The Etiology of Community-Acquired Pneumonia Correlates with Serum Inflammatory Markers in Children
Abstract
1. Introduction
2. Materials and Methods
3. Results
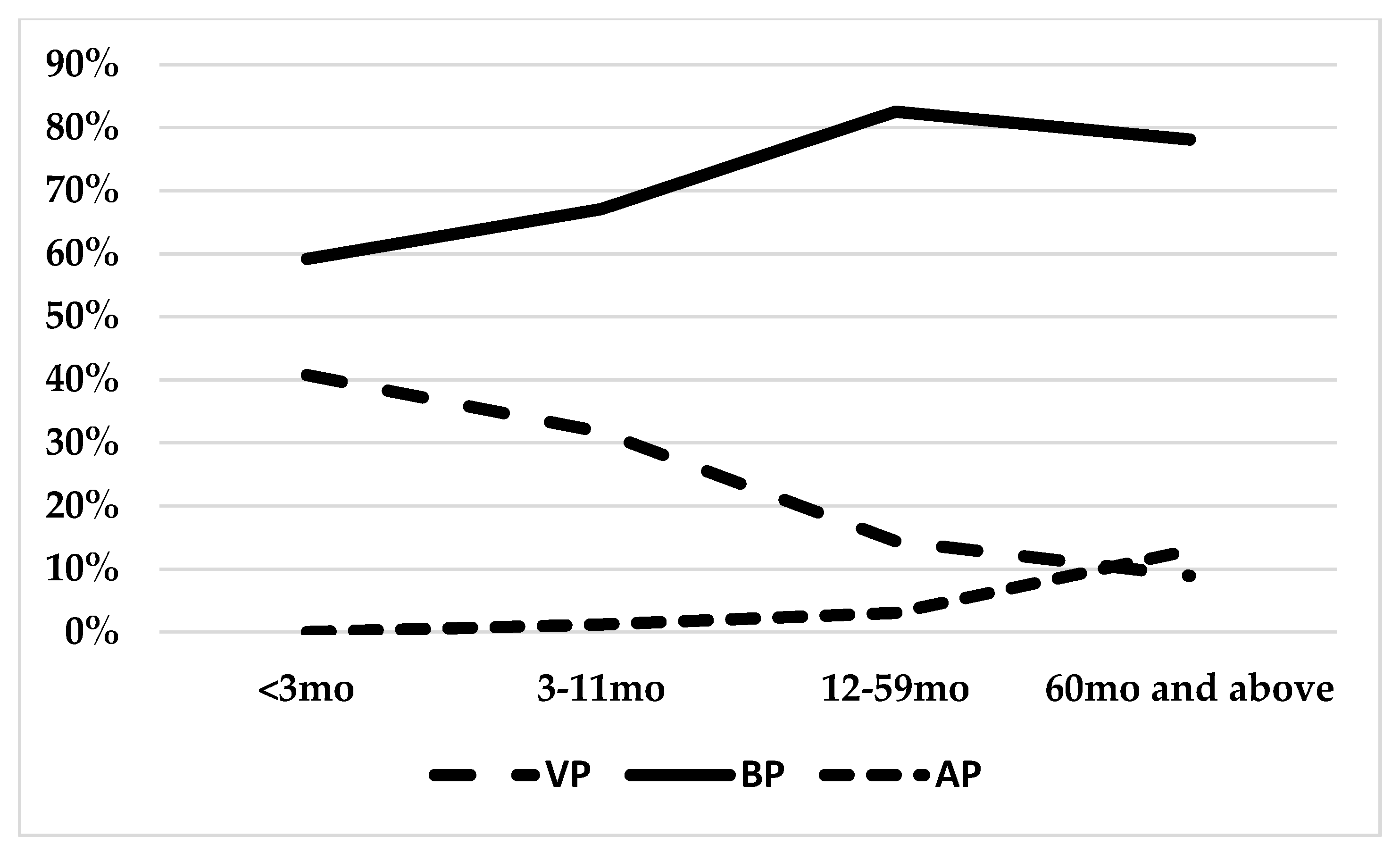
3.1. Etiology of Childhood Pneumonia
3.2. Inflammatory Markers
3.3. Pneumonia Impact on Hospitalizations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD2017 LRIC. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: An analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2020, 20, 60–79. [Google Scholar] [CrossRef]
- Nair, H.; Simões, E.A.; Rudan, I.; Gessner, B.D.; Azziz-Baumgartner, E.; Zhang, J.S.F.; Feikin, D.R.; Mackenzie, G.A.; Moiïsi, J.C.; Roca, A.; et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet 2013, 381, 1380–1390. [Google Scholar] [CrossRef]
- Tahilramani, H.; Misra, M. Clinico-Epidemiologic Study of Acute Lower Respiratory Tract Infections in Children Less Than 5 Years of Age Needing Hospital Admission. Int. J. Med. Sci. Educ. 2019, 6, 85–91. [Google Scholar]
- Cilloniz, C.; Martin-Loeches, I.; Garcia-Vidal, C.; San Jose, A.; Torres, A. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Patterns. Int. J. Mol. Sci. 2016, 17, 2120. [Google Scholar] [CrossRef] [PubMed]
- FIRS FoIRS. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Sheffield, UK, 2017. [Google Scholar]
- McAllister, D.A.; Liu, L.; Shi, T.; Chu, Y.; Reed, C.; Burrows, J.; Adeloye, D.; Rudan, I.; Black, R.E.; Campbell, H.; et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: A systematic analysis. Lancet Glob. Health 2019, 7, e47–e57. [Google Scholar] [CrossRef]
- WHO. Pneumonia. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 8 January 2021).
