsopoulos, H.M. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002, 46, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Suresh, L. Autoantibodies, detection methods and panels for diagnosis of Sjögren’s syndrome. Clin. Immunol. 2017, 182, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Seror, R.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.E.; Mariette, X.; Theander, E.; Bombardieri, S.; et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology 2015, 54, 2230–2238. [Google Scholar] [CrossRef]
- Sène, D.; Ismael, S.; Forien, M.; Charlotte, F.; Kaci, R.; Cacoub, P.; Diallo, A.; Dieudé, P.; Lioté, F. Ectopic Germinal Center-Like Structures in Minor Salivary Gland Biopsy Tissue Predict Lymphoma Occurrence in Patients with Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 1481–1488. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Font, J.; Garcia-Carrasco, M.; Brito, M.P.; Rosas, J.; Calvo-Alen, J.; Pallares, L.; Cervera, R.; Ingelmo, M. Primary Sjögren syndrome: Hematologic patterns of disease expression. Medicine 2002, 81, 281–292. [Google Scholar] [CrossRef]
- Wei, L.; Zhifei, X.; Xiaoran, N.; Meilu, L.; Yang, L.; Yixuan, L.; Xiuying, R.; Yashuang, S.; Jingjing, C.; Shaoying, G.; et al. Patients with early-onset primary Sjögren’s syndrome have distinctive clinical manifestations and circulating lymphocyte profiles. Rheumatology 2022, 61, 597–605. [Google Scholar] [CrossRef]
- Huang, H.; Xie, W.; Geng, Y.; Fan, Y.; Zhang, Z. Mortality in patients with primary Sjögren’s syndrome: A systematic review and meta-analysis. Rheumatology 2021, 60, 4029–4038. [Google Scholar] [CrossRef]
- Li, Z.; Fu, T.; Li, L.; Cui, Y.; Dong, C.; Li, J.; Gu, Z. Prevalence, severity, and predictors of dry eye and dry mouth in Chinese patients with primary Sjögren syndrome. Clin. Rheumatol. 2018, 37, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, E.; Amato, L.; Parodi, A.; Cottoni, F.; Rubegni, P.; De Pità, O.; Papini, M.; Rebora, A.; Bombardieri, S.; Fabbri, P. Sjögren’s syndrome: A retrospective review of the cutaneous features of 93 patients by the Italian Group of Immunodermatology. Clin. Exp. Rheumatol. 2004, 22, 55–62. [Google Scholar] [PubMed]
- Ramos-Casals, M.; Anaya, J.M.; García-Carrasco, M.; Rosas, J.; Bové, A.; Claver, G.; Diaz, L.A.; Herrero, C.; Font, J. Cutaneous vasculitis in primary Sjögren syndrome: Classification and clinical significance of 52 patients. Medicine 2004, 83, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, O.D.; Tzioufas, A.G. Common and rare forms of vasculitis associated with Sjögren’s syndrome. Curr. Opin. Rheumatol. 2020, 32, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Generali, E.; Costanzo, A.; Mainetti, C.; Selmi, C. Cutaneous and Mucosal Manifestations of Sjögren’s Syndrome. Clin. Rev. Allergy Immunol. 2017, 53, 357–370. [Google Scholar] [CrossRef]