- Rudan, I.; Boschi-Pinto, C.; Biloglav, Z.; Mulholland, K.; Campbell, H. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ. 2008, 86, 408–416. [Google Scholar] [CrossRef]
- Mapy Potrzeb Zdrowotnych. Available online: https://analizy.mz.gov.pl/app_direct/mpz_2020_szpital/#tab-6081-7 (accessed on 29 August 2022).
- Ruuskanen, O.; Lahti, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef]
- Chi, H.; Huang, Y.C.; Liu, C.C.; Chang, K.Y.; Huang, Y.C.; Lin, H.C.; Chang, L.Y.; Ho, Y.H.; Tsao, K.C.; Mu, J.J.; et al. Characteristics and etiology of hospitalized pediatric community-acquired pneumonia in Taiwan. J. Formos. Med. Assoc. 2020, 119, 1490–1499. [Google Scholar] [CrossRef]
- Shin, E.J.; Kim, Y.; Jeong, J.Y.; Jung, Y.M.; Lee, M.H.; Chung, E.H. The changes of prevalence and etiology of pediatric pneumonia from National Emergency Department Information System in Korea, between 2007 and 2014. Korean J. Pediatr. 2018, 61, 291–300. [Google Scholar] [CrossRef]
- Oumei, H.; Xuefeng, W.; Jianping, L.; Kunling, S.; Rong, M.; Zhenze, C.; Li, D.; Huimin, Y.; Lining, W.; Zhaolan, L.; et al. Etiology of community-acquired pneumonia in 1500 hospitalized children. J. Med. Virol. 2018, 90, 421–428. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Rodríguez, O.; Estrella, B.; Sabin, L.L.; Sempértegui, F.; Hamer, D.H. Etiology of severe pneumonia in Ecuadorian children. PLoS ONE 2017, 12, e0171687. [Google Scholar] [CrossRef]
- El Seify, M.Y.; Fouda, E.M.; Ibrahim, H.M.; Fathy, M.M.; Husseiny Ahmed, A.A.; Khater, W.S.; El Deen, N.N.; Abouzeid, H.G.; Hegazy, N.R.; Elbanna, H.S. Microbial Etiology of Community-Acquired Pneumonia among Infants and Children Admitted to the Pediatric Hospital, Ain Shams University. Eur. J. Microbiol. Immunol. 2016, 6, 206–214. [Google Scholar] [CrossRef]
- Berg, A.S.; Inchley, C.S.; Aase, A.; Fjaerli, H.O.; Bull, R.; Aaberge, I.; Leegaard, T.M.; Nakstad, B. Etiology of Pneumonia in a Pediatric Population with High Pneumococcal Vaccine Coverage: A Prospective Study. Pediatr. Infect. Dis. J. 2016, 35, e69–e75. [Google Scholar] [CrossRef]
- Black, S.B.; Shinefield, H.R.; Ling, S.; Hansen, J.; Fireman, B.; Spring, D.; Noyes, J.; Lewis, E.; Ray, P.; Lee, J.; et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 2002, 21, 810–815. [Google Scholar] [CrossRef]
- Neuman, M.I.; Hall, M.; Lipsett, S.C.; Hersh, A.L.; Williams, D.J.; Gerber, J.S.; Brogan, T.V.; Blaschke, A.J.; Grijalva, C.G.; Parikh, K.; et al. Utility of Blood Culture Among Children Hospitalized With Community-Acquired Pneumonia. Pediatrics 2017, 140. [Google Scholar] [CrossRef]
- Fritz, C.Q.; Edwards, K.M.; Self, W.H.; Grijalva, C.G.; Zhu, Y.; Arnold, S.R.; McCullers, J.A.; Ampofo, K.; Pavia, A.T.; Wunderink, R.G.; et al. Prevalence, Risk Factors, and Outcomes of Bacteremic Pneumonia in Children. Pediatrics 2019, 144, e20183090. [Google Scholar] [CrossRef]
- Iroh Tam, P.Y.; Bernstein, E.; Ma, X.; Ferrieri, P. Blood Culture in Evaluation of Pediatric Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Hosp. Pediatr. 2015, 5, 324–336. [Google Scholar] [CrossRef]
- Huong Ple, T.; Hien, P.T.; Lan, N.T.; Binh, T.Q.; Tuan, D.M.; Anh, D.D. First report on prevalence and risk factors of severe atypical pneumonia in Vietnamese children aged 1–15 years. BMC Public Health 2014, 14, 1304. [Google Scholar] [CrossRef]
- Bradley, J.S.; Byington, C.L.; Shah, S.S.; Alverson, B.; Carter, E.R.; Harrison, C.; Kaplan, S.L.; Mace, S.E.; McCracken, G.H., Jr.; Moore, M.R.; et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, e25–e76. [Google Scholar] [CrossRef]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin assay in systemic inflammation, infection, and sepsis: Clinical utility and limitations. Crit. Care Med. 2008, 36, 941–952. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Biomarkers in Pediatric Community-Acquired Pneumonia. Int. J. Mol. Sci. 2017, 18, 447. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Fujita, I.; Hamasaki, Y.; Miyazaki, S. Differentiating between bacterial and viral infection by measuring both C-reactive protein and 2’-5’-oligoadenylate synthetase as inflammatory markers. J. Infect. Chemother. 2002, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; Nylén, E.S.; White, J.C.; Müller, B.; Snider, R.H., Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: A journey from calcitonin back to its precursors. J. Clin. Endocrinol. Metab. 2004, 89, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M. Pathobiochemistry and clinical use of procalcitonin. Clin. Chim. Acta 2002, 323, 17–29. [Google Scholar] [CrossRef]
- Nora, D.; Salluh, J.; Martin-Loeches, I.; Póvoa, P. Biomarker-guided antibiotic therapy-strengths and limitations. Ann. Transl. Med. 2017, 5, 208. [Google Scholar] [CrossRef]
- Branche, A.; Neeser, O.; Mueller, B.; Schuetz, P. Procalcitonin to guide antibiotic decision making. Curr. Opin. Infect. Dis. 2019, 32, 130–135. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Jaccard-Stolz, D.; Bingisser, R.; Gencay, M.M.; Huber, P.R.; Tamm, M.; Müller, B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet 2004, 363, 600–607. [Google Scholar] [CrossRef]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; González Del Castillo, J.; Jensen, J.U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 2019, 57, 1308–1318. [Google Scholar] [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst. Rev. 2017, 10, Cd007498. [Google Scholar] [CrossRef]
- Townsend, J.; Adams, V.; Galiatsatos, P.; Pearse, D.; Pantle, H.; Masterson, M.; Kisuule, F.; Jacob, E.; Kiruthi, C.; Ortiz, P.; et al. Procalcitonin-Guided Antibiotic Therapy Reduces Antibiotic Use for Lower Respiratory Tract Infections in a United States Medical Center: Results of a Clinical Trial. Open Forum Infect. Dis. 2018, 5, ofy327. [Google Scholar] [CrossRef]
- Wu, G.; Wu, G.; Wu, S.; Wu, H. Comparison of Procalcitonin Guidance-Administered Antibiotics with Standard Guidelines on Antibiotic Therapy in Children with Lower Respiratory Tract Infections: A Retrospective Study in China. Med. Princ. Pract. 2017, 26, 316–320. [Google Scholar] [CrossRef]