- Jordán-González, P.; Gago-Piñero, R.; Varela-Rosario, N.; Pérez-Ríos, N.; Vilá, L.M. Characterization of a subset of patients with primary Sjögren’s syndrome initially presenting with C3 or C4 hypocomplementemia. Eur. J. Rheumatol. 2020, 7, 112–117. [Google Scholar] [CrossRef]
- Tengnér, P.; Halse, A.K.; Haga, H.J.; Jonsson, R.; Wahren-Herlenius, M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjögren’s syndrome. Arthritis Rheum. 1998, 41, 2238–2248. [Google Scholar] [CrossRef]
- Quartuccio, L.; Isola, M.; Baldini, C.; Priori, R.; Bartoloni, E.; Carubbi, F.; Gregoraci, G.; Gandolfo, S.; Salvin, S.; Luciano, N.; et al. Clinical and biological differences between cryoglobulinaemic and hypergammaglobulinaemic purpura in primary Sjögren’s syndrome: Results of a large multicentre study. Scand. J. Rheumatol. 2015, 44, 36–41. [Google Scholar] [CrossRef]
- Nocturne, G.; Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef]
- Roguedas, A.M.; Pers, J.O.; Lemasson, G.; Devauchelle, V.; Tobón, G.J.; Saraux, A.; Misery, L.; Youinou, P. Memory B-cell aggregates in skin biopsy are diagnostic for primary Sjögren’s syndrome. J. Autoimmun. 2010, 35, 241–247. [Google Scholar] [CrossRef]
- Fu, H.; Qin, B.; Hu, Z.; Ma, N.; Yang, M.; Wei, T.; Tang, Q.; Huang, Y.; Huang, F.; Liang, Y.; et al. Neutrophil- and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin. Lab. 2015, 61, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Shehata, O.Z.; Abdel-Latif, S.M.; El-Din, A.M.M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in Behçet’s disease: Which and when to use? Clin. Rheumatol. 2018, 37, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Du, J.; Li, T.; Liao, H. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio associated with disease activity in patients with Takayasu’s arteritis: A case-control study. BMJ Open 2017, 7, e014451. [Google Scholar] [CrossRef] [PubMed]
- Yayla, M.E.; İlgen, U.; Okatan, İ.E.; UsluYurteri, E.; Torgutalp, M.; Keleşoğlu Dinçer, A.B.; Aydemir Gülöksüz, E.G.; Sezer, S.; Turgay, T.M.; Kınıklı, G.; et al. Association of simple hematological parameters with disease manifestations, activity, and severity in patients with systemic sclerosis. Clin. Rheumatol. 2020, 39, 77–83. [Google Scholar] [CrossRef]
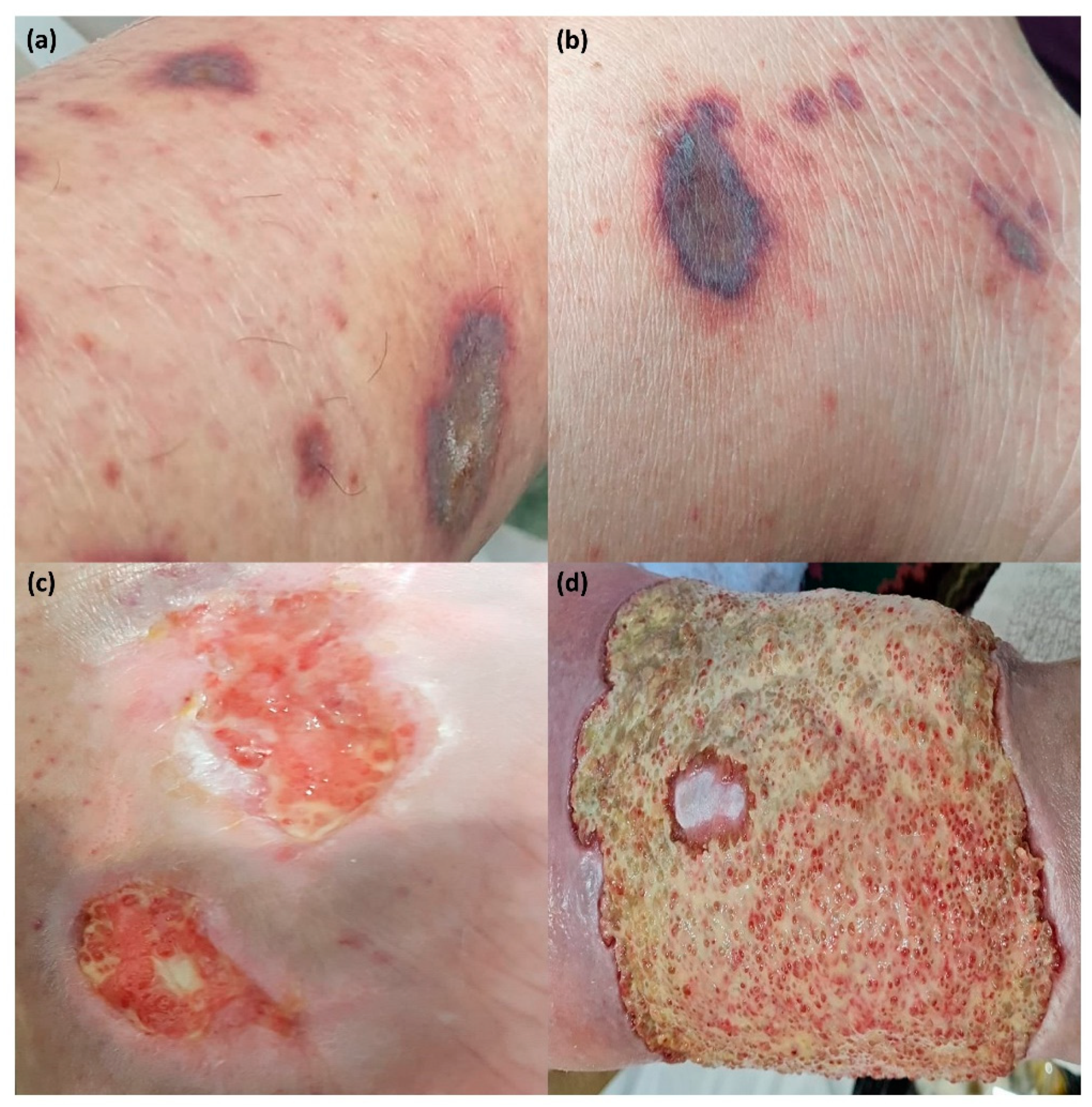


| Demographic Characteristics | Patients Group No = 124 | Control Group No = 121 | p-Value |
|---|---|---|---|
| Mean ± SD/Median | Mean ± SD/Median | ||
| Age (years) | 48.55 ± 10.45 | 48.37 ± 8.92 | 0.887 b |
| Sex Female (n, %) Male (n, %) | 121 (97.6%) | 119 (98.3%) | 0.671 c |
| 3 (2.4%) | 2 (1.7%) | ||
| Laboratory findings Cell counts | |||