- Nascimento-Carvalho, C.M.; Cardoso, M.R.; Barral, A.; Araújo-Neto, C.A.; Guerin, S.; Saukkoriipi, A.; Paldanius, M.; Vainionpää, R.; Lebon, P.; Leinonen, M.; et al. Procalcitonin is useful in identifying bacteraemia among children with pneumonia. Scand. J. Infect. Dis. 2010, 42, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, C.; Ampofo, K.; Killpack, J.; Williams, D.J.; Edwards, K.M.; Grijalva, C.G.; Arnold, S.R.; McCullers, J.A.; Anderson, E.J.; Wunderink, R.G.; et al. Procalcitonin Accurately Identifies Hospitalized Children with Low Risk of Bacterial Community-Acquired Pneumonia. J. Pediatric Infect. Dis. Soc. 2018, 7, 46–53. [Google Scholar] [CrossRef]
- Li, F.; Kong, S.; Xie, K.; Zhang, Y.; Yan, P.; Zhao, W. High ratio of C-reactive protein/procalcitonin predicts Mycoplasma pneumoniae infection among adults hospitalized with community acquired pneumonia. Scand. J. Clin. Lab. Invest. 2021, 81, 65–71. [Google Scholar] [CrossRef]
- Neeser, O.L.; Vukajlovic, T.; Felder, L.; Haubitz, S.; Hammerer-Lercher, A.; Ottiger, C.; Mueller, B.; Schuetz, P.; Fux, C.A. A high C-reactive protein/procalcitonin ratio predicts Mycoplasma pneumoniae infection. Clin. Chem. Lab. Med. 2019, 57, 1638–1646. [Google Scholar] [CrossRef]
- Kraj, G.; Peradzyńska, J.; Chądzyńska, J.; Kulus, M.; Wołoszyn, K.; Jackowska, T.; Krajewska, M.; Mołdoch-Łukasik, A.; Czubik-Przybyła, A.; Górska-Kot, A.; et al. The Influence of National Guidelines on the Management of Community-Acquired Pneumonia in Children. Do Pediatricians Follow the Recommendations? Adv. Exp. Med. Biol. 2019, 1211, 103–110. [Google Scholar] [CrossRef]
- Hryniewicz, W.; Albrechta, P.; Radzikowski, A. Rekomendacje Postępowania w Pozaszpitalnych Zakażeniach Układu Oddechowego. 2016. Available online: http://antybiotyki.edu.pl/wp-content/uploads/Rekomendacje/Rekomendacje2016.pdf (accessed on 23 March 2021).
- Rueda, Z.V.; Aguilar, Y.; Maya, M.A.; López, L.; Restrepo, A.; Garcés, C.; Morales, O.; Roya-Pabón, C.; Trujillo, M.; Arango, C.; et al. Etiology and the challenge of diagnostic testing of community-acquired pneumonia in children and adolescents. BMC Pediatr. 2022, 22, 169. [Google Scholar] [CrossRef]
- Nolan, V.G.; Arnold, S.R.; Bramley, A.M.; Ampofo, K.; Williams, D.J.; Grijalva, C.G.; Self, W.H.; Anderson, E.J.; Wunderink, R.G.; Edwards, K.M.; et al. Etiology and Impact of Coinfections in Children Hospitalized With Community-Acquired Pneumonia. J. Infect. Dis. 2018, 218, 179–188. [Google Scholar] [CrossRef]
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Yao, S.; Zha, H.; Huang, B.; Liu, D.; Wu, K. Prevalence and clinical significance of common respiratory pathogens in the upper respiratory tract of children with community-acquired pneumonia in Zunyi, China. Pediatr. Pulmonol. 2020, 55, 2437–2443. [Google Scholar] [CrossRef]
- Farah, M.M.; Padgett, L.B.; McLario, D.J.; Sullivan, K.M.; Simon, H.K. First-time wheezing in infants during respiratory syncytial virus season: Chest radiograph findings. Pediatr. Emerg. Care 2002, 18, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Lalani, A.; Allen, U.; Manson, D.; Babyn, P.; Stephens, D.; MacPhee, S.; Mokanski, M.; Khaikin, S.; Dick, P. Evaluation of the utility of radiography in acute bronchiolitis. J. Pediatr. 2007, 150, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Wrotek, A.; Czajkowska, M.; Jackowska, T. Chest Radiography in Children Hospitalized with Bronchiolitis. Adv. Exp. Med. Biol. 2019, 1222, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Jackowska, T.; Wrotek, A. Etiology of community-acquired pneumonia in children hospitalized in the period of 2009–2012. Dev. Period. Med. 2014, 18, 209–215. [Google Scholar]
- Zar, H.J.; Polack, F.P. Childhood pneumonia: The role of viruses. Thorax 2015, 70, 811–812. [Google Scholar] [CrossRef][Green Version]
- Ben-Shimol, S.; Dagan, R.; Givon-Lavi, N.; Avital, D.; Bar-Ziv, J.; Greenberg, D. Use of Chest Radiography Examination as a Probe for Pneumococcal Conjugate Vaccine Impact on Lower Respiratory Tract Infections in Young Children. Clin. Infect. Dis. 2020, 71, 177–187. [Google Scholar] [CrossRef]
- Wagner, C.E.; Prentice, J.A.; Saad-Roy, C.M.; Yang, L.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Economic and Behavioral Influencers of Vaccination and Antimicrobial Use. Front. Public Health 2020, 8, 614113. [Google Scholar] [CrossRef]
- Cantais, A.; Mory, O.; Plat, A.; Bourmaud, A.; Giraud, A.; Costille, M.; Pozzetto, B.; Pillet, S. Impact of bedside diagnosis of influenza in the paediatric emergency ward. Clin. Microbiol. Infect. 2019, 25, 898–903. [Google Scholar] [CrossRef]
- Finelli, L.; Fiore, A.; Dhara, R.; Brammer, L.; Shay, D.K.; Kamimoto, L.; Fry, A.; Hageman, J.; Gorwitz, R.; Bresee, J.; et al. Influenza-associated pediatric mortality in the United States: Increase of Staphylococcus aureus coinfection. Pediatrics 2008, 122, 805–811. [Google Scholar] [CrossRef]
- Kumar, K.J.; Ashok Chowdary, K.V.; Usha, H.C.; Kulkarni, M.; Manjunath, V.G. Etiology of community acquired pneumonia among children in India with special reference to atypical pathogens. Lung India Off. Organ Indian Chest Soc. 2018, 35, 116–120. [Google Scholar] [CrossRef]
- Miyashita, N.; Akaike, H.; Teranishi, H.; Kawai, Y.; Ouchi, K.; Kato, T.; Hayashi, T.; Okimoto, N.; Group, A.P.S. Chlamydophila pneumoniae serology: Cross-reaction with Mycoplasma pneumoniae infection. J. Infect. Chemother. 2013, 19, 256–260. [Google Scholar] [CrossRef]
- Virkki, R.; Juven, T.; Rikalainen, H.; Svedström, E.; Mertsola, J.; Ruuskanen, O. Differentiation of bacterial and viral pneumonia in children. Thorax 2002, 57, 438–441. [Google Scholar] [CrossRef]
- Korppi, M.; Kröger, L. C-reactive protein in viral and bacterial respiratory infection in children. Scand. J. Infect. Dis. 1993, 25, 207–213. [Google Scholar] [CrossRef]