| Erythrocytes (×106/µL) | 4.22 ± 0.38 | 4.44 ± 0.38 | 0.001 a |
| Leucocytes (109/µL) | 5.1 ± 1.71 | 6.94 ± 2.17 | 0.001 a |
| Neutrophils (109/µL) | 3.11 ± 0.67 | 4.27 ± 1.96 | 0.001 a |
| Lymphocytes (109/µL) | 1.12 ± 0.09 | 1.97 ± 1.21 | 0.001 a |
| Monocyte (109/µL) | 0.33 ± 0.14 | 0.64 ± 0.16 | 0.001 a |
| Platelets (109/L) | 25.8 ± 51.4 | 29.6 ± 68.18 | 0.001 a |
| RDW-CV (%) | 12.97 ± 1.16 | 13.13 ± 0.84 | 0.086 a |
| Cellular ratios | |||
| MPVPCTR | 39.33 ± 10.93 | 42.22 ± 25.36 | 0.246 b |
| PDWPCTR | 46.46 ± 12.7 | 49.76 ± 29.45 | 0.255 b |
| MLR | 0.29 ± 0.12 | 0.1 ± 0.52 | 0.001 a |
| NLR | 2.8 ± 0.71 | 2.2 ± 0.96 | 0.001 a |
| PLR | 233.75 ± 56.34 | 153.7 ± 60.8 | 0.001 a |
| Immunological results | |||
| RF (U/mL) | 52.18 ± 61 | 9.32 ± 18.55 | 0.001 a |
| IgA (mg/dL) | 293.67 ± 15.98 | 222.9 ± 93.9 | 0.001 a |
| IgG (mg/dL) | 1656.94 ± 708.73 | 1200.5 ± 315.2 | 0.001 a |
| IgM (mg/dL) | 168.4 ± 121.31 | 116.84 ± 45.75 | 0.001 a |
| Gammaglobulins (g/dL) | 1.45 ± 0.4 | 1.05 ± 0.16 | 0.001 a |
| C3 (mg/dL) | 133.48 ± 116.22 | 131.33 ± 16.27 | 0.003 a |
| C4 (mg/dL) | 22.02 ± 10.33 | 32.32 ± 10.7 | 0.001 a |
| ANA Positive (n, %) Negative (n, %) | 105 (84.67%) | - | - |
| 19 (15.32%) | - | - | |
| Anti-Ro/SSA Positive (n, %) Negative (n, %) | 105 (84.67%) | - | - |
| 19 (15.32%) | - | - | |
| Anti-La/SSB Positive (n, %) Negative (n, %) | 76 (61.29%) | - | - |
| 48 (38.70%) | - | - | |
| Inflammatory results | |||
| hs-CRP (mg/L) | 24.22 ± 25.53 | 5.75 ± 4.74 | 0.001 a |
| Clinical scores | |||
| ESSDAI score | 16.96 ± 7.8 | - | - |
| ESSDAI score 5–13 | 50 (40.32%) | - | - |
| ESSDAI score ≥14 | 74 (59.67%) | - | - |
| VAS pain score (mm) | 18.87 ± 26.9 | - | - |
| Clinical Manifestations | Number of Patients (%) |
|---|---|
| Xerostomia (n, %) | 113 (91.12%) |
| Xerophthalmia (n, %) | 75 (60.48%) |
| CM (n, %) | 50 (40.32%) |
| Cutaneous vasculitis lesions (n, %) | 34 (27.41%) |
| Non–palpable purpura | 23 (18.54%) |
| Palpable purpura | 10 (8.06%) |
| Cryoglobulinemic vasculitis (n, %) | 8 (6.45%) |
| Leukocytoclastic vasculitis (n, %) | 2 (1.61%) |
| Urticarial vasculitis (n, %) | 1 (0.8%) |
| Non vasculitis lesions | 66 (48.38%) |
| Xeroderma (n, %) | 44 (35.48%) |
| Raynaud syndrome (n, %) | 22 (17.74%) |
| Cutaneous Vasculitis Lesions | ||
|---|---|---|
| Correlation Coefficient (r) | p-Value | |
| Lymphocytes (109/µL) | −0.432 | 0.001 * |
| Neutrophils (109/µL) | 0.389 | 0.001 * |
| Monocytes (109/L) | −0.150 | 0.097 |
| Platelets (109/L) | 0.467 | 0.001 * |
| NLR | 0.481 | 0.001 * |
| PLR | 0.524 | 0.001 * |
| MLR | 0.375 | 0.001 * |
| MPWPCTR | −0.080 | 0.380 |
| PDWPCTR | −0.029 | 0.753 |
| C3 (mg/dL) | −0.101 | 0.267 |
| C4 (mg/dL) | −0.123 | 0.174 |
| IgA (mg/dL) | −0.067 | 0.457 |