- Nohynek, H.; Valkeila, E.; Leinonen, M.; Eskola, J. Erythrocyte sedimentation rate, white blood cell count and serum C-reactive protein in assessing etiologic diagnosis of acute lower respiratory infections in children. Pediatr. Infect. Dis. J. 1995, 14, 484–490. [Google Scholar] [CrossRef]
- Heiskanen-Kosma, T.; Korppi, M. Serum C-reactive protein cannot differentiate bacterial and viral aetiology of community-acquired pneumonia in children in primary healthcare settings. Scand. J. Infect. Dis. 2000, 32, 399–402. [Google Scholar] [CrossRef]
- Flood, R.G.; Badik, J.; Aronoff, S.C. The utility of serum C-reactive protein in differentiating bacterial from nonbacterial pneumonia in children: A meta-analysis of 1230 children. Pediatric Infect. Dis. J. 2008, 27, 95–99. [Google Scholar] [CrossRef]
- Esposito, S.; Di Gangi, M.; Cardinale, F.; Baraldi, E.; Corsini, I.; Da Dalt, L.; Tovo, P.A.; Correra, A.; Villani, A.; Sacco, O.; et al. Sensitivity and Specificity of Soluble Triggering Receptor Expressed on Myeloid Cells-1, Midregional Proatrial Natriuretic Peptide and Midregional Proadrenomedullin for Distinguishing Etiology and to Assess Severity in Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0163262. [Google Scholar] [CrossRef]
- Moulin, F.; Raymond, J.; Lorrot, M.; Marc, E.; Coste, J.; Iniguez, J.L.; Kalifa, G.; Bohuon, C.; Gendrel, D. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch. Dis. Child. 2001, 84, 332–336. [Google Scholar] [CrossRef]
- Korppi, M. Non-specific host response markers in the differentiation between pneumococcal and viral pneumonia: What is the most accurate combination? Pediatr. Int. 2004, 46, 545–550. [Google Scholar] [CrossRef]
- Hedlund, J.; Hansson, L.O. Procalcitonin and C-reactive protein levels in community-acquired pneumonia: Correlation with etiology and prognosis. Infection 2000, 28, 68–73. [Google Scholar] [CrossRef]
- Jereb, M.; Kotar, T. Usefulness of procalcitonin to differentiate typical from atypical community-acquired pneumonia. Wien. Klin. Wochenschr. 2006, 118, 170–174. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Yin, L.; Deng, Y.; Chu, G.; Liu, S. Utilization of serum procalcitonin as a biomarker in the diagnosis and treatment of children with bacterial hospital-acquired pneumonia. Mol. Cell. Biochem. 2021, 476, 261–267. [Google Scholar] [CrossRef]
- Krüger, S.; Ewig, S.; Papassotiriou, J.; Kunde, J.; Marre, R.; von Baum, H.; Suttor, N.; Welte, T.; the, C.s.g. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP—Results from the German competence network CAPNETZ. Respir. Res. 2009, 10, 65. [Google Scholar] [CrossRef]
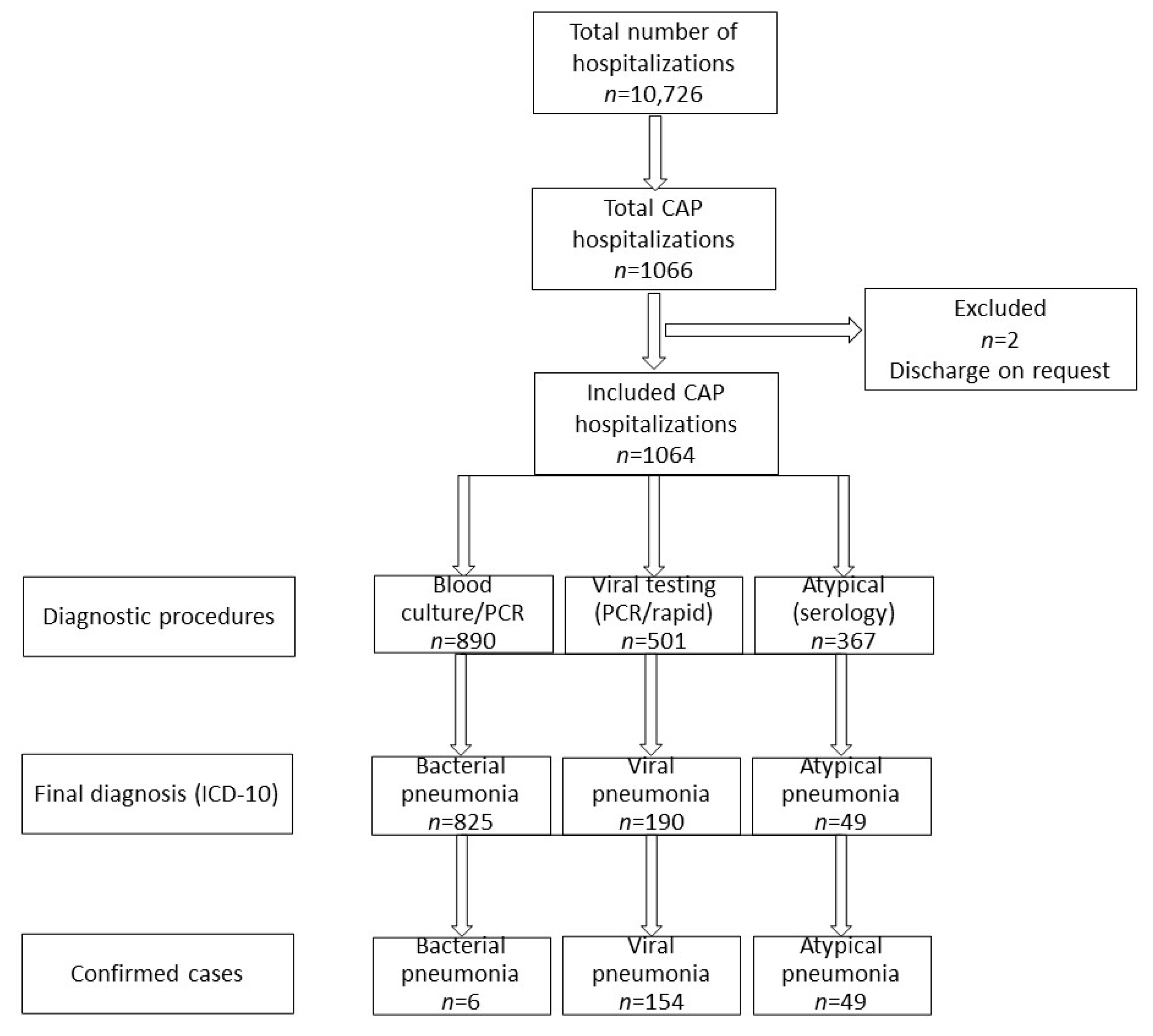
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Age: 0–18 years old 2. Hospitalization due to CAP between January 2013 and December 2018 3. CAP criteria: (a) community-acquired pneumonia: signs/symptoms (see below) present prior to hospitalization or up to the first 48 h after hospital admission (b) 2 or more of the following signs/symptoms: fever (38 degrees Celsius or more), cough, tachypnea (according to age, i.e., 0–1 months > 60 breaths/min, >1–12 months > 50 breaths/min, >12–59 months > 40 breaths/min, 60 months or older > 25 breaths/min), intercostal retractions, a dull percussion note and (c) crackles or a bronchial murmur on auscultation OR positive chest X-ray (i.e., presence of consolidation, or parenchymal infiltrates, or linear densities, or patchy densities, or pleural effusion) OR positive lung ultrasound (hypoechogenic lung lesions, or pleural line abnormalities—locally absent or hypoechogenic, or bronchogram sign-hyperechogenic area within the consolidation, or impaired lung respiratory mobility-absent or decreased “lung sliding”) | 1. immunodeficiency (congenital or acquired or drug-related), 2. hemodynamically significant heart disease 3. disease worsening the course of respiratory tract infection (cystic fibrosis, neuromuscular disease) 4. lack of full knowledge on the clinical course of the disease (e.g., a discharge on parent’s/tutor’s request) |
| VP (n = 190) | BP (n = 825) | AP (n = 49) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Statistical Significance | ||||
| age (months) | 16.63 | 4.77 | 35.47 | 30.90 | 16.13 | 55.30 | 75.15 | 43.43 | 108.46 | VP vs. BP vs. AP |
| WBC (10 × 3/μL) n = 1061 | 10.95 | 7.30 | 15.13 | 12.82 | 8.99 | 18.40 | 12.27 | 8.49 | 15.50 | VP vs. BP |
| ANC (10 × 3/μL) n = 1061 | 4.41 | 2.33 | 8.08 | 7.10 | 3.91 | 12.14 | 6.39 | 4.82 | 11.59 | VP vs. BP VP vs. AP |
| CRP (mg/L) n = 1061 | 7.07 | 2.33 | 22.66 | 24.26 | 7.67 | 66.94 | 14.57 | 6.32 | 32.34 | VP vs. BP |
| PCT (ng/mL) n = 936 | 0.22 | 0.10 | 0.52 | 0.36 | 0.12 | 1.50 | 0.11 | 0.08 | 0.16 | VP vs. BP vs. AP |
| CRP/ PCT n = 936 | 36.46 | 12.98 | 83.90 | 55.89 | 19.19 | 141.78 | 120.41 | 65.33 | 190.06 | VP vs. BP vs. AP |
| LOS (days) | 7 | 5 | 10 | 7 | 4 | 9 | 7 | 5 | 10 | VP vs. BP |
| BP vs. VP | |||||||||
| AUC | 95% CI | p | Optimal Cut-Off (Youden Index) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | ||
| WBC | 0.606 | 0.56 | 0.65 | <0.01 | 11.96 | 56.33% | 59.47% | 85.74% | 23.94% |
| 52.86% to 59.75% | (52.13% to 66.52%) | (83.36% to 87.83%) | (21.47% to 26.60%) | ||||||
| ANC | 0.658 | 0.62 | 0.70 | <0.01 | 5.22 | 65.69% | 58.42% | (87.24% | 28.24% |
| 62.34% to 68.94% | (51.06% to 65.51%) | 85.15% to 89.07%) | (25.25% to 31.44%) | ||||||
| CRP | 0.675 | 0.63 | 0.72 | <0.01 | 12.94 | 64.56% | 64.55% | 88.78% | 29.54% |
| 61.17% to 67.83% | (57.28% to 71.36%) | (86.64% to 90.61%) | (26.71% to 32.54%) | ||||||
| PCT | 0.589 | 0.55 | 0.63 | <0.01 | 0.33 | 52.12% | 66.46% | 87.59% | 23.41% |
| 48.43% to 55.80% | (58.60% to 73.70%) | (84.88% to 89.86%) | (21.11% to 25.89%) | ||||||
| CRP/PCT | 0.592 | 0.55 | 0.64 | <0.01 | 74.332 | 43.82% | 73.12% | 88.12% | 22.24% |
| 40.18% to 47.51% | (65.55% to 79.82%) | (85.01% to 90.66%) | (20.34% to 24.27%) | ||||||
| BP vs. AP | |||||||||