| IgG (mg/dL) | −0.168 | 0.063 |
| IgM (mg/dL) | 0.057 | 0.531 |
| Gammaglobulins g/dL) | 0.674 | 0.001 * |
| RF (U/mL) | 0.176 | 0.051 |
| hs-CRP (mg/L) | 0.077 | 0.398 |
| Anti-Ro/SSA (U/mL) | 0.211 | 0.280 |
| Anti-La/SSB (U/mL) | 0.414 | 0.791 |
| VAS pain score (mm) | 0.571 | 0.001 * |
| ESSDAI score | 0.444 | 0.001 * |
| Laboratory Findings | Unstandardized Coefficients | Standardized Coefficients | T | p | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|---|
| Β | Standard Error | Beta | Lower Bound | Upper Bound | |||
| (Constant) | −1.585 | 0.176 | − | −9.012 | 0.000 | −1.934 | −1.237 |
| NLR | 0.142 | 0.045 | 0.226 | 3.156 | 0.002 * | 0.053 | 0.2 |
| PLR | 0.002 | 0.001 | 0.230 | 3.064 | 0.003 * | 0.001 | 0.003 |
| MLR | 0.510 | 0.214 | 0.147 | 2.380 | 0.019 * | 0.086 | 0.935 |
| Gammaglobulins (g/dL) | 0.555 | 0.067 | 0.499 | 8.291 | 0.001 * | 0.423 | 0.688 |
| Anti-Ro/SSA | 0.000 | 0.000 | 0.074 | 1.247 | 0.215 | 0.000 | 0.001 |
| Anti-La/SSB | −2.15 × 10−5 | 0.000 | −0.005 | −0.079 | 0.937 | −0.001 | 0.000 |
| Age | 0.000 | 0.002 | 0.009 | 0.154 | 0.878 | −0.004 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, A.; Caruntu, A.; Opris-Belinski, D.; Jurcut, C.; Dima, A.; Caruntu, C.; Ionescu, R. The Predictive Role of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Monocytes-to-Lymphocyte Ratio (MLR) and Gammaglobulins for the Development of Cutaneous Vasculitis Lesions in Primary Sjögren’s Syndrome. J. Clin. Med. 2022, 11, 5525. https://doi.org/10.3390/jcm11195525
Mihai A, Caruntu A, Opris-Belinski D, Jurcut C, Dima A, Caruntu C, Ionescu R. The Predictive Role of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Monocytes-to-Lymphocyte Ratio (MLR) and Gammaglobulins for the Development of Cutaneous Vasculitis Lesions in Primary Sjögren’s Syndrome. Journal of Clinical Medicine. 2022; 11(19):5525. https://doi.org/10.3390/jcm11195525
Chicago/Turabian StyleMihai, Ancuta, Ana Caruntu, Daniela Opris-Belinski, Ciprian Jurcut, Alina Dima, Constantin Caruntu, and Ruxandra Ionescu. 2022. "The Predictive Role of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Monocytes-to-Lymphocyte Ratio (MLR) and Gammaglobulins for the Development of Cutaneous Vasculitis Lesions in Primary Sjögren’s Syndrome" Journal of Clinical Medicine 11, no. 19: 5525. https://doi.org/10.3390/jcm11195525
APA StyleMihai, A., Caruntu, A., Opris-Belinski, D., Jurcut, C., Dima, A., Caruntu, C., & Ionescu, R. (2022). The Predictive Role of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Monocytes-to-Lymphocyte Ratio (MLR) and Gammaglobulins for the Development of Cutaneous Vasculitis Lesions in Primary Sjögren’s Syndrome. Journal of Clinical Medicine, 11(19), 5525. https://doi.org/10.3390/jcm11195525