| AUC | 95% CI | p | Optimal Cut-Off (Youden Index) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | ||
| WBC | 0.563 | 0.49 | 0.64 | 0.1047 | |||||
| ANC | 0.507 | 0.43 | 0.58 | 0.8582 | |||||
| CRP | 0.604 | 0.54 | 0.67 | <0.01 | 22.19 | 52.50% | 69.39% | 96.64% | 8.02% |
| 49.02% to 55.96% | (54.58% to 81.75%) | (94.94% to 97.78%) | (6.67% to 9.62%) | ||||||
| PCT | 0.733 | 0.67 | 0.79 | <0.01 | 0.187 | 64.16% | 82.22% | 98.32% | 12.37% |
| 60.56% to 67.64% | (67.95% to 92.00%) | (96.89% to 99.10%) | (10.68% to 14.30%) | ||||||
| CRP/PCT | 0.656 | 0.58 | 0.73 | <0.01 | 65 | 53.16% | 77.78% | 97.48% | 9.31% |
| 49.46% to 56.83% | (62.91% to 88.80%) | (95.71% to 98.53%) | (7.94% to 10.89%) | ||||||
| VP vs. AP | |||||||||
| AUC | 95% CI | p | Optimal Cut-Off (Youden Index) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | ||
| WBC | 0.449 | 0.36 | 0.54 | 0.2434 | |||||
| ANC | 0.667 | 0.59 | 0.75 | <0.01 | 4.42 | 50.53% | 79.59% | 90.57% | 29.32% |
| 43.19% to 57.84% | (65.66% to 89.76%) | (84.44% to 94.44%) | (25.32% to 33.67%) | ||||||
| CRP | 0.607 | 0.53 | 0.69 | <0.01 | 69.17 | 9.52% | 95.92% | 90.00% | 21.56% |
| 5.74% to 14.63% | (86.02% to 99.50%) | (68.36% to 97.40%) | (20.33% to 22.84%) | ||||||
| PCT | 0.68 | 0.60 | 0.76 | <0.01 | 0.19 | 22.98% | 95.56% | 94.87% | 25.75% |
| 16.73% to 30.26% | (84.85% to 99.46%) | (82.26% to 98.66%) | (23.79% to 27.81%) | ||||||
| CRP/PCT | 0.752 | 0.67 | 0.83 | <0.01 | 63.895 | 68.12% | 77.78% | 91.60% | 40.70% |
| 60.30% to 75.26% | (62.91% to 88.80%) | (86.20% to 95.01%) | (34.26% to 47.47%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrotek, A.; Robakiewicz, J.; Pawlik, K.; Rudzinski, P.; Pilarska, I.; Jaroń, A.; Imiełowska, A.; Jarzębowska, M.; Zabłocka, K.; Jackowska, T. The Etiology of Community-Acquired Pneumonia Correlates with Serum Inflammatory Markers in Children. J. Clin. Med. 2022, 11, 5506. https://doi.org/10.3390/jcm11195506
Wrotek A, Robakiewicz J, Pawlik K, Rudzinski P, Pilarska I, Jaroń A, Imiełowska A, Jarzębowska M, Zabłocka K, Jackowska T. The Etiology of Community-Acquired Pneumonia Correlates with Serum Inflammatory Markers in Children. Journal of Clinical Medicine. 2022; 11(19):5506. https://doi.org/10.3390/jcm11195506
Chicago/Turabian StyleWrotek, August, Julita Robakiewicz, Katarzyna Pawlik, Patryk Rudzinski, Izabela Pilarska, Aleksandra Jaroń, Aleksandra Imiełowska, Małgorzata Jarzębowska, Katarzyna Zabłocka, and Teresa Jackowska. 2022. "The Etiology of Community-Acquired Pneumonia Correlates with Serum Inflammatory Markers in Children" Journal of Clinical Medicine 11, no. 19: 5506. https://doi.org/10.3390/jcm11195506
APA StyleWrotek, A., Robakiewicz, J., Pawlik, K., Rudzinski, P., Pilarska, I., Jaroń, A., Imiełowska, A., Jarzębowska, M., Zabłocka, K., & Jackowska, T. (2022). The Etiology of Community-Acquired Pneumonia Correlates with Serum Inflammatory Markers in Children. Journal of Clinical Medicine, 11(19), 5506. https://doi.org/10.3390/jcm11195506







